An Update on Translating Stem Cell Therapy for Stroke from Bench to Bedside
Abstract
:1. Translational Gating Items of Stem Cell Therapy
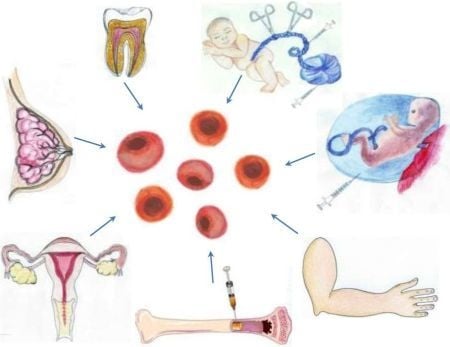
2. Tailoring Stem Cells for Therapeutic Applications in Stroke
3. Searching for Safe and Effective Stem Cell Therapy

3.1. A Stem Cell Source with a Long History of Transplant Use: Bone Marrow-Derived Stem Cells
3.2. Harvesting Neural Stem Cells for Neural Repair in Stroke
3.3. Recapitulating Cell Developmental Growth in Other Adult Stem Cells
3.3.1. Mimicking Bone Marrow Therapeutic Transplant Potential: Umbilical Cord Blood (UCB)
3.3.2. Shedding the Fat for Stem Cells: Adipose Tissue
3.3.3. Gender-Specific Stem Cells: Menstrual Blood-Derived Stem Cells
3.3.4. Mother Knows Best: Breast Milk-Derived Stem Cells
3.3.5. A Wisdom Tooth: Dental Tissue-Derived Stem Cells
3.3.6. Reverting Differentiated Tissues to Stem Cells: Induced-Pluripotent Stem Cells
4. Stem Cell Therapy is Not a Magic Bullet: Exploring Co-Adjunctive Therapies
5. Conclusions
Conflicts of Interest
References
- Saino, O.; Taguchi, A.; Nakagomi, T.; Nakano-Doi, A.; Kashiwamura, S.; Doe, N.; Nakagomi, N.; Soma, T.; Yoshikawa, H.; Stern, D.M.; et al. Immunodeficiency reduces neural stem/progenitor cell apoptosis and enhances neurogenesis in the cerebral cortex after stroke. J. Neurosci. Res. 2010, 88, 2385–2397. [Google Scholar]
- Erlandsson, A.; Lin, C.H.; Yu, F.; Morshead, C.M. Immunosuppression promotes endogenous neural stem and progenitor cell migration and tissue regeneration after ischemic injury. Exp. Neurol. 2011, 230, 48–57. [Google Scholar] [CrossRef]
- Willing, A.E.; Eve, D.J.; Sanberg, P.R. Umbilical cord blood transfusions for prevention of progressive brain injury and induction of neural recovery: An immunological perspective. Regen. Med. 2007, 2, 457–464. [Google Scholar] [CrossRef]
- Hunt, J.S.; Petroff, M.G.; McIntire, R.H.; Ober, C. HLA-G and immune tolerance in pregnancy. FASEB J. 2005, 19, 681–693. [Google Scholar] [CrossRef]
- Lee, J.M.; Jung, J.; Lee, H.J.; Jeong, S.J.; Cho, K.J.; Hwang, S.G.; Kim, G.J. Comparison of immunomodulatory effects of placenta mesenchymal stem cells with bone marrow and adipose mesenchymal stem cells. Int. Immunopharmacol. 2012, 13, 219–224. [Google Scholar] [CrossRef]
- Menier, C.; Rouas-Freiss, N.; Favier, B.; LeMaoult, J.; Moreau, P.; Carosella, E.D. Recent advances on the non-classical major histocompatibility complex class I HLA-G molecule. Tissue Antigens 2010, 75, 201–206. [Google Scholar] [CrossRef]
- Fazekasova, H.; Lechler, R.; Langford, K.; Lombardi, G. Placenta-derived MSCs are partially immunogenic and less immunomodulatory than bone marrow-derived MSCs. J. Tissue Eng. Regen. Med. 2011, 5, 684–694. [Google Scholar] [CrossRef]
- Newcomb, J.D.; Ajmo, C.T., Jr.; Sanberg, C.D.; Sanberg, P.R.; Pennypacker, K.R.; Willing, A.E. Timing of cord blood treatment after experimental stroke determines therapeutic efficacy. Cell Transpl. 2006, 15, 213–223. [Google Scholar] [CrossRef]
- Huang, H.; Chen, L.; Sanberg, P. Cell therapy from bench to bedside translation in CNS neurorestoratology era. Cell Med. 2010, 1, 15–46. [Google Scholar] [CrossRef]
- Oyamada, N.; Itoh, H.; Sone, M.; Yamahara, K.; Miyashita, K.; Park, K.; Taura, D.; Inuzuka, M.; Sonoyama, T.; Tsujimoto, H.; et al. Transplantation of vascular cells derived from human embryonic stem cells contributes to vascular regeneration after stroke in mice. J. Transl. Med. 2008, 6, 1–14. [Google Scholar] [CrossRef]
- Hayashi, J.; Takagi, Y.; Fukuda, H.; Imazato, T.; Nishimura, M.; Fujimoto, M.; Takahashi, J.; Hashimoto, N.; Nozaki, K. Primate embryonic stem cell-derived neuronal progenitors transplanted into ischemic brain. J. Cereb. Blood Flow Metab. 2006, 26, 906–914. [Google Scholar] [CrossRef]
- Yanagisawa, D.; Qi, M.; Kim, D.H.; Kitamura, Y.; Inden, M.; Tsuchiya, D.; Takata, K.; Taniguchi, T.; Yoshimoto, K.; Shimoama, S.; et al. Improvement of focal ischemia-induced rat dopaminergic dysfunction by striatal transplantation of mouse embryonic stem cells. Neurosci. Lett. 2006, 407, 74–79. [Google Scholar] [CrossRef]
- Wei, L.; Cui, L.; Snider, B.J.; Rivkin, M.; Yu, S.S.; Lee, C.S.; Adams, L.D.; Gottlieb, D.I.; Johnson, E.M.; Yu, S.P.; et al. Transplantation of embryonic stem cells overexpressing Bcl-2 promotes functional recovery after transient cerebral ischemia. Neurobiol. Dis. 2005, 19, 183–193. [Google Scholar] [CrossRef]
- Pignataro, G.; Studer, F.E.; Wilz, A.; Simon, R.P.; Boison, D. Neuroprotection in ischemic mouse brain induced by stem cell-derived brain implants. J. Cereb. Blood Flow Metab. 2007, 27, 919–927. [Google Scholar]
