Protective Effects of Labisia pumila var. alata on Biochemical and Histopathological Alterations of Cardiac Muscle Cells in Isoproterenol-Induced Myocardial Infarction Rats
Abstract
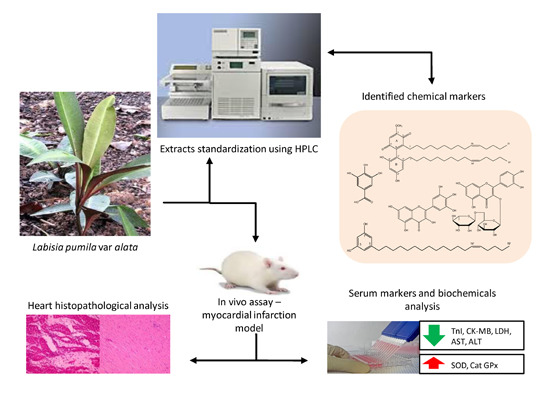
:1. Introduction
2. Results and Discussion
2.1. Identification of Chemical Markers of L. pumila Extracts



2.2. Quantification of Aqueous and 80% Ethanol Extracts of L. pumila var. alata by Validated RP-HPLC Method
| Extract (10 mg/mL) | Amounts (µg/mL) | ||||
|---|---|---|---|---|---|
| 5-(Z-nonadec-14-enyl)resorcinol | Demethyl-belamcandaquinone B | Myricetin | Rutin | Gallic Acid | |
| 80%-EtOH extract | 1006.87 ± 0.1 | 2793.61 ± 0.08 | 15.96 ± 0.16 | 16.89 ± 0.08 | 77.57 ± 0.11 |
| Water extract | Not detected | Not detected | 1.16 ± 0.01 | 3.51 ± 0.49 | 170.06 ± 0.01 |
| Standard | Conc. (µg/mL) | Recovery * (%) | Intra-Day Precision ** | Inter-Day Precision ** | ||
|---|---|---|---|---|---|---|
| RT | Response | RT | Response | |||
| 5-(Z-nonadec-14-enyl)resorcinol | 31.25 | 104.89 ± 3.04 | 39.392 ± 0.02 | 52,392 ± 0.01 | 39.309 ± 0.06 | 52,690 ± 0.08 |
| 62.5 | 103.74 ± 3.14 | 39.400 ± 0.04 | 106,716 ± 0.05 | 39.325 ± 0.06 | 106,589 ± 0.12 | |
| 125 | 107.93 ± 5.88 | 39.384 ± 0.04 | 241,753 ± 0.04 | 39.292 ± 0.08 | 236,970 ± 0.02 | |
| Demethylbelamcanda-quinone B | 31.25 | 98.22 ± 3.67 | 61.752 ± 0.52 | 710,140 ± 0.11 | 61.584 ± 0.42 | 754,214 ± 0.03 |
| 62.5 | 100.41 ± 3.77 | 61.537 ± 0.44 | 1,318,115 ±0.10 | 61.921 ± 0.08 | 1,394,224 ± 0.03 | |
| 125 | 100.36 ± 1.49 | 61.754 ± 0.28 | 2,495,414 ± 0.06 | 61.735 ± 0.30 | 2,539,039 ± 0.07 | |
| Gallic acid | 31.25 | 94.60 ± 5.67 | 3.607 ± 0.01 | 46,449 ± 0.05 | 3.613 ± 0.01 | 45,190 ± 0.05 |
| 62.5 | 97.07 ± 3.14 | 3.663 ± 0.01 | 81,207 ± 0.03 | 3.659 ± 0.02 | 82,606 ± 0.02 | |
| 125 | 98.22 ± 3.67 | 3.675 ± 0.05 | 169,362 ± 0.04 | 3.699 ± 0.03 | 165,185 ± 0.02 | |
| Myricetin | 31.25 | 91.21 ± 2.66 | 20.590 ± 0.03 | 569,867 ± 0.05 | 20.619 ± 0.08 | 596,883 ± 0.04 |
| 62.5 | 104.03 ± 2.76 | 20.551 ± 0.03 | 1,213,120 ±0.14 | 20.542 ± 0.11 | 1,227,027 ± 0.05 | |
| 125 | 99.15 ± 1.56 | 20.541 ± 0.04 | 2,132,241 ± 0.05 | 20.616 ± 0.20 | 2,159,026 ± 0.03 | |
| Rutin | 31.25 | 99.81 ± 2.83 | 16.607 ± 0.23 | 722,316 ± 0.01 | 17.017 ± 0.35 | 788,152 ± 0.04 |
