The Herbal Medicine KIOM-MA128 Inhibits the Antigen/IgE-Mediated Allergic Response in Vitro and in Vivo
Abstract
:1. Introduction
2. Results
2.1. KIOM-MA128 Did Not Affect Cell Viability in RBL-2H3 Mast Cells
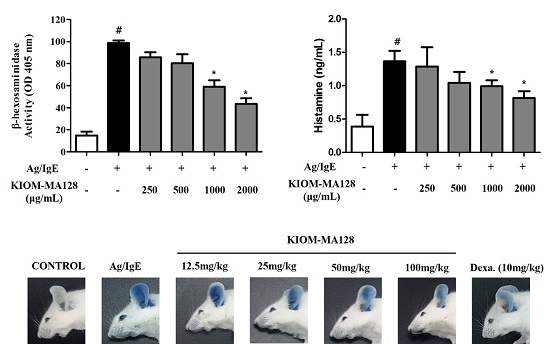
2.2. KIOM-MA128 Inhibits Ag/IgE-Mediated Degranulation in RBL-2H3 Cells
2.3. KIOM-MA128 Inhibits the IgE-Induced Release of Pro-Inflammatory Cytokines in RBL-2H3 Cells
2.4. KIOM-MA128 Inhibits the Arachidonate Signaling Pathway in RBL-2H3 Cells
2.5. KIOM-MA128 Inhibits the FcεRI Signaling Pathway in Ag/IgE-Activated RBL-2H3 Cells
2.6. KIOM-MA128 Inhibits the Allergic Response in the PCA Model
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Preparation of KIOM-MA128
4.3. Animals
4.4. Passive Cutaneous Anaphylaxis
4.5. Cell Culture
4.6. Cell Viability
4.7. β-Hexosaminidase Activity
4.8. Evaluation of Inflammatory Mediators
4.9. Immunoblot Analysis
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bischoff, S.C. Role of mast cells in allergic and non-allergic immune responses: Comparison of human and murine data. Nat. Rev. Immunol. 2007, 7, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Newton, K.; Dixit, V.M. Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 2012, 4. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Yamaki, K.; Tong, X.; Fu, L.; Zhang, R.; Cai, Y.; Yanagisawa, R.; Inoue, K.; Takano, H.; Yoshino, S. Inhibition of the antigen-induced activation of RBL-2H3 cells by sinomenine. Int. Immunopharmacol. 2008, 8, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Aketani, S.; Teshima, R.; Umezawa, Y.; Sawada, J. Correlation between cytosolic calcium concentration and degranulation in RBL-2H3 cells in the presence of various concentrations of antigen-specific IgEs. Immunol. Lett. 2001, 75, 185–189. [Google Scholar] [CrossRef]
- Oh, Y.C.; Cho, W.K.; Jeong, Y.H.; Im, G.Y.; Kim, A.; Hwang, Y.H.; Kim, T.; Song, K.H.; Ma, J.Y. A Novel Herbal Medicine KIOM-MA Exerts an Anti-Inflammatory Effect in LPS-Stimulated RAW 264.7 Macrophage Cells. Evid. Based Complement Altern. Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.H.; Kang, T.J.; Cho, W.K.; Im, G.Y.; Lee, G.S.; Yang, M.C.; Cho, C.W.; Ma, J.Y. Effectiveness of the Novel Herbal Medicine, KIOM-MA, and Its Bioconversion Product, KIOM-MA128, on the Treatment of Atopic Dermatitis. Evid. Based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Hotz, C.; Gibson, R.S. Traditional food-processing and preparation practices to enhance the bioavailability of micronutrients in plant-based diets. J. Nutr. 2007, 137, 1097–1100. [Google Scholar] [PubMed]
- Kim, A.; Im, M.; Yim, N.H.; Hwang, Y.H.; Yang, H.J.; Ma, J.Y. The novel herbal cocktail MA128 suppresses tumor growth and the metastatic potential of highly malignant tumor cells. Oncol. Rep. 2015, 34, 900–912. [Google Scholar] [CrossRef] [PubMed]
- Halova, I.; Draberova, L.; Draber, P. Mast cell chemotaxis-chemoattractants and signaling pathways. Front. Immunol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Shakoory, B.; Fitzgerald, S.M.; Lee, S.A.; Chi, D.S.; Krishnaswamy, G. The role of human mast cell-derived cytokines in eosinophil biology. J. Interferon Cytokine Res. 2004, 24, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Tada, K.; Murakami, M.; Kambe, T.; Kudo, I. Induction of cyclooxygenase-2 by secretory phospholipases A2 in nerve growth factor-stimulated rat serosal mast cells is facilitated by interaction with fibroblasts and mediated by a mechanism independent of their enzymatic functions. J. Immunol. 1998, 161, 5008–5015. [Google Scholar] [PubMed]