- Theus, M.H.; Wei, L.; Cui, L.; Francis, K.; Hu, X.Y.; Keogh, C.; Yu, S.P. In vitro hypoxic preconditioning of embryonic stem cells as a strategy of promoting cell survival and functional benefits after transplantation into the ischemic rat brain. Exp. Neurol. 2008, 210, 656–670. [Google Scholar] [CrossRef]
- Yang, T.; Tsang, K.S.; Poon, W.S.; Ng, H.K. Neurotrophism of bone marrow stromal cells to embryonic stem cells: Noncontact induction and transplantation to a mouse ischemic stroke model. Cell Transpl. 2009, 18, 391–404. [Google Scholar] [CrossRef]
- Li, Z.; McKercher, S.R.; Cui, J.; Nie, Z.G.; Soussou, W.; Roberts, A.J.; Sallmen, T.; Lipton, J.H.; Talantova, M.; Okamoto, S.I.; et al. Myocyte enhancer factor 2C as a neurogenic and antiapoptotic transcription factor in murine embryonic stem cells. J. Neurosci. 2008, 28, 6557–6568. [Google Scholar] [CrossRef]
- Hoehn, M.; Kustermann, E.; Blunk, J.; Wiedermann, D.; Trapp, T.; Wecker, S.; Focking, M.; Arnold, H.; Hescheler, J.; Fleischmann, B.K.; et al. Monitoring of implanted stem cell migration in vivo: A highly resolved in vivo magnetic resonance imaging investigation of experimental stroke in rat. Proc. Natl. Acad. Sci. USA 2002, 99, 16267–16272. [Google Scholar] [CrossRef]
- Lappalainen, R.S.; Narkilahti, S.; Huhtala, T.; Liimatainen, T.; Suuronen, T.; Narvanen, A.; Suuronen, R.; Hovatta, O.; Jolkkonen, J. The SPECT imaging shows the accumulation of neural progenitor cells into internal organs after systemic administration in middle cerebral artery occlusion rats. Neurosci. Lett. 2008, 440, 246–250. [Google Scholar] [CrossRef]
- Newman, M.B.; Misiuta, I.; Willing, A.E.; Zigova, T.; Karl, R.C.; Borlongan, C.V.; Sanberg, P.R. Tumorigenicity issues of embryonic carcinoma-derived stem cells: Relevance to surgical trials using NT2 and hNT neural cells. Stem Cells Dev. 2005, 14, 29–43. [Google Scholar] [CrossRef]
- Kawai, H.; Yamashita, T.; Ohta, Y.; Deguchi, K.; Nagotani, S.; Zhang, X.; Ikeda, Y.; Matsuura, T.; Abe, K. Tridermal tumorigenesis of induced pluripotent stem cells transplanted in ischemic brain. J. Cereb. Blood Flow Metabol. 2010, 30, 1487–1493. [Google Scholar] [CrossRef]
- Przyborski, S. Differentiation of human embryonic stem cells after transplantation in immune-deficient mice. Stem Cells 2005, 23, 1242–1250. [Google Scholar] [CrossRef]
- Hovatta, O.; Jaconi, M.; Thnen, V.; Bna, F.; Gimelli, S.; Bosman, A.; Holm, F.; Wyder, S.; Zdobnov, E.M.; Irion, O.; et al. A teratocarcinoma-like human embryonic stem cell (hESC) line and four hESC lines reveal potentially oncogenic genomic changes. PloS One 2010, 5, e10263. [Google Scholar] [CrossRef]
- Hara, K.; Yasuhara, T.; Maki, M.; Matsukawa, N.; Masuda, T.; Yu, S.J.; Ali, M.; Yu, G.; Xu, L.; Kim, S.U.; et al. Neural progenitor NT2N cell lines from teratocarcinoma for transplantation therapy in stroke. Prog. Neurobiol. 2008, 3, 318–334. [Google Scholar]
- Ghosh, Z.; Huang, M.; Hu, S.; Wilson, K.D.; Dey, D.; Wu, J.C. Dissecting the oncogenic and tumorigenic potential of differentiated human induced pluripotent stem cells and human embryonic stem cells. Cancer Res. 2011, 14, 5030–5039. [Google Scholar]
- Snyder, E.Y. The risk of putting something where it does not belong: Mesenchymal stem cells produce masses in the brain. Exp. Neurol. 2011, 1, 75–77. [Google Scholar] [CrossRef]
- Marcus, A.J.; Woodbury, D. Fetal stem cells from extra-embryonic tissues: Do not discard. J. Cell Mol. Med. 2008, 12, 730–742. [Google Scholar] [CrossRef]
- Yu, S.J.; Soncini, M.; Kaneko, Y.; Hess, D.C.; Parolini, O.; Borlongan, C.V. Amnion: A potent graft source for cell therapy in stroke. Cell Transpl. 2009, 18, 111–118. [Google Scholar] [CrossRef]
- Konig, J.; Huppertz, B.; Desoye, G.; Parolini, O.; Frohlich, J.D.; Weiss, G.; Dohr, G.; Sedlmayr, P.; Lang, I. Amnion-derived mesenchymal stromal cells show angiogenic properties but resist differentiation into mature endothelial cells. Stem Cells Dev. 2012, 21, 1309–1320. [Google Scholar] [CrossRef]
- Yarygin, K.N.; Kholodenko, I.V.; Konieva, A.A.; Burunova, V.V.; Tairova, R.T.; Gubsky, L.V.; Cheglakov, I.B.; Pirogov, Y.A.; Yarygin, V.N.; Skvortsova, V.I. Mechanisms of positive effects of transplantation of human placental mesenchymal stem cells on recovery of rats after experimental ischemic stroke. Bull. Exp. Biol. Med. 2009, 148, 862–868. [Google Scholar] [CrossRef]
- Chen, J.; Shehadah, A.; Pal, A.; Zacharek, A.; Cui, X.; Cui, Y.; Roberts, C.; Lu, M.; Zeitlin, A.; Hariri, R.; et al. Neuroprotective effect of human placenta-derived cell treatment of stroke in rats. Cell Transpl. 2012, 22, 871–879. [Google Scholar]
- Kranz, A.; Wagner, D.C.; Kamprad, M.; Scholz, M.; Schmidt, U.R.; Nitzsche, F.; Aberman, Z.; Emmrich, F.; Riegelsberger, U.M.; Boltze, J. Transplantation of placenta-derived mesenchymal stromal cells upon experimental stroke in rats. Brain Res. 2010, 1315, 128–136. [Google Scholar] [CrossRef]