| 62.5 | 93.44 ± 2.39 | 16.962 ± 0.18 | 1,712,277 ±0.01 | 17.007 ± 0.47 | 1,617,376 ± 0.06 | |
| 125 | 98.51 ± 3.21 | 17.208 ± 0.14 | 2,830,868 ± 0.01 | 17.089 ± 0.36 | 2,987,506 ± 0.11 | |
| Standard | Conc Range (µg/mL) | Regression Equation | R2 | LOD (ng/mL) | LOQ (ng/mL) |
|---|---|---|---|---|---|
| 5-(Z-nonadec-14-enyl)resorcinol | 31.25–500 | y = 1672x + 20,149 | 0.998 | 0.69 | 2.08 |
| Demethylbelamcandaquinone B | 31.25–500 | y = 16,378x + 45,378 | 0.999 | 0.89 | 2.70 |
| Gallic acid | 31.25–500 | y = 1051.8x + 18,227 | 0.998 | 0.35 | 1.05 |
| Myricetin | 7.81–125 | y = 22,051x − 274,315 | 0.995 | 0.03 | 0.08 |
| Rutin | 7.81–125 | y = 24,591x + 21,313 | 0.999 | 0.14 | 0.43 |
2.3. Effects of L. pumila var alata Extracts on Serum Cardiac Troponin I (cTnI) Level

2.4. Effects of L. pumila var alata Extracts on Other Cardiac Serum Marker Enzymes
2.5. Effect of L. pumila var alata Extracts on Serum and Cardiac Antioxidant System
| Group | Serum Cardiac Marker Enzymes | Serum and Heart Antioxidant System | |||||||
|---|---|---|---|---|---|---|---|---|---|
| LDH | AST | ALT | SOD | CAT | GPx | ||||
| Serum | Heart | Serum | Heart | Serum | Heart | ||||
| Group I | 127.83 ± 13.52 a,c | 51.40 ± 2.26 a | 21.10 ± 0.73 a | 290.37 ± 3.68 a,c | 15.05 ± 0.46 a,c | 296.45 ± 15.60 a,c | 19.13 ± 1.23 a,c | 81.69 ± 11.04 a,c | 340 ± 7.68 a,c |
| Group II | 215.11 ± 18.11 b,c | 92.44 ± 4.22 b,c | 55.91 ± 1.70 b,c | 217.91 ± 8.91 b,c | 9.30 ± 0.70 b,c | 63.63 ± 12.86 b,c | 9.37 ± 1.42 b,c | 15.52 ± 3.37 b,c | 38.14 ± 0.86 b,c |
| Group III | 157.10 ± 5.28 a,b | 56.48 ± 0.67 a | 18.46 ± 0.96 a | 282.33 ± 1.42 a,b | 13.06 ± 0.32 a,b | 240.33 ± 16.89 a,b | 15.06 ± 0.28 a,b | 58.05 ± 5.78 a,b | 218.52 ± 20.13 a,b |
| Group IV | 190.36 ± 1.08 a,b,c | 71.14 ± 0.83 a,b,c | 35.98 ± 0.49 a,b,c | 238.42 ± 4.50 a,b,c | 10.06 ± 0.12 a,b,c | 131.38 ± 2.99 a,b,c | 13.71 ± 0.13 a,b,c | 22.81 ± 2.21 b,c | 52.85 ± 4.93 b,c |
| Group V | 185.44 ± 1.36 a,b,c | 64.75 ± 1.52 a,b,c | 28.22 ± 0.35 a,b | 250.86 ± 4.11 a,b,c | 10.62 ± 0.30 a,b,c | 176.41 ± 5.67 a,b,c | 14.49 ± 0.10 a,b,c | 27.65 ± 0.40 a,b,c | 69.66 ± 4.33 a,b,c |
| Group VI | 181.04 ± 1.10 a,b,c | 59.01 ± 1.51 a,b | 26.92 ± 1.26 a | 272.16 ± 1.58 a,b,c | 12.12 ± 0.04 a,b,c | 201.65 ± 9.10 a,b,c | 14.78 ± 0.13 a,b,c | 30.22 ± 1.17 a,b,c | 94.76 ± 1.86 a,b,c |
| Group VII | 178.10 ± 8.51 a,b,c | 84.57 ± 2.42 a,b,c | 47.21 ± 1.50 a | 260.25 ± 1.70 a,b,c | 11.11 ± 0.14 a,b,c | 94.78 ± 8.37 a,b,c | 13.13 ± 0.15 a,b,c | 26.14 ± 0.63 a,b,c | 62.59 ± 3.02 a,b,c |
| Group VIII | 173.99 ± 1.42 a,b,c | 75.57 ± 2.42 a,b,c | 42.62 ± 0.30 a | 266.50 ± 2.19 a,b,c | 11.81 ± 0.21 a,b,c | 119.57 ± 4.08 a,b,c | 13.46 ± 0.09 a,b,c | 28.63 ± 0.32 a,b,c | 85.83 ± 1.28 a,b,c |
| Group IX | 167.96 ± 2.14 a,b | 68.23 ± 0.76 a,b,c | 32.89 ± 1.90 a | 277.81 ± 1.15 a,b | 12.45 ± 0.16 a,b | 150.20 ± 10.56 a,b,c | 14.18 ± 0.29 a,b,c | 41.14 ± 4.92 a,b,c | 121.43 ± 10.93 a,b,c |
2.6. Effects of L. pumila var alata on Cardiac Histopathology

3. Experimental Section
3.1. General Information
3.2. Sample Collection and Extracts Preparation
3.3. Isolation Work
3.4. Quantitative Determination of the Major Components of Plant Extracts by HPLC
3.5. Validation Procedures for HPLC Analysis
3.6. In Vivo Experimental Design and Protocol
- Group I
- normal-control group.
- Group II
- ISO-control group (received 85 mg/kg of ISO, s.c. on 29th and 30th day).
- Group III
- rats treated with 10 mg/kg of propranolol, orally, for 28 days and received 85 mg/kg ISO, s.c. on 29th and 30th day.
- Group IV
- rats treated with 100 mg/kg of LPva water extract orally, for 28 days and received 85 mg/kg of ISO, s.c. on 29th and 30th day.
- Group V
- rats treated with 200 mg/kg of LPva water extract, orally, for 28 days and received 85 mg/kg of ISO, s.c. on 29th and 30th day.
- Group VI
- rats treated with 400 mg/kg of LPva water extract, orally, for 28 days and received 85 mg/kg of ISO, s.c. on 29th and 30th day.
- Group VII
- rats treated with 100 mg/kg of LPva 80% ethanol extract, orally, for 28 days and received 85 mg/kg of ISO, s.c. on 29th and 30th day.
- Group VIII
- rats treated with 200 mg/kg of LPva 80% ethanol extract, orally, for 28 days and received 85 mg/kg of ISO, s.c. on 29th and 30th day.
- Group IX
- rats treated with 400 mg/kg of LPva 80% ethanol extract, orally, for 28 days and received 85 mg/kg of ISO, s.c. on 29th and 30th day.
