Therapeutic Potential of 47Sc in Comparison to 177Lu and 90Y: Preclinical Investigations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Radionuclides
2.2. Preparation of 47Sc-Folate, 177Lu-Folate, and 90Y-Folate
2.3. Tumor Cell Culture and Internalization Experiments
2.4. Cell Viability Assay
2.5. Animal Experiments
2.6. Biodistribution Studies
2.7. Dosimetric Calculations
2.8. Tumor Therapy Studies
2.9. Determination of Blood Plasma Parameters
2.10. Histopathological Investigations
3. Results
3.1. Radiofolate Preparation and Tumor Cell Internalization Studies
3.2. Cell Viability Assay
3.3. Biodistribution and Dosimetry
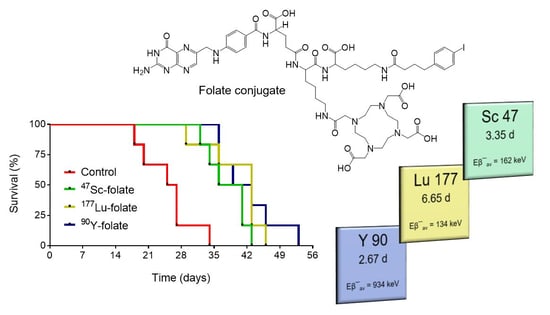
3.4. Therapy Experiments
3.5. Histopathological Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sudhakar, A. History of cancer, ancient and modern treatment methods. J. Cancer Sci. Ther. 2009, 1, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.E.; Chester, J.D. Personalised cancer medicine. Int. J. Cancer 2015, 137, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, J.; Falzone, N.; Lee, B.Q.; Vallis, K.A. Targeted radionuclide therapy: New advances for improvement of patient management and response. Cancers (Basel) 2019, 11, 268. [Google Scholar] [CrossRef] [PubMed]
- Enger, S.A.; Hartman, T.; Carlsson, J.; Lundqvist, H. Cross-fire doses from beta-emitting radionuclides in targeted radiotherapy. A theoretical study based on experimentally measured tumor characteristics. Phys. Med. Biol. 2008, 53, 1909–1920. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Pillai, M.R.; Knapp, F.F. Lutetium-177 therapeutic radiopharmaceuticals: Linking chemistry, radiochemistry, and practical applications. Chem. Rev. 2015, 115, 2934–2974. [Google Scholar] [CrossRef] [PubMed]
- Bodei, L.; Cremonesi, M.; Grana, C.; Rocca, P.; Bartolomei, M.; Chinol, M.; Paganelli, G. Receptor radionuclide therapy with 90Y-[DOTA]0-Tyr3-octreotide (90Y-DOTATOC) in neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Nisa, L.; Savelli, G.; Giubbini, R. Yttrium-90 DOTATOC therapy in GEP-NET and other SST2 expressing tumors: A selected review. Ann. Nucl. Med. 2011, 25, 75–85. [Google Scholar] [CrossRef]
- Moll, S.; Nickeleit, V.; Müller-Brand, J.; Brunner, F.P.; Maecke, H.R.; Mihatsch, M.J. A new cause of renal thrombotic microangiopathy: 90Y-DOTATOC internal radiotherapy. Am. J. Kidney Dis. 2001, 37, 847–851. [Google Scholar] [CrossRef]
- Valkema, R.; Pauwels, S.A.; Kvols, L.K.; Kwekkeboom, D.J.; Jamar, F.; de Jong, M.; Barone, R.; Walrand, S.; Kooij, P.P.; Bakker, W.H.; et al. Long-term follow-up of renal function after peptide receptor radiation therapy with 90Y-DOTA0, Tyr3-octreotide and 177Lu-DOTA0, Tyr3-octreotate. J. Nucl. Med. 2005, 46, 83S–91S. [Google Scholar]
- Kunikowska, J.; Krolicki, L.; Hubalewska-Dydejczyk, A.; Mikolajczak, R.; Sowa-Staszczak, A.; Pawlak, D. Clinical results of radionuclide therapy of neuroendocrine tumours with 90Y-DOTATATE and tandem 90Y/177Lu-DOTATATE: Which is a better therapy option? Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1788–1797. [Google Scholar] [CrossRef]
- Frost, S.H.; Frayo, S.L.; Miller, B.W.; Orozco, J.J.; Booth, G.C.; Hylarides, M.D.; Lin, Y.; Green, D.J.; Gopal, A.K.; Pagel, J.M.; et al. Comparative efficacy of 177Lu and 90Y for anti-CD20 pretargeted radioimmunotherapy in murine lymphoma xenograft models. PLoS ONE 2015, 10, e0120561. [Google Scholar] [CrossRef] [PubMed]
- Domnanich, K.A.; Müller, C.; Benešová, M.; Dressler, R.; Haller, S.; Koster, U.; Ponsard, B.; Schibli, R.; Türler, A.; van der Meulen, N.P. 47Sc as useful β¯-emitter for the radiotheragnostic paradigm: A comparative study of feasible production routes. EJNMMI Radiopharm. Chem. 2017, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Domnanich, K.A.; Umbricht, C.A.; van der Meulen, N.P. Scandium and terbium radionuclides for radiotheranostics: Current state of development towards clinical application. Br. J. Radiol. 2018, 91, 20180074. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Bunka, M.; Haller, S.; Köster, U.; Groehn, V.; Bernhardt, P.; van der Meulen, N.; Türler, A.; Schibli, R. Promising prospects for 44Sc-/47Sc-based theragnostics: Application of 47Sc for radionuclide tumor therapy in mice. J. Nucl. Med. 2014, 55, 1658–1664. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Torano, E.; Peyres, V.; Roteta, M.; Sanchez-Cabezudo, A.I.; Romero, E.; Martinez Ortega, A. Standardisation and precise determination of the half-life of 44Sc. Appl. Radiat. Isot. 2016, 109, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Van der Meulen, N.P.; Bunka, M.; Domnanich, K.A.; Müller, C.; Haller, S.; Vermeulen, C.; Türler, A.; Schibli, R. Cyclotron production of 44Sc: From bench to bedside. Nucl. Med. Biol. 2015, 42, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Bunka, M.; Reber, J.; Fischer, C.; Zhernosekov, K.; Türler, A.; Schibli, R. Promises of cyclotron-produced 44Sc as a diagnostic match for trivalent b--emitters: In vitro and in vivo study of a 44Sc-DOTA-folate conjugate. J. Nucl. Med. 2013, 54, 2168–2174. [Google Scholar] [CrossRef] [PubMed]
- Domnanich, K.A.; Müller, C.; Farkas, R.; Schmid, R.M.; Ponsard, B.; Schibli, R.; Türler, A.; van der Meulen, N.P. 44Sc for labeling of DOTA- and NODAGA-functionalized peptides: Preclinical in vitro and in vivo investigations. EJNMMI Radiopharm. Chem. 2017, 1, 8. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; van der Meulen, N.P.; Müller, C.; Klette, I.; Kulkarni, H.R.; Türler, A.; Schibli, R.; Baum, R.P. First-in-human PET/CT imaging of metastatic neuroendocrine neoplasms with cyclotron-produced 44Sc-dotatoc: A proof-of-concept study. Cancer Biother. Radiopharm. 2017, 32, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Eppard, E.; de la Fuente, A.; Benešová, M.; Khawar, A.; Bundschuh, R.A.; Gartner, F.C.; Kreppel, B.; Kopka, K.; Essler, M.; Rösch, F. Clinical translation and first in-human use of [44Sc]Sc-PSMA-617 for PET imaging of metastasized castrate-resistant prostate cancer. Theranostics 2017, 7, 4359–4369. [Google Scholar] [CrossRef] [PubMed]
- Khawar, A.; Eppard, E.; Sinnes, J.P.; Rösch, F.; Ahmadzadehfar, H.; Kurpig, S.; Meisenheimer, M.; Gaertner, F.C.; Essler, M.; Bundschuh, R.A. [44Sc]Sc-PSMA-617 biodistribution and dosimetry in patients with metastatic castration-resistant prostate carcinoma. Clin. Nucl. Med. 2018, 43, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Low, P.S.; Kularatne, S.A. Folate-targeted therapeutic and imaging agents for cancer. Curr. Opin. Chem. Biol. 2009, 13, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Siwowska, K.; Schmid, R.M.; Cohrs, S.; Schibli, R.; Müller, C. Folate receptor-positive gynecological cancer cells: In vitro and in vivo characterization. Pharmaceuticals (Basel) 2017, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Siwowska, K.; Haller, S.; Bortoli, F.; Benešová, M.; Groehn, V.; Bernhardt, