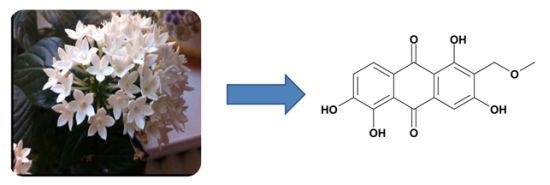
Anthraquinones of the Roots of Pentas micrantha
Abstract
:1. Introduction
2. Results and Discussion
| Atom | δH (I, mult, J in Hz) | δC | HMBC |
|---|---|---|---|
| 1 | - | 164.4 | - |
| 1a | - | 109.1 | - |
| 2 | - | 117.4 | - |
| 3 | - | 163.6 | - |
| 4 | 7.20 (1H, s) | 107.2 | 1a, 2, 10 |
| 4a | - | 134.2 | - |
| 5 | - | 152.4 | - |
| 5a | - | 116.2 | - |
| 6 | - | 152.9 | - |
| 7 | 7.06 (1H, d, 8.1) | 120.9 | 5,8a |
| 8 | 7.63 (1H, d, 8.1) | 121.8 | 5a,6,9 |
| 8a | - | 123.3 | - |
| 9 | - | 184.0 | - |
| 10 | - | 188.6 | - |
| 11 | 4.42 (2H, s) | 61.7 | 1,2,3,12 |
| 12 | 3.25 (3H, s) | 58.0 | 11 |
| 1-OH | 13.92 (1H, br s) | - | 1,1a,2 |
| 3-OH | - | - | - |
| 5-OH | - | - | - |
| 6-OH | - | - | - |

| Sample | R1 | R2 | R3 | R5 | R6 | IC50 (μmol/mL) a | CC50b | |
|---|---|---|---|---|---|---|---|---|
| D6 | W2 | (μmol/mL) | ||||||
| Crude (CH3OH) | 4.00 ± 1.86c | 3.37 ± 0.74c | >450 c | |||||
| 1 | H | CH3 | H | H | H | 30.36 ± 0.01 | 48.56 ± 0.01 | >100 |
| 2 | OH | CH2OCH3 | OH | H | H | 42.54 ± 0.01 | 46.44 ± 0.01 | >352 |
| 3 | OCH3 | CH2OH | OH | H | H | 56.58 ± 0.00 | 110.6 ± 0.01 | 238 |
| 4 | OCH3 | CH3 | OH | H | H | 45.07 ± 0.01 | 70.56 ± 0.00 | 208 |
| 5 | OH | CH3 | OH | H | H | 21.54 ± 0.00 | 31.91 ± 0.01 | 310 |
| 6 | OH | CH2OCH3 | OH | OH | OH | 31.84 ± 0.01 | 35.41 ± 0.01 | 258 |
| 7 | OCH3 | CHO | OH | H | H | 27.19 ± 0.00 | 38.58 ± 0.00 | 316 |
| 8 | OCH3 | CH2OH | OH | OH | OH | 47.53 ± 0.01 | 61.17 ± 0.02 | >450 |
| 9 | OH | COOCH3 | OH | H | H | n.d.d | n.d. d | n.d. d |

3. Experimental
3.1. General Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Drugs
3.5. Drug Susceptibility Testing
3.6. Cytotoxicity Assay
3.7. Spectral Data
4. Conclusions
Supplementary Materials
Acknowledgments
Supplementary Files
References
- Njoroge, G.N.; Bussmann, R.W. Diversity and utilization of antimalarial ethnophytotherapeutic remedies among the Kikuyus (Central Kenya). J. Ethnobiol. Ethnomed. 2006, 2, 8. [Google Scholar] [CrossRef]
- Han, Y.S.; van der Heijden, R.; Verpoorte, R. Biosynthesis of anthraquinones in cell cultures of the Rubiaceae. Plant. Cell Tiss. Org. 2001, 67, 201–220. [Google Scholar] [CrossRef]
- Koyama, J.; Nisino, Y.; Morita, I.; Kobayashi, N.; Osakai, T.; Tokuda, H. Correlation between reduction potentials and inhibitions of Epstein-Barr virus activation by anthraquinone derivatives. Bioorg. Med. Chem. Lett. 2008, 18, 4106–4109. [Google Scholar]
- Chan, K.Y.; Zhang, J.; Chang, C.W. Mode of action investigation for the antibacterial cationic anthraquinone analogs. Bioorg. Med. Chem. Lett. 2011, 21, 6353–6356. [Google Scholar]