3.7. Estimation of Cardiac Troponin I (cTn1)
3.8. Assay of Cardiac Marker Enzymes
3.9. Antioxidant System Assays
3.10. Histopathological Examination
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- O’Brien, P.J.; Smith, D.E.C.; Knechtel, T.J.; Marchak, M.A.; Pruimboom-Brees, I.; Brees, D.J.; Spratt, D.P.; Archer, F.J.; Butler, P.; Potter, A.N.; et al. Cadiac troponin I is a sensitive, specific biomarker of cardiac injury in laboratory animals. Lab. Anim. 2006, 40, 153–171. [Google Scholar] [CrossRef] [PubMed]
- Balazs, T.; Ferrans, V.J. Cardiac lesions induced by chemicals. Environ. Health Perspect. 1978, 26, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Kung, H.F.; Blau, M. Subcutaneous isoproterenol: A convenient rat model for early detection of myocardial necrosis. J. Nucl. Med. 1978, 19, 948–951. [Google Scholar] [PubMed]
- Ravichandran, L.V.; Puvanakrishnan, R.; Joseph, K.T. Influence of isoproterenol-induced myocardial infarction on certain glycohydrolases and cathepsins in rats. Biochem. Med. Metab. Biol. 1991, 45, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Upaganlawar, A.; Gandhi, H.; Balaraman, R. Isoproterenol induced mypcardial infarction: Protective role of natural products. J. Pharmacol. Toxicol. 2011, 6, 1–17. [Google Scholar]
- Hori, M.; Nishida, K. Oxidative stress and left ventricular remodeling after myocardial infarction. Cardiovasc. Res. 2009, 81, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Burkill, I.H. Dictionary of the Economic Products of the Malay Peninsula; Ministry of Agriculture and Cooperatives: Kuala Lumpur, Malaysia, 1966; Volume I.
- Norhaiza, M.; Maziah, M.; Hakiman, M. Antioxidative properties of leaf extracts of a popular Malaysian herb, Labisia pumila. J. Med. Plants Res. 2009, 3, 217–223. [Google Scholar]
- Chua, L.S.; Latiff, N.A.; Lee, S.Y.; Lee, C.T.; Sarmidi, M.R.; Aziz, R.A. Flavonoids and phenolic acids from Labisia pumila (Kacip Fatimah). Food Chem. 2011, 127, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.; Khan, I.A. Alkyl phenols and saponins from the roots of Labisia pumila (Kacip Fatimah). Phytochemistry 2011, 72, 2075–2080. [Google Scholar] [CrossRef] [PubMed]
- Stasiuk, M.; Kozubek, A. Biological activity of phenolic lipids. Cell. Mol. Life Sci. 2010, 67, 841–860. [Google Scholar] [CrossRef] [PubMed]
- Saputri, F.C.; Jantan, I. Effects of selected medicinal plants on human low-density lipoprotein oxidation, 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals and human platelet aggregation. J. Med. Plants Res. 2011, 5, 6182–6191. [Google Scholar]
- Nguyen, H.A.; Ripperger, H.; Schmidt, J.; Porzel, A.; Tran, V.S.; Adam, G. Resorcinol derivatives from two Ardisia species. Planta Med. 1996, 62, 479–480. [Google Scholar] [CrossRef] [PubMed]
- Avula, B.; Wang, Y.H.; Ali, Z.; Smillie, T.J.; Khan, I.A. Quantitative determination of triterpene saponins and alkenated-phenolics from Labisia pumila using an LC-UV/ELSD method and confirmation by LC-ESI-TOF. Planta Med. 2011, 77, 1742–1748. [Google Scholar] [CrossRef] [PubMed]
- Karimi, E.; Jaafar, H.W.E. HPLC and GC-MS determination of bioactive compounds in microwave obtained extracts of three varieties of Labisia pumila Benth. Molecules 2011, 16, 6791–6805. [Google Scholar] [CrossRef] [PubMed]