- Liao, W.B.; Xie, J.; Zhong, J.; Liu, Y.J.; Du, L.; Zhou, B.; Xu, J.; Liu, P.X.; Yang, S.G.; Wang, J.M.; et al. Therapeutic effect of human umbilical cord multipotent mesenchymal stromal cells in a rat model of stroke. Transplantation 2009, 87, 350–359. [Google Scholar] [CrossRef]
- Deuse, T.; Stubbendorff, M.; Tang-Quan, K.; Phillips, N.; Kay, M.A.; Eiermann, T.; Phan, T.T.; Volk, H.D.; Reichenspurner, H.; Robbins, R.C.; et al. Immunogenicity and immunomodulatory properties of umbilical cord lining mesenchymal stem cells. Cell Transpl. 2011, 20, 655–667. [Google Scholar] [CrossRef]
- Borlongan, C.V.; Glover, L.E.; Tajiri, N.; Kaneko, Y.; Freeman, T.B. The great migration of bone marrow-derived stem cells toward the ischemic brain: Therapeutic implications for stroke and other neurological disorders. Prog. Neurobiol. 2011, 95, 213–228. [Google Scholar] [CrossRef]
- Herzog, E.L.; Chai, L.; Krause, D.S. Plasticity of marrow-derived stem cells. Blood 2003, 102, 3483–3493. [Google Scholar] [CrossRef]
- Lapidot, T.; Dar, A.; Kollet, O. How do stem cells find their way home? Blood 2005, 106, 1901–1910. [Google Scholar] [CrossRef]
- Lapidot, T.; Kollet, O. The brain-bone-blood triad: Traffic lights for stem-cell homing and mobilization. Hematology 2010, 2010, 1–6. [Google Scholar] [CrossRef]
- Nervi, B.; Link, D.C.; DiPersio, J.F. Cytokines and hematopoietic stem cell mobilization. J. Cell. Biochem. 2006, 99, 690–705. [Google Scholar] [CrossRef]
- Papayannopoulou, T.; Scadden, D.T. Stem-cell ecology and stem cells in motion. Blood 2008, 111, 3923–3930. [Google Scholar] [CrossRef]
- Kalinkovich, A.; Spiegel, A.; Shivtiel, S.; Kollet, O.; Jordaney, N.; Piacibello, W.; Lapidot, T. Blood-forming stem cells are nervous: Direct and indirect regulation of immature human CD34+ cells by the nervous system. Brain Behav. Immun. 2009, 23, 1059–1065. [Google Scholar] [CrossRef]
- Moniche, F.; Gonzalez, A.; Gonzalez-Marcos, J.R.; Carmona, M.; Pinero, P.; Espigado, I.; Garcia-Solis, D.; Cayuela, A.; Montaner, J.; Boada, C.; et al. Intra-arterial bone marrow mononuclear cells in ischemic stroke a pilot clinical trial. Stroke 2012, 43, 2242–2244. [Google Scholar] [CrossRef]
- Savitz, S.I.; Misra, V.; Kasam, M.; Juneja, H.; Cox, C.S.; Alderman, S.; Aisiku, I.; Kar, S.; Gee, A.; Grotta, J.C. Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Ann. Neurol. 2011, 70, 59–69. [Google Scholar] [CrossRef]
- Battistella, V.; de Freitas, G.R.; da Fonseca, L.M.B.; Mercante, D.; Gutfilen, B.; Goldenberg, R.C.S.; Dias, J.V.; Kasai-Brunswick, T.H.; Wajnberg, E.; Rosado-de-Castro, P.H.; et al. Safety of autologous bone marrow mononuclear cell transplantation in patients with nonacute ischemic stroke. Regen. Med. 2011, 6, 45–52. [Google Scholar] [CrossRef]
- Friedrich, M.A.G.; Martins, M.P.; Araujo, M.D.; Klamt, C.; Vedolin, L.; Garicochea, B.; Raupp, E.F.; El Ammar, J.S.; Machado, D.C.; da Costa, J.C.; et al. Intra-arterial infusion of autologous bone marrow mononuclear cells in patients with moderate to severe middle cerebral artery acute ischemic stroke. Cell Transpl. 2012, 21, 13–21. [Google Scholar] [CrossRef]
- Felfly, H.; Muotri, A.; Yao, H.; Haddad, G.G. Hematopoietic stem cell transplantation protects mice from lethal stroke. Exp. Neurol. 2010, 225, 284–293. [Google Scholar] [CrossRef]
- Schwarting, S.; Litwak, S.; Hao, W.; Bähr, M.; Weise, J.; Neumann, H. Hematopoietic stem cells reduce postischemic inflammation and ameliorate ischemic brain injury. Stroke 2008, 39, 2867–2875. [Google Scholar] [CrossRef]
- Chopp, M.; Li, Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002, 1, 92–100. [Google Scholar] [CrossRef]
- Rempe, D.A.; Kent, T.A. Using bone marrow stromal cells for treatment of stroke. Neurology 2002, 59, 486–487. [Google Scholar] [CrossRef]
- Song, S.; Kamath, S.; Mosquera, D.; Zigova, T.; Sanberg, P.; Vesely, D.L.; Sanchez-Ramos, J. Expression of brain natriuretic peptide by human bone marrow stromal cells. Exp. Neurol. 2004, 185, 191–197. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Wang, L.; Lu, M.; Chopp, M. Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology 2001, 56, 1666–1672. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Katakowski, M.; Chen, X.; Wang, L.; Lu, D.; Lu, M.; Gautam, S.C.; Chopp, M. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J. Neurosci. Res. 2003, 73, 778–786. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Wang, L.; Lu, M.; Chopp, M. Caspase inhibition by Z-VAD increases the survival of grafted bone marrow cells and improves functional outcome after MCAo in rats. J. Neurol. Sci. 2002, 199, 17–24. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Chen, J.; Yang, M.; Katakowski, M.; Lu, M.; Chopp, M. Expression of insulin-like growth factor 1 and receptor in ischemic rats treated with human marrow stromal cells. Brain Res. 2004, 1030, 19–27. [Google Scholar] [CrossRef]