- Singh, D.N.; Verma, N.; Raghuwanshi, S.; Shukla, P.K.; Kulshreshtha, D.K. Antifungal anthraquinones from Saprosma fragrans. Bioorg. Med. Chem. Lett. 2006, 16, 4512–4514. [Google Scholar]
- Suzuki, A.; Hasegawa, M.; Ishii, M.; Matsumura, S.; Toshima, K. Anthraquinone derivatives as a new family of protein photocleavers. Bioorg. Med. Chem. Lett. 2005, 15, 4624–4627. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Q.; Wang, Y.; Liu, L.; Weng, X.; Li, G.; Zhang, X.; Zhou, X. Novel anthraquinone derivatives: Synthesis via click chemistry approach and their induction of apoptosis in BGC gastric cancer cells via reactive oxygen species (ROS)-dependent mitochondrial pathway. Bioorg. Med. Chem. Lett. 2008, 18, 6505–6508. [Google Scholar] [CrossRef]
- Winter, R.W.; Cornell, K.A.; Johnson, L.L.; Isabelle, L.M.; Hinrichs, D.J.; Riscoe, M.K. Hydroxy-Anthraquinones as Antimalarial Agents. Bioorg. Med. Chem. Lett. 1995, 5, 1927–1932. [Google Scholar] [CrossRef]
- Abu el Heiga, L.A.; Katzhendler, J.; Gean, K.F.; Bachrach, U. Antimalarial activity of substituted anthraquinones. Biochem. Pharmacol. 1990, 39, 1620–1623. [Google Scholar]
- El-Hady, S.; Bukuru, J.; Kesteleyn, B.; van Puyvelde, L.; van, T.N.; de Kimpe, N. New pyranonaphthoquinone and pyranonaphthohydroquinone from the roots of Pentas longiflora. J. Nat. Prod. 2002, 65, 1377–1379. [Google Scholar]
- Endale, M.; Alao, J.P.; Akala, H.M.; Rono, N.K.; Eyase, F.L.; Derese, S.; Ndakala, A.; Mbugua, M.; Walsh, D.S.; Sunnerhagen, P.; et al. Antiplasmodial Quinones from Pentas longiflora and Pentas lanceolata. Planta Med. 2012, 78, 31–35. [Google Scholar]
- Muthaura, C.N.; Rukunga, G.M.; Chhabra, S.C.; Mungai, G.M.; Njagi, E.N. Traditional antimalarial phytotherapy remedies used by the Kwale community of the Kenyan Coast. J. Ethnopharmacol. 2007, 114, 377–386. [Google Scholar] [CrossRef]
- Wanyoike, G.N.; Chhabra, S.C.; Lang’at-Thoruwa, C.C.; Omar, S.A. Brine shrimp toxicity and antiplasmodial activity of five Kenyan medicinal plants. J. Ethnopharmacol. 2004, 90, 129–133. [Google Scholar] [CrossRef]
- Endale, M.; Ekberg, A.; Akala, H.; Alao, J.P.; Sunnerhagen, P.; Yenesew, A.; Erdelyi, M. Busseihydroquinines A–D from the Roots of Pentas bussei. J. Nat. Prod. 2012, 75, 1299–1304. [Google Scholar]
- Kokowaro, J.O. Medicinal Plants of East Africa; University of Nairobi Press: Nairobi, Kenya, 2010; pp. 247–248. [Google Scholar]
- Wu, T.S.; Lin, D.M.; Shi, L.S.; Damu, A.G.; Kuo, P.C.; Kuo, Y.H. Cytotoxic anthraquinones from the stems of Rubia wallichiana Decne. Chem. Pharm. Bull. 2003, 51, 948–950. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Goda, Y.; Yoshihira, K. The mutagenic constituents of Rubia tinctorum. Chem. Pharm. Bull. 1992, 40, 1504–1509. [Google Scholar]