- Gerbec, Z.J.; Thakar, M.S.; Malarkannan, S. The Fyn-ADAP Axis: Cytotoxicity Versus Cytokine Production in Killer Cells. Front. Immunol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Kim, S.H.; Kim, B.K.; Yang, M.C.; Ma, J.Y. Antiasthmatic Effects of Herbal Complex MA and Its Fermented Product MA128. Evid. Based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Ma, J.Y. Anti-melanogenic activity of the novel herbal medicine, MA128, through inhibition of tyrosinase activity mediated by the p38 mitogen-activated protein kinases and protein kinase signaling pathway in B16F10 cells. Pharmacogn. Mag. 2014, 10, 463–471. [Google Scholar]
- Lim, B.O.; Lee, J.H.; Ko, N.Y.; Mun, S.H.; Kim, J.W.; Kim, D.K.; Kim, J.D.; Kim, B.K.; Kim, H.S.; Her, E.; et al. Polygoni cuspidati radix inhibits the activation of Syk kinase in mast cells for antiallergic activity. Exp. Biol. Med. 2007, 232, 1425–1431. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.H.; Lee, J.Y.; Jung, H.; Jin, D.H.; Go, H.Y.; Kim, J.H.; Jang, B.H.; Shin, Y.C.; Ko, S.G. Sophora flavescens Aiton inhibits the production of pro-inflammatory cytokines through inhibition of the NF kappaB/IkappaB signal pathway in human mast cell line (HMC-1). Toxicol. In Vitro 2009, 23, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.M.; Yang, J.H.; Yang, H.J.; Cho, W.K.; Ma, J.Y. Inhibitory effect of fermented Arctium lappa fruit extract on the IgE-mediated allergic response in RBL2H3 cells. Int. J. Mol. Med. 2016, 37, 501–508. [Google Scholar] [PubMed]
- Sheih, I.C.; Fang, T.J.; Wu, T.K.; Chang, C.H.; Chen, R.Y. Purification and properties of a novel phenolic antioxidant from Radix astragali fermented by Aspergillus oryzae M29. J. Agric. Food Chem. 2011, 59, 6520–6525. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, T.S.; Yang, S.H.; Suh, J.W.; Shim, S.M. Microbial bioconversion and processing methods enhance the phenolic acid and flavonoids and the radical scavenging capacity of Smilax china L. leaf. J. Sci. Food Agric. 2016, 96, 878–885. [Google Scholar] [PubMed]
- Gilfillan, A.M.; Tkaczyk, C. Integrated signalling pathways for mast-cell activation. Nat. Rev. Immunol. 2006, 6, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Oettgen, H.C.; Burton, O.T. IgE receptor signaling in food allergy pathogenesis. Curr. Opin. Immunol. 2015, 36, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Kinet, J.P. The high-affinity IgE receptor (Fc epsilon RI): From physiology to pathology. Annu. Rev. Immunol. 1999, 17, 931–972. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.; Craxton, A.; Kurosaki, T.; Clark, E.A. Different protein tyrosine kinases are required for B cell antigen receptor-mediated activation of extracellular signal-regulated kinase, c-Jun NH2-terminal kinase 1, and p38 mitogen-activated protein kinase. J. Exp. Med. 1998, 188, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, D.D. Mast cells and mastocytosis. Blood 2008, 112, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Siraganian, R.P. Mast cell signal transduction from the high-affinity IgE receptor. Curr. Opin. Immunol. 2003, 15, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Gomez, G.; Gonzalez-Espinosa, C.; Odom, S.; Baez, G.; Cid, M.E.; Ryan, J.J.; Rivera, J. Impaired FcepsilonRI-dependent gene expression and defective eicosanoid and cytokine production as a consequence of Fyn deficiency in mast cells. J. Immunol. 2005, 175, 7602–7610. [Google Scholar] [CrossRef] [PubMed]
- Fanning, L.B.; Boyce, J.A. Lipid mediators and allergic diseases. Ann. Allergy Asthma. Immunol. 2013, 111, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Arima, M.; Fukuda, T. Prostaglandin D2 and T(H)2 inflammation in the pathogenesis of bronchial asthma. Korean J. Intern. Med. 2011, 26, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Nettis, E.; D'Erasmo, M.; Di Leo, E.; Calogiuri, G.; Montinaro, V.; Ferrannini, A.; Vacca, A. The employment of leukotriene antagonists in cutaneous diseases belonging to allergological field. Mediat. Inflamm. 2010, 2010, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Gijon, M.A.; Leslie, C.C. Regulation of arachidonic acid release and cytosolic phospholipase A2 activation. J. Leukoc. Biol. 1999, 65, 330–336. [Google Scholar] [PubMed]
- Mitchell, J.A.; Larkin, S.; Williams, T.J. Cyclooxygenase-2: Regulation and relevance in inflammation. Biochem. Pharmacol. 1995, 50, 1535–1542. [Google Scholar] [CrossRef]
- National Toxicology Program. NTP Toxicology and Carcinogenesis Studies of o-Phenylphenol (CAS No. 90-43-7) Alone and with 7,12-Dimethylbenz(a)anthracene (CAS No. 57-97-6) in Swiss CD-1 Mice (Dermal Studies). Natl. Toxicol. Program. Tech. Rep. Ser. 1986, 301, 1–141. [Google Scholar]
- Yoo, J.M.; Sok, D.E.; Kim, M.R. Anti-allergic action of aged black garlic extract in RBL-2H3 cells and passive cutaneous anaphylaxis reaction in mice. J. Med. Food 2014, 17, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Siraganian, R.P. Inhibition of IgE-mediated histamine release from rat basophilic leukemia cells and rat mast cells by inhibitors of transmethylation. J. Immunol. 1981, 127, 1339–1344. [Google Scholar] [PubMed]
- Ishiyama, M.; Tominaga, H.; Shiga, M.; Sasamoto, K.; Ohkura, Y.; Ueno, K. A combined assay of cell viability and in vitro cytotoxicity with a highly water-soluble tetrazolium salt, neutral red and crystal violet. Biol. Pharm. Bull. 1996, 19, 1518–1520. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.M.; Kim, N.Y.; Seo, J.M.; Kim, S.J.; Lee, S.Y.; Kim, S.K.; Kim, H.D.; Lee, S.W.; Kim, M.R. Inhibitory effects of mulberry fruit extract in combination with naringinase on the allergic response in IgE-activated RBL-2H3 cells. Int. J. Mol. Med. 2014, 33, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.






© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, K.I.; Kim, D.G.; Yoo, J.M.; Ma, J.Y. The Herbal Medicine KIOM-MA128 Inhibits the Antigen/IgE-Mediated Allergic Response in Vitro and in Vivo. Molecules 2016, 21, 1015. https://doi.org/10.3390/molecules21081015
Park KI, Kim DG, Yoo JM, Ma JY. The Herbal Medicine KIOM-MA128 Inhibits the Antigen/IgE-Mediated Allergic Response in Vitro and in Vivo. Molecules. 2016; 21(8):1015. https://doi.org/10.3390/molecules21081015
Chicago/Turabian StylePark, Kwang Il, Dong Gun Kim, Jae Myung Yoo, and Jin Yeul Ma. 2016. "The Herbal Medicine KIOM-MA128 Inhibits the Antigen/IgE-Mediated Allergic Response in Vitro and in Vivo" Molecules 21, no. 8: 1015. https://doi.org/10.3390/molecules21081015