- Bang, O.Y.; Lee, J.S.; Lee, P.H.; Lee, G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann. Neurol. 2005, 57, 874–882. [Google Scholar] [CrossRef]
- Lee, J.S.; Hong, J.M.; Moon, G.J.; Lee, P.H.; Ahn, Y.H.; Bang, O.Y.; Collaborators, S. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells 2010, 28, 1099–1106. [Google Scholar] [CrossRef]
- Shen, L.H.; Li, Y.; Chen, J.; Zacharek, A.; Gao, Q.; Kapke, A.; Lu, M.; Raginski, K.; Vanguri, P.; Smith, A.; et al. Therapeutic benefit of bone marrow stromal cells administered 1 month after stroke. J. Cereb. Blood Flow Metab. 2007, 27, 6–13. [Google Scholar] [CrossRef]
- Barlow, S.; Brooke, G.; Chatterjee, K.; Price, G.; Pelekanos, R.; Rossetti, T.; Doody, M.; Venter, D.; Pain, S.; Gilshenan, K.; et al. Comparison of human placenta- and bone marrow-derived multipotent mesenchymal stem cells. Stem Cells Dev. 2008, 17, 1095–1107. [Google Scholar] [CrossRef]
- Jansen, B.J.; Gilissen, C.; Roelofs, H.; Schaap-Oziemlak, A.; Veltman, J.A.; Raymakers, R.A.; Jansen, J.H.; Kogler, G.; Figdor, C.G.; Torensma, R.; et al. Functional differences between mesenchymal stem cell populations are reflected by their transcriptome. Stem Cells Dev. 2010, 19, 481–490. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, Y.S.; Lee, S.Y.; Kim, K.H.; Lee, Y.M.; Kim, W.K.; Lee, Y.K. Gene expression profile in mesenchymal stem cells derived from dental tissues and bone marrow. J. Periodontal Implant Sci. 2011, 41, 192–200. [Google Scholar] [CrossRef]
- Dmitrieva, R.I.; Minullina, I.R.; Bilibina, A.A.; Tarasova, O.V.; Anisimov, S.V.; Zaritskey, A.Y. Bone marrow- and subcutaneous adipose tissue-derived mesenchymal stem cells: Differences and similarities. Cell Cycle 2012, 11, 377–383. [Google Scholar] [CrossRef]
- Strioga, M.; Viswanathan, S.; Darinskas, A.; Slaby, O.; Michalek, J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012, 21, 2724–2752. [Google Scholar] [CrossRef]
- Lin, R.Z.; Moreno-Luna, R.; Zhou, B.; Pu, W.T.; Melero-Martin, J.M. Equal modulation of endothelial cell function by four distinct tissue-specific mesenchymal stem cells. Angiogenesis 2012, 15, 443–455. [Google Scholar] [CrossRef] [Green Version]
- Egashira, Y.; Sugitani, S.; Suzuki, Y.; Mishiro, K.; Tsuruma, K.; Shimazawa, M.; Yoshimura, S.; Iwama, T.; Hara, H. The conditioned medium of murine and human adipose-derived stem cells exerts neuroprotective effects against experimental stroke model. Brain Res. 2012, 1461, 87–95. [Google Scholar] [CrossRef]
- Ribeiro, C.A.; Fraga, J.S.; Graos, M.; Neves, N.M.; Reis, R.L.; Gimble, J.M.; Sousa, N.; Salgado, A.J. The secretome of stem cells isolated from the adipose tissue and Wharton jelly acts differently on central nervous system derived cell populations. Stem Cell Res. Ther. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Borlongan, C.; Hadman, M.; Sanberg, C.; Sanberg, O. Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke 2004, 35, 2385–2389. [Google Scholar] [CrossRef]
- Aloe, L.; Rocco, M.L.; Bianchi, P.; Manni, L. Nerve growth factor: From the early discoveries to the potential clinical use. J. Transl. Med. 2012, 10, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Ball, S.; Marangell, L.B.; Lipsius, S.; Russell, J.M. Brain-derived neurotrophic factor in generalized anxiety disorder: Results from a duloxetine clinical trial. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 3, 217–221. [Google Scholar]
- Kim, W.S.; Lim, J.Y.; Shin, J.H.; Park, H.K.; Tan, S.A.; Park, K.U.; Paik, N.J. Effect of the presence of brain-derived neurotrophic factor val(66)met polymorphism on the recovery in patients with acute subcortical stroke. Ann. Rehabil. Med. 2013, 37, 311–319. [Google Scholar] [CrossRef]
- Stanzani, L.; Zoia, C.; Sala, G.; Appollonio, I.; Frattola, L.; de Simoni, M.G.; Ferrarese, C. Nerve growth factor and transforming growth factor-beta serum levels in acute stroke patients. Possible involvement of neurotrophins in cerebrovascular disease. Cerebrovasc. Dis. 2001, 12, 240–244. [Google Scholar] [CrossRef]
- Tolar, J.; Nauta, A.J.; Osborn, M.J.; Panoskaltsis Mortari, A.; McElmurry, R.T.; Bell, S.; Xia, L.; Zhou, N.; Riddle, M.; Schroeder, T.M.; et al. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells 2007, 25, 371–379. [Google Scholar] [CrossRef]
- De Luca, A.; Lamura, L.; Gallo, M.; Maffia, V.; Normanno, N. Mesenchymal stem cell-derived interleukin-6 and vascular endothelial growth factor promote breast cancer cell migration. J. Cell. Biochem. 2012, 113, 3363–3370. [Google Scholar] [CrossRef]
- Karnoub, A.E.; Dash, A.B.; Vo, A.P.; Sullivan, A.; Brooks, M.W.; Bell, G.W.; Richardson, A.L.; Polyak, K.; Tubo, R.; Weinberg, R.A. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007, 449, 557–563. [Google Scholar] [CrossRef]
- Asahara, T.; Murohara, T.; Sullivan, A.; Silver, M.; van der Zee, R.; Li, T.; Witzenbichler, B.; Schatteman, G.; Isner, J.M. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997, 275, 964–967. [Google Scholar] [CrossRef]
- Kawamoto, A.; Losordo, D.W. Endothelial progenitor cells for cardiovascular regeneration. Trends Cardiovasc. Med. 2008, 18, 33–37. [Google Scholar] [CrossRef]
- Masuda, H.; Asahara, T. Post-natal endothelial progenitor cells for neovascularization in tissue regeneration. Cardiovasc. Res. 2003, 58, 390–398. [Google Scholar] [CrossRef]
- Yip, H.K.; Chang, L.T.; Chang, W.N.; Lu, C.H.; Liou, C.W.; Lan, M.Y.; Liu, J.S.; Youssef, A.A.; Chang, H.W. Level and value of circulating endothelial progenitor cells in patients after acute ischemic stroke. Stroke 2008, 39, 69–74. [Google Scholar] [CrossRef]
- Chen, J.; Chen, S.Z.; Chen, Y.S.; Zhang, C.; Wang, J.J.; Zhang, W.F.; Liu, G.; Zhao, B.; Chen, Y.F. Circulating endothelial progenitor cells and cellular membrane microparticles in db/db diabetic mouse: Possible implications in cerebral ischemic damage. Am. J. Physiol. Endocrinol. Metabol. 2011, 301, 62–71. [Google Scholar] [CrossRef]
- Decano, J.L.; Moran, A.M.; Giordano, N.; Ruiz-Opazo, N.; Herrera, V.L. Analysis of CD45− [CD34+/KDR+] endothelial progenitor cells as juvenile protective factors in a rat model of ischemic-hemorrhagic stroke. PLoS One 2013, 8, e55222. [Google Scholar]
- Chen, Z.Z.; Jiang, X.D.; Zhang, L.L.; Shang, J.H.; Du, M.X.; Xu, G.; Xu, R.X. Beneficial effect of autologous transplantation of bone marrow stromal cells and endothelial progenitor cells on cerebral ischemia in rabbits. Neurosci. Lett. 2008, 445, 36–41. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Machalinski, B.; Wojakowski, W.; Ratajczak, J.; Kucia, M. A hypothesis for an embryonic origin of pluripotent Oct-4(+) stem cells in adult bone marrow and other tissues. Leukemia 2007, 21, 860–867. [Google Scholar]
- Kucia, M.; Wysoczynski, M.; Ratajczak, J.; Ratajczak, M.Z. Identification of very small embryonic like (VSEL) stem cells in bone marrow. Cell Tissue Res. 2008, 331, 125–134. [Google Scholar] [CrossRef]
- Kucia, M.; Ratajczak, J.; Ratajczak, M.Z. Are bone marrow stem cells plastic or heterogenous—That is the question. Exp. Hematol. 2005, 33, 613–623. [Google Scholar] [CrossRef]
- Ratajczak, J.; Shin, D.M.; Wan, W.; Liu, R.; Masternak, M.M.; Piotrowska, K.; Wiszniewska, B.; Kucia, M.; Bartke, A.; Ratajczak, M.Z. Higher number of stem cells in the bone marrow of circulating low Igf-1 level Laron dwarf mice—Novel view on Igf-1, stem cells and aging. Leukemia 2011, 25, 729–733. [Google Scholar] [CrossRef]
- Barkho, B.Z.; Munoz, A.E.; Li, X.; Li, L.; Cunningham, L.A.; Zhao, X. Endogenous matrix metalloproteinase (MMP)-3 and MMP-9 promote the differentiation and migration of adult neural progenitor cells in response to chemokines. Stem Cells 2008, 26, 3139–3149. [Google Scholar] [CrossRef]
- Liu, X.S.; Chopp, M.; Zhang, R.L.; Hozeska-Solgot, A.; Gregg, S.C.; Buller, B.; Lu, M.; Zhang, Z.G. Angiopoietin 2 mediates the differentiation and migration of neural progenitor cells in the subventricular zone after stroke. J. Biol. Chem. 2009, 284, 22680–22689. [Google Scholar]
- Zhang, R.L.; Chopp, M.; Gregg, S.R.; Toh, Y.; Roberts, C.; Letourneau, Y.; Buller, B.; Jia, L.; Davarani, S.P.; Zhang, Z.G. Patterns and dynamics of subventricular zone neuroblast migration in the ischemic striatum of the adult mouse. J. Cereb. Blood Flow Metab. 2009, 29, 1240–1250. [Google Scholar] [CrossRef]
- Carbajal, K.S.; Schaumburg, C.; Strieter, R.; Kane, J.; Lane, T.E. Migration of engrafted neural stem cells is mediated by CXCL12 signaling through CXCR4 in a viral model of multiple sclerosis. Proc. Natl. Acad. Sci. USA 2010, 107, 11068–11073. [Google Scholar] [CrossRef]
- Arvidsson, A.; Collin, T.; Kirik, D.; Kokaia, Z.; Lindvall, O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 2002, 8, 963–970. [Google Scholar] [CrossRef]
- Nygren, J.; Wieloch, T.; Pesic, J.; Brundin, P.; Deierborg, T. Enriched environment attenuates cell genesis in subventricular zone after focal ischemia in mice and decreases migration of newborn cells to the striatum. Stroke 2006, 37, 2824–2829. [Google Scholar] [CrossRef]
- Deierborg, T.; Staflin, K.; Pesic, J.; Roybon, L.; Brundin, P.; Lundberg, C. Absence of striatal newborn neurons with mature phenotype following defined striatal and cortical excitotoxic brain injuries. Exp. Neurol. 2009, 219, 363–367. [Google Scholar] [CrossRef]
- Deierborg, T.; Roybon, L.; Inacio, A.R.; Pesic, J.; Brundin, P. Brain injury activates microglia that induce neural stem cell proliferation ex vivo and promote differentiation of neurosphere-derived cells into neurons and oligodendrocytes. Neuroscience 2010, 171, 1386–1396. [Google Scholar] [CrossRef]
- Bachstetter, A.D.; Pabon, M.M.; Cole, M.J.; Hudson, C.E.; Sanberg, P.R.; Willing, A.E.; Bickford, P.C.; Gemma, C. Peripheral injection of human umbilical cord blood stimulates neurogenesis in the aged rat brain. BMC Neurosci. 2008, 9. [Google Scholar] [CrossRef]
- Park, D.H.; Eve, D.J.; Sanberg, P.R.; Musso, J., III; Bachstetter, A.D.; Wolfson, A.; Schlunk, A.; Baradez, M.O.; Sinden, J.D.; Gemma, C. Increased neuronal proliferation in the dentate gyrus of aged rats following neural stem cell implantation. Stem Cells Dev. 2010, 19, 175–180. [Google Scholar] [CrossRef]
- Van Velthoven, C.T.; Kavelaars, A.; van Bel, F.; Heijnen, C.J. Mesenchymal stem cell treatment after neonatal hypoxic-ischemic brain injury improves behavioral outcome and induces neuronal and oligodendrocyte regeneration. Brain Behav. Immun. 2010, 24, 387–393. [Google Scholar] [CrossRef]
- Jin, K.; Xie, L.; Mao, X.; Greenberg, M.B.; Moore, A.; Peng, B.; Greenberg, R.B.; Greenberg, D.A. Effect of human neural precursor cell transplantation on endogenous neurogenesis after focal cerebral ischemia in the rat. Brain Res. 2010, 1374, 56–62. [Google Scholar]
- Chen, N.; Newcomb, J.; Garbuzova-Davis, S.; Davis Sanberg, C.; Sanberg, P.R.; Willing, A.E. Human umbilical cord blood cells have trophic effects on young and aging hippocampal neurons in vitro. Aging Dis. 2010, 1, 173–190. [Google Scholar]
- Ikegame, Y.; Yamashita, K.; Hayashi, S.I.; Mizuno, H.; Tawada, M.; You, F.; Yamada, K.; Tanaka, Y.; Egashira, Y.; Nakashima, S.; et al. Comparison of mesenchymal stem cells from adipose tissue and bone marrow for ischemic stroke therapy. Cytotherapy 2011, 13, 675–685. [Google Scholar] [CrossRef]
- Vendrame, M.; Gemma, C.; de Mesquita, D.; Collier, L.; Bickford, P.C.; Sanberg, C.D.; Sanberg, P.R.; Pennypacker, K.R.; Willing, A.E. Anti-inflammatory effects of human cord blood cells in a rat model of stroke. Stem Cells Dev. 2005, 14, 595–604. [Google Scholar] [CrossRef]
- Willing, A.E.; Lixian, J.; Milliken, M.; Poulos, S.; Zigova, T.; Song, S.; Hart, C.; Sanchez-Ramos, J.; Sanberg, P.R. Intravenous versus intrastriatal cord blood administration in a rodent model of stroke. J. Neurosci. Res. 2003, 73, 296–307. [Google Scholar] [CrossRef]
- Xiao, J.; Nan, Z.H.; Motooka, Y.; Low, W.C. Transplantation of a novel cell line population of umbilical cord blood stem cells ameliorates neurological deficits associated with ischemic brain injury. Stem Cells Dev. 2005, 14, 722–733. [Google Scholar] [CrossRef]
- Chung, D.J.; Choi, C.B.; Lee, S.H.; Kang, E.H.; Lee, J.H.; Hwang, S.H.; Han, H.; Lee, J.H.; Choe, B.Y.; Lee, S.Y.; et al. Intraarterially delivered human umbilical cord blood-derived mesenchymal stem cells in canine cerebral ischemia. J. Neurosci. Res. 2009, 87, 3554–3567. [Google Scholar] [CrossRef]
- Vendrame, M.; Cassady, J.; Newcomb, J.; Butler, T.; Pennypacker, K.R.; Zigova, T.; Sanberg, C.D.; Sanberg, P.R.; Willing, A.E. Infusion of human umbilical cord blood cells in a rat model of stroke dose-dependently rescues behavioral deficits and reduces infarct volume. Stroke 2004, 35, 2390–2395. [Google Scholar] [CrossRef]
- Lu, J.; Kanji, S.; Aggarwal, R.; Das, M.; Joseph, M.; Wu, L.C.; Mao, H.Q.; Pompili, V.J.; Hadjiconstantinou, M.; Das, H. Umbilical cord blood-derived hematopoietic stem cells improve dopaminergic neuron morphology in the MPTP-mice. Front. Biosci. 2013, 18, 970–981. [Google Scholar] [CrossRef]
- Kim, J.M.; Lee, S.T.; Chu, K.; Jung, K.H.; Song, E.C.; Kim, S.J.; Sinn, D.I.; Kim, J.H.; Park, D.K.; Kang, K.M.; et al. Systemic transplantation of human adipose stem cells attenuated cerebral inflammation and degeneration in a hemorrhagic stroke model. Brain Res. 2007, 1183, 43–50. [Google Scholar] [CrossRef]
- Leu, S.; Lin, Y.C.; Yuen, C.M.; Yen, C.H.; Kao, Y.H.; Sun, C.K.; Yip, H.K. Adipose-derived mesenchymal stem cells markedly attenuate brain infarct size and improve neurological function in rats. J. Transl. Med. 2010, 8. [Google Scholar] [CrossRef]
- Rubio, D.; Garcia-Castro, J.; Martin, M.C.; de la Fuente, R.; Cigudosa, J.C.; Lloyd, A.C.; Bernad, A. Spontaneous human adult stem cell transformation. Cancer Res. 2005, 65, 3035–3039. [Google Scholar]
- De la Fuente, R.; Bernad, A.; Garcia-Castro, J.; Martin, M.C.; Cigudosa, J.C. Retraction: Spontaneous human adult stem cell transformation. Cancer Res. 2010, 70. [Google Scholar] [CrossRef]
- Ra, J.C.; Shin, I.S.; Kim, S.H.; Kang, S.K.; Kang, B.C.; Lee, H.Y.; Kim, Y.J.; Jo, J.Y.; Yoon, E.J.; Choi, H.J.; et al. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2011, 20, 1297–1308. [Google Scholar] [CrossRef]
- Borlongan, C.V.; Kaneko, Y.; Maki, M.; Yu, S.J.; Ali, M.; Allickson, J.G.; Sanberg, C.D.; Kuzmin-Nichols, N.; Sanberg, P.R. Menstrual blood cells display stem cell-like phenotypic markers and exert neuroprotection following transplantation in experimental stroke. Stem Cells Dev. 2010, 19, 439–452. [Google Scholar] [CrossRef]
- Meng, X.; Ichim, T.; Zhong, J.; Rogers, A.; Yin, Z.; Jackson, J.; Wang, H.; Ge, W.; Bogin, V.; Chan, K.; et al. Endometrial regenerative cells: A novel stem cell population. J. Transl. Med. 2007, 5. [Google Scholar] [CrossRef]
- Cregan, M.D.; Fan, Y.P.; Appelbee, A.; Brown, M.L.; Klopcic, B.; Koppen, J.; Mitoulas, L.R.; Piper, K.M.E.; Choolani, M.A.; Chong, Y.S.; et al. Identification of nestin-positive putative mammary stem cells in human breastmilk. Cell Tissue Res. 2007, 329, 129–136. [Google Scholar] [CrossRef]
- Hassiotou, F.; Beltran, A.; Chetwynd, E.; Stuebe, A.M.; Twigger, A.J.; Metzger, P.; Trengove, N.; Lai, C.T.; Filgueira, L.; Blancafort, P.; et al. Breastmilk is a novel source of stem cells with multilineage differentiation potential. Stem Cells 2012, 30, 2164–2174. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar]
- Seo, B.M.; Miura, M.; Gronthos, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Sonoyama, W.; Liu, Y.; Fang, D.A.J.; Yamaza, T.; Seo, B.M.; Zhang, C.M.; Liu, H.; Gronthos, S.; Wang, C.Y.; Shi, S.T.; et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PloS One 2006, 1, e79. [Google Scholar] [CrossRef]
- Sonoyama, W.; Liu, Y.; Yamaza, T.; Tuan, R.S.; Wang, S.; Shi, S.; Huang, G.T.J. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: A pilot study. J. Endod. 2008, 34, 166–171. [Google Scholar] [CrossRef]
- Morsczeck, C.; Gotz, W.; Schierholz, J.; Zellhofer, F.; Kuhn, U.; Mohl, C.; Sippel, C.; Hoffmann, K.H. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005, 24, 155–165. [Google Scholar] [CrossRef]
- Huang, G.T.J.; Gronthos, S.; Shi, S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef]
- Yang, K.L.; Chen, M.F.; Liao, C.H.; Pang, C.Y.; Lin, P.Y. A simple and efficient method for generating Nurr1-positive neuronal stem cells from human wisdom teeth (tNSC) and the potential of tNSC for stroke therapy. Cytotherapy 2009, 11, 606–617. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Li, W.; Su, H.; Qin, D.; Yang, J.; Zhu, F.; Xu, J.; He, W.; Guo, X.; Labuda, K.; et al. Generation of human induced pluripotent stem cells from umbilical cord matrix and amniotic membrane mesenchymal cells. J. Biol. Chem. 2010, 285, 11227–11234. [Google Scholar]
- Tat, P.A.; Sumer, H.; Jones, K.L.; Upton, K.; Verma, P.J. The efficient generation of induced pluripotent stem (iPS) cells from adult mouse adipose tissue-derived and neural stem cells. Cell Transpl. 2010, 19, 525–536. [Google Scholar] [CrossRef]
- Chen, S.J.; Chang, C.M.; Tsai, S.K.; Chang, Y.L.; Chou, S.J.; Huang, S.S.; Tai, L.K.; Chen, Y.C.; Ku, H.H.; Li, H.Y.; et al. Functional improvement of focal cerebral ischemia injury by subdural transplantation of induced pluripotent stem cells with fibrin glue. Stem Cells Dev. 2010, 19, 1757–1767. [Google Scholar] [CrossRef]
- Jiang, M.; Lv, L.; Ji, H.; Yang, X.; Zhu, W.; Cai, L.; Gu, X.; Chai, C.; Huang, S.; Sun, J.; et al. Induction of pluripotent stem cells transplantation therapy for ischemic stroke. Mol. Cell. Biochem. 2011, 354, 67–75. [Google Scholar] [CrossRef]
- Yamashita, T.; Kawai, H.; Tian, F.F.; Ohta, Y.; Abe, K. Tumorigenic development of induced pluripotent stem cells in ischemic mouse brain. Cell Transpl. 2011, 20, 883–891. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, Z.N.; Rong, Z.; Xu, Y. Immunogenicity of induced pluripotent stem cells. Nature 2011, 474, 212–215. [Google Scholar] [CrossRef]
- Mohamad, O.; Drury-Stewart, D.; Song, M.; Faulkner, B.; Chen, D.; Yu, S.P.; Wei, L. Vector-free and transgene-free human iPS cells differentiate into functional neurons and enhance functional recovery after ischemic stroke in mice. PLoS One 2013, 8, e64160. [Google Scholar]
- Oh, J.S.; Kim, K.N.; An, S.S.; Pennant, W.A.; Kim, H.J.; Gwak, S.J.; do Yoon, H.; Lim, M.H.; Choi, B.H.; Ha, Y. Cotransplantation of mouse neural stem cells (mNSCs) with adipose tissue-derived mesenchymal stem cells improves mNSC survival in a rat spinal cord injury model. Cell Transpl. 2011, 20, 837–849. [Google Scholar] [CrossRef]
- Matsuda, R.; Yoshikawa, M.; Kimura, H.; Ouji, Y.; Nakase, H.; Nishimura, F.; Nonaka, J.; Toriumi, H.; Yamada, S.; Nishiofuku, M.; et al. Cotransplantation of mouse embryonic stem cells and bone marrow stromal cells following spinal cord injury suppresses tumor development. Cell Transpl. 2009, 18, 39–54. [Google Scholar] [CrossRef]
- Nakagomi, N.; Nakagomi, T.; Kubo, S.; Nakano-Doi, A.; Saino, O.; Takata, M.; Yoshikawa, H.; Stern, D.M.; Matsuyama, T.; Taguchi, A. Endothelial cells support survival, proliferation, and neuronal differentiation of transplanted adult ischemia-induced neural stem/progenitor cells after cerebral infarction. Stem Cells 2009, 27, 2185–2195. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, Q.; Zeng, Y.S.; Zhang, X.B.; Xiong, Y.; Wang, J.M.; Chen, S.J.; Li, Y.; Bruce, I.C.; Wu, W. Implantation of adult bone marrow-derived mesenchymal stem cells transfected with the neurotrophin-3 gene and pretreated with retinoic acid in completely transected spinal cord. Brain Res. 2010, 1359, 256–271. [Google Scholar] [CrossRef]
- Jin, K.; Mao, X.; Xie, L.; Galvan, V.; Lai, B.; Wang, Y.; Gorostiza, O.; Wang, X.; Greenberg, D.A. Transplantation of human neural precursor cells in Matrigel scaffolding improves outcome from focal cerebral ischemia after delayed postischemic treatment in rats. J. Cereb. Blood Flow Metab. 2010, 30, 534–544. [Google Scholar] [CrossRef]
- Kaneko, Y.; Pabon, M.M.; Dailey, T.; Weinbren, N.L.; Rizzi, J.; Tamboli, C.; Vasconcellos, J.; Kuzmin-Nichols, N.; Sanberg, P.R.; Eve, D.J.; et al. The battle of thesexes for stroke therapy: Female- versus male-derived stem cells. CNS Neurol. Disord. Drug. Targets 2013, 12, 405–412. [Google Scholar] [CrossRef]
- Sanberg, P.R.; Borlongan, C.V.; Saporta, S.; Cameron, D.F. Testis-derived Sertoli cells survive and provide localized immunoprotection for xenografts in rat brain. Nat. Biotechnol. 1996, 14, 1692–1695. [Google Scholar] [CrossRef]
- Sanberg, P.R.; Othberg, A.I.; Borlongan, C.V.; Saporta, S.; Anton, A.; Freeman, T.B.; Cahill, D.W.; Allen, R.C.; Cameron, D.F. Transplantation of testis-derived Sertoli cells into the mammalian brain. Transpl. Proc. 1997, 29, 1926–1928. [Google Scholar] [CrossRef]
- Saporta, S.; Cameron, D.F.; Borlongan, C.V.; Sanberg, P.R. Survival of rat and porcine Sertoli cell transplants in the rat striatum without cyclosporine-A immunosuppression. Exp. Neurol. 1997, 146, 299–304. [Google Scholar] [CrossRef]
- Smith, G.A.; Snyder, E.Y. Two cells are better than one: Optimizing stem cell survival by co-grafting “helper” cells that offer regulated trophic support. Exp. Neurol. 2013, 247, 751–754. [Google Scholar] [CrossRef]
- Liang, Y.; Agren, L.; Lyczek, A.; Walczak, P.; Bulte, J.W. Neural progenitor cell survival in mouse brain can be improved by co-transplantation of helper cells expressing bFGF under doxycycline control. Exp. Neurol. 2013, 247, 73–79. [Google Scholar] [CrossRef]
- Liang, Y.; Walczak, P.; Bulte, J.W. The survival of engrafted neural stem cells within hyaluronic acid hydrogels. Biomaterials 2013, 34, 5521–5529. [Google Scholar] [CrossRef]
- Sanberg, P.R.; Eve, D.J.; Cruz, L.E.; Borlongan, C.V. Neurological disorders and the potential role for stem cells as a therapy. Br. Med. Bull. 2012, 101, 163–181. [Google Scholar] [CrossRef]
- Borlongan, C.V.; Chopp, M.; Steinberg, G.K.; Bliss, T.M.; Li, Y.; Lu, M.; Hess, D.C.; Kondziolka, D. Potential of stem/progenitor cells in treating stroke: The missing steps in translating cell therapy from laboratory to clinic. Regen. Med. 2008, 3, 249–250. [Google Scholar] [CrossRef]
- Borlongan, C.V. Cell therapy for stroke: Remaining issues to address before embarking on clinical trials. Stroke 2009, 40, 146–148. [Google Scholar] [CrossRef]
- Chopp, M.; Steinberg, G.K.; Kondziolka, D.; Lu, M.; Bliss, T.M.; Li, Y.; Hess, D.C.; Borlongan, C.V. Who’s in favor of translational cell therapy for stroke: STEPS forward please? Cell Transpl. 2009, 18, 691–693. [Google Scholar] [CrossRef]
- Borlongan, C.V.; Weiss, M.D. Baby STEPS: A giant leap for cell therapy in neonatal brain injury. Pediatr. Res. 2011, 70, 3–9. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dailey, T.; Metcalf, C.; Mosley, Y.I.; Sullivan, R.; Shinozuka, K.; Tajiri, N.; Pabon, M.; Acosta, S.; Kaneko, Y.; Loveren, H.V.; et al. An Update on Translating Stem Cell Therapy for Stroke from Bench to Bedside. J. Clin. Med. 2013, 2, 220-241. https://doi.org/10.3390/jcm2040220
Dailey T, Metcalf C, Mosley YI, Sullivan R, Shinozuka K, Tajiri N, Pabon M, Acosta S, Kaneko Y, Loveren HV, et al. An Update on Translating Stem Cell Therapy for Stroke from Bench to Bedside. Journal of Clinical Medicine. 2013; 2(4):220-241. https://doi.org/10.3390/jcm2040220
Chicago/Turabian StyleDailey, Travis, Christopher Metcalf, Yusef I. Mosley, Robert Sullivan, Kazutaka Shinozuka, Naoki Tajiri, Mibel Pabon, Sandra Acosta, Yuji Kaneko, Harry Van Loveren, and et al. 2013. "An Update on Translating Stem Cell Therapy for Stroke from Bench to Bedside" Journal of Clinical Medicine 2, no. 4: 220-241. https://doi.org/10.3390/jcm2040220