- Scott, A.I. Interpretation of the Ultraviolet Spectra of Natural Products; Pergamon Press: Oxford, UK, 1964; Volume 7, pp. 286–289. [Google Scholar]
- Jia, J.; Zhu, F.; Ma, X.; Cao, Z.; Li, Y.; Chen, Y.Z. Mechanisms of drug combinations: Interaction and network perspectives. Nat. Rev. Drug Discov. 2009, 8, 111–128. [Google Scholar]
- Osman, C.P.; Ismail, N.H.; Ahmad, R.; Ahmat, N.; Awang, K.; Jaafar, F.M. Anthraquinones with antiplasmodial activity from the roots of Rennellia elliptica Korth. (Rubiaceae). Molecules 2010, 15, 7218–7226. [Google Scholar] [CrossRef]
- Koumaglo, K.; Gbeassor, M.; Nikabu, O.; de Souza, C.; Werner, W. Effects of three compounds extracted from Morinda lucida on Plasmodium falciparum. Planta Med. 1992, 58, 533–534. [Google Scholar] [CrossRef]
- Fotie, J. Quinones and Malaria. Antiinflamm.Antiallergy Agents Med. Chem. 2006, 5, 357–366. [Google Scholar]
- Onegi, B.; Kraft, C.; Kohler, I.; Freund, M.; Jenett-Siems, K.; Siems, K.; Beyer, G.; Melzig, M.F.; Bienzle, U.; Eich, E. Antiplasmodial activity of naphthoquinones and one anthraquinone from Stereospermum kunthianum. Phytochemistry 2002, 60, 39–44. [Google Scholar]
- Locatelli, M. Anthraquinones: Analytical techniques as a novel tool to investigate on the triggering of biological targets. Curr. Drug Targets 2011, 12, 366–380. [Google Scholar] [CrossRef]
- Shamma, M.; Rahimizadeh, M. The Identity of Chileninone with Berberrubine the Problem of True Natural-Products Vs Artifacts of Isolation. J. Nat. Prod. 1986, 49, 398–405. [Google Scholar] [CrossRef]
- Diner, P.; Alao, J.P.; Soderlund, J.; Sunnerhagen, P.; Grotli, M. Preparation of 3-substituted-1-isopropyl-1H-pyrazolo[3,4-d]pyrimidin-4-amines as RET kinase inhibitors. J. Med. Chem. 2012, 55, 4872–4876. [Google Scholar]
- Sample Availability: Samples of the compounds 1–9 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Endale, M.; Ekberg, A.; Alao, J.P.; Akala, H.M.; Ndakala, A.; Sunnerhagen, P.; Erdélyi, M.; Yenesew, A. Anthraquinones of the Roots of Pentas micrantha. Molecules 2013, 18, 311-321. https://doi.org/10.3390/molecules18010311
Endale M, Ekberg A, Alao JP, Akala HM, Ndakala A, Sunnerhagen P, Erdélyi M, Yenesew A. Anthraquinones of the Roots of Pentas micrantha. Molecules. 2013; 18(1):311-321. https://doi.org/10.3390/molecules18010311
Chicago/Turabian StyleEndale, Milkyas, Annabel Ekberg, John Patrick Alao, Hoseah M. Akala, Albert Ndakala, Per Sunnerhagen, Máté Erdélyi, and Abiy Yenesew. 2013. "Anthraquinones of the Roots of Pentas micrantha" Molecules 18, no. 1: 311-321. https://doi.org/10.3390/molecules18010311