Effect of Consuming Oat Bran Mixed in Water before a Meal on Glycemic Responses in Healthy Humans—A Pilot Study
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Subjects
2.2. Study Outline
2.3. Measurements
2.4. Data and Statistical Analysis
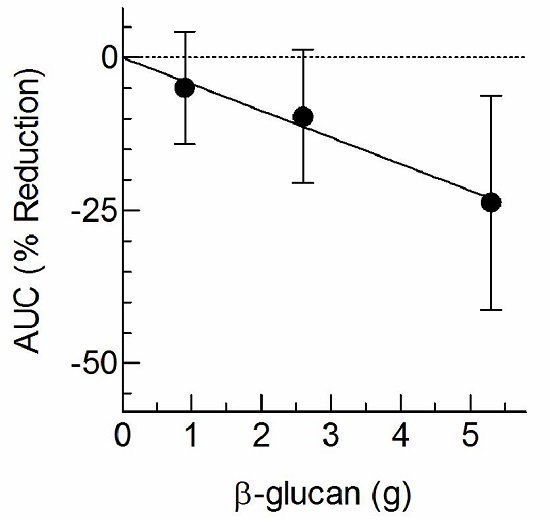
3. Results
4. Discussion
Author Contributions
Conflicts of Interest
References
- Monnier, L.; Lapinski, H.; Colette, C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: Variations with increasing levels of HbA(1c). Diabetes Care 2003, 26, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Peter, R.; Dunseath, G.; Luzio, S.D.; Owens, D.R. Estimates of the relative and absolute diurnal contributions of fasting and post-prandial plasma glucose over a range of hyperglycaemia in type 2 diabetes. Diabetes Metab. 2013, 39, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Monnier, L.; Colette, C.; Owens, D. Postprandial and basal glucose in type 2 diabetes: Assessment and respective impacts. Diabetes Technol. Ther. 2011, 13, S25–S32. [Google Scholar] [CrossRef] [PubMed]
- Tosh, S.M. Review of human studies investigating the post-prandial blood-glucose lowering ability of oat and barley food products. Eur. J. Clin. Nutr. 2013, 67, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Würsch, P.; Pi-Sunyer, F.X. The role of viscous soluble fiber in the metabolic control of diabetes: A review with special emphasis on cereals rich in beta-glucan. Diabetes Care 1997, 20, 1774–1780. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, M.; Edelbroek, M.A.; Wishart, J.M.; Straathof, J.W. Relationship between oral glucose tolerance and gastric emptying in normal healthy subjects. Diabetologia 1993, 36, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Marathe, C.S.; Rayner, C.K.; Jones, K.L.; Horowitz, M. Relationships between gastric emptying, postprandial glycemia, and incretin hormones. Diabetes Care 2013, 36, 1396–1405. [Google Scholar] [CrossRef] [PubMed]
- Fuessl, S.; Adrian, T.E.; Bacarese-Hamilton, A.J.; Bloom, S.R. Guar in NIDD: Effect of different modes of administration on plasma glucose and insulin responses to a starch meal. Pract. Diabetes Int. 1986, 3, 258–260. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Nineham, R.; Craddock, C.; Craig-McFeely, P.; Donaldson, K.; Leigh, T.; Snook, J. Fibre in diabetes. Lancet 1979, 1, 434–435. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 7th ed.; Iowa State University Press: Ames, IA, USA, 1980. [Google Scholar]
- Ren, Y.; Ellis, P.R.; Sutherland, I.W.; Ross-Murphy, S.B. Dilute and semi-dilute solution properties of an exopolysaccharide from Escherichia coli strain S61. Carbohydr. Polym. 2003, 52, 189–195. [Google Scholar] [CrossRef]
- Wolever, T.M.; Vuksan, V.; Eshuis, H.; Spadafora, P.; Peterson, R.D.; Chao, E.S.; Storey, M.L.; Jenkins, D.J. Effect of method of administration of psyllium on glycemic response and carbohydrate digestibility. J. Am. Coll. Nutr. 1991, 10, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.; Leong, V.W.; Salmon, E.; Martin, F.I. Role of guar and dietary fibre in the management of diabetes mellitus. Med. J. Aust. 1980, 1, 59–61. [Google Scholar] [PubMed]
- Gentilcore, D.; Chaikomin, R.; Jones, K.L.; Russo, A.; Feinle-Bisset, C.; Wishart, J.M.; Rayner, C.K.; Horowitz, M. Effects of fat on gastric emptying of and the glycemic, insulin, and incretin responses to a carbohydrate meal in type 2 diabetes. J. Clin. Endocrinol. Metab. 2006, 91, 2062–2067. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Stevens, J.E.; Cukier, K.; Maddox, A.F.; Wishart, J.M.; Jones, K.L.; Clifton, P.M.; Horowitz, M.; Rayner, C.K. Effects of a protein preload on gastric emptying, glycemia, and gut hormones after a carbohydrate meal in diet-controlled type 2 diabetes. Diabetes Care 2009, 32, 1600–1602. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Jesudason, D.R.; Stevens, J.E.; Keogh, J.B.; Jones, K.L.; Clifton, P.M.; Horowitz, M.; Rayner, C.K. Sustained effects of a protein “preload” on glycaemia and gastric emptying over 4 weeks in patients with type 2 diabetes: A randomized clinical trial. Diabetes Res. Clin. Pract. 2015, 108, e31–e34. [Google Scholar] [CrossRef] [PubMed]
- Lan-Pidhainy, X.; Wolever, T.M.S. The hypoglycemic effect of fat and protein is not attenuated by insulin resistance. Am. J. Clin. Nutr. 2010, 91, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to beta-glucans from oats and barley and maintenance of normal blood LDL-cholesterol concentrations (ID 1236, 1299), increase in satiety leading to a reduction in energy intake (ID 851, 852), reduction of post-prandial glycaemic responses (ID 821, 824), and “digestive function” (ID 850) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9. [Google Scholar] [CrossRef] [Green Version]
- Wanders, A.J.; van den Borne, J.J.G.C.; de Graaf, C.; Hulshof, T.; Jonathan, M.C.; Kristensen, M.; Mars, M.; Schols, H.A.; Feskens, E.J.M. Effects of dietary fibre on subjective appetite, energy intake and body weight: A systematic review of randomized controlled trials. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2011, 12, 724–739. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, A.; Astbury, N.M.; Olli, K.; Alhoniemi, E.; Tiihonen, K. Effect of polydextrose on subjective feelings of appetite during the satiation and satiety periods: A systematic review and meta-analysis. Nutrients 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Howarth, N.C.; Saltzman, E.; Roberts, S.B. Dietary fiber and weight regulation. Nutr. Rev. 2001, 59, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, M.A.; Pomerleau, J.; Grace, D.M.; Anderson, L. Fiber intake of normal weight, moderately obese and severely obese subjects. Obes. Res. 1995, 3, 541–547. [Google Scholar] [CrossRef] [PubMed]

| Test Meal | Energy (kcal) | Weight (g) | Protein (g) | Fat (g) | tCHO 1 (g) | Fibre (g) | avCHO 1 (g) | |
|---|---|---|---|---|---|---|---|---|
| Total | β-Glucan | |||||||
| White Bread 1 | 245 | 119 | 9.0 | 1.0 | 52.6 | 2.6 | 0 | 50.0 |
| OatWell®22 2 | 13.1 | 4.5 | 1.0 | 0.2 | 3.0 | 2.2 | 0.9 | 0.8 |
| 39.7 | 13.6 | 3.1 | 0.6 | 8.8 | 6.5 | 2.6 | 2.3 | |
| 79.7 | 27.3 | 6.2 | 1.1 | 17.7 | 13.1 | 5.3 | 4.6 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steinert, R.E.; Raederstorff, D.; Wolever, T.M.S. Effect of Consuming Oat Bran Mixed in Water before a Meal on Glycemic Responses in Healthy Humans—A Pilot Study. Nutrients 2016, 8, 524. https://doi.org/10.3390/nu8090524
Steinert RE, Raederstorff D, Wolever TMS. Effect of Consuming Oat Bran Mixed in Water before a Meal on Glycemic Responses in Healthy Humans—A Pilot Study. Nutrients. 2016; 8(9):524. https://doi.org/10.3390/nu8090524
Chicago/Turabian StyleSteinert, Robert E., Daniel Raederstorff, and Thomas M. S. Wolever. 2016. "Effect of Consuming Oat Bran Mixed in Water before a Meal on Glycemic Responses in Healthy Humans—A Pilot Study" Nutrients 8, no. 9: 524. https://doi.org/10.3390/nu8090524