- Collard, C.D.; Gelman, S. Pathophysiology, clinical manifestations, and prevention of ischemia-reperfusion injury. Anesthesiology 2001, 94, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Adamcová, M.; Šimůnek, T.; Kaiserová, H.; Popelová, O.; Štĕrba, M.; Potáčová, A.; Vávrová, J.; Maláková, J.; Geršl, V. In vitro and in vivo examination of cardiac troponins as biochemical markers of drug-induced cardiotoxiciy. Toxicology 2007, 237, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Fredericks, S.; Merton, G.K.; Lerena, M.J.; Haining, P.; Carter, N.D.; Holt, D.W. Cardiac troponin and creatine kinase content of striated muscle in common laboratory animals. Clin. Chim. Act. 2001, 304, 65–74. [Google Scholar] [CrossRef]
- Bertinchant, J.P.; Larue, C.; Pernel, I.; Ledermann, B.; Fabbro-peray, P.; Beck, L.; Calzolari, C.; Trinquier, S.; Nigond, J.; Pau, B. Release kinetics of serum cardiac troponin I in ischemic myocardial injury. Clin. Biochem. 1996, 29, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, A.S.; Babuin, L.; Apple, F.S. Biomarkers in acute cardiac disease: The present and the future. J. Am. Coll. Cardiol. 2006, 48, 1–11. [Google Scholar] [CrossRef] [PubMed]
- York, M.; Scudamore, C.; Brady, S.; Chen, C.; Wilson, S.; Curtis, M.; Evans, G.; Griffiths, W.; Whayman, M.; Williams, T.; et al. Characterization of troponin responses in isoproterenol-induced cardiac injury in the Hannover Wistar rat. Toxicol. Pathol. 2007, 35, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Lewandrowski, K.; Chen, A.; Januzzi, J. Cardiac markers for myocardial infarction. Am. J. Clin. Pathol. 2002, 118 (Suppl. 1), S93–S99. [Google Scholar] [CrossRef] [PubMed]
- Sobel, B.E.; Shell, W.E. Serum enzyme determinations in the diagnosis and assessment of myocardial infarction. Circulation 1972, 45, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.A.; Alhomida, A.S.; Sobki, S.H.; Habib, S.S.; Al Aseri, Z.; Khan, A.A.; Al Moghairi, A. Serum markers of tissue damage and antioxidative stress in patients with acute myocardial infarction. Biomed. Res. 2013, 24, 15–20. [Google Scholar]
- Benjamin, I.J.; Jalil, J.E.; Tan, L.B.; Cho, K.; Weber, K.T.; Clark, A. Isoproterenol-induced myocardial fibrosis in relation to myocyte necrosis. Circ. Res. 1989, 65, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Ithayarasi, A.P.; Padmavathy, V.N.; Shyamala Devi, C.S. Effect of α-tocopherol on isoproterenol induced myocardial infarction in rats—Electrocardiographic, biochemical and histological evidences. Indian J. Physiol. Pharmacol. 1996, 40, 297–302. [Google Scholar] [PubMed]
- Patel, D.K.; Desai, S.N.; Gandhi, H.P.; Devkar, R.V.; Ramachandran, A.V. Cardioprotective effect of Coriandrum sativum L. on isoproterenol induced myocardial necrosis in rats. Food Chem. Toxicol. 2012, 50, 3120–3125. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xie, Y.H.; Yang, Q.; Wang, S.W.; Zhang, B.L.; Wang, J.B.; Cao, W.; Bi, L.L.; Sun, J.Y.; Miao, S.; et al. Cardiprotective effect of paeonol and danshensu combination on isoproterenol-induced myocardial injury in rats. PLoS One 2012, 7, e48872. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.P.; Downing, S.E. Catecholamine cardiomyopathy: Review and analysis of pathogenetic mechanism. Yale J. Biol. Med. 1990, 63, 581–591. [Google Scholar] [PubMed]
- Padmanabhan, M.; Prince, P.S.M. Preventive effect of S-allylcysteine on lipid peroxides and antioxidants in normal and isoproterenol-induced cardiotoxicity in rats: A histopathological study. Toxicology 2006, 224, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.F.; Singal, P.K. Antioxidant and oxidative stress changes during heart failure subsequent to myocardial infarction in rats. Am. J. Pathol. 1996, 148, 291–300. [Google Scholar] [PubMed]
- Forman, M.B.; Puett, D.W.; Cates, C.U.; McCroskey, D.E.; Beckman, J.K.; Greene, H.L.; Virmani, R. Glutathione redox pathway and reperfusion injury. Effect of N-acetylcysteine on infarct size and ventricular function. Circulation 1988, 78, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Usui, A.; Kato, K.; Tsubol, H.; Sone, T.; Sassa, H.; Abe, T. Concentration of Mn-superoxide dismutase in serum in acute myocardial infarction. Clin. Chem. 1991, 37, 458–461. [Google Scholar] [PubMed]
- Chakraborty, M.; Asdaq, S.M.B. Interaction of Semecarpus anacardium L. with propranolol against isoproterenol induced myocardial damage in rats. Indian J. Exp. Biol. 2011, 49, 200–206. [Google Scholar] [PubMed]
- Zhang, G.X.; Kimura, S.; Nishiyama, A.; Shokoji, T.; Rahman, M.; Yao, L.; Nagai, Y.; Fujisawa, Y.; Miyatake, A.; Abe, Y. Cardiac oxidative stress in acute and chronic isoproterenol-infused rats. Cardiovasc. Res. 2005, 65, 230–238. [Google Scholar] [CrossRef] [PubMed]
- ICH Harmonised Tripartite Guideline. Validation of Analytical Procedures: Text and Methodology Q2(R1). (2005, Step 4 Version). Available online: http://www.ich.org/products/guidelines/quality/article/quality-guidelines.html (accessed on 10 March 2015).
- Tiwari, R.; Mohan, M.; Kasture, S.; Maxia, A.; Ballero, M. Cardioprotective potential of myricetin in isoproterenol-induced myocardial infarction in Wistar rats. Phytother. Res. 2009, 23, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- Prince, P.S.M.; Priya, S. Preventive effects of rutin on lyosomal enzymes in isoproterenol induced cardiotoxic rats: Biochemical, histological and in vitro evidences. Eur. J. Pharmacol. 2010, 649, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Prince, P.S.M.; Pricilla, H.; Devika, P.T. Gallic acid prevents lyosomal damage in isoproterenol induced cardiotoxicity in Wistar rats. Eur. J. Pharmacol. 2009, 139, 139–143. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 5-(Z-nonadec-14-enyl)resorcinol and demethylbelamcandaquinone B are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dianita, R.; Jantan, I.; Amran, A.Z.; Jalil, J. Protective Effects of Labisia pumila var. alata on Biochemical and Histopathological Alterations of Cardiac Muscle Cells in Isoproterenol-Induced Myocardial Infarction Rats. Molecules 2015, 20, 4746-4763. https://doi.org/10.3390/molecules20034746
Dianita R, Jantan I, Amran AZ, Jalil J. Protective Effects of Labisia pumila var. alata on Biochemical and Histopathological Alterations of Cardiac Muscle Cells in Isoproterenol-Induced Myocardial Infarction Rats. Molecules. 2015; 20(3):4746-4763. https://doi.org/10.3390/molecules20034746
Chicago/Turabian StyleDianita, Roza, Ibrahim Jantan, Athirah Z. Amran, and Juriyati Jalil. 2015. "Protective Effects of Labisia pumila var. alata on Biochemical and Histopathological Alterations of Cardiac Muscle Cells in Isoproterenol-Induced Myocardial Infarction Rats" Molecules 20, no. 3: 4746-4763. https://doi.org/10.3390/molecules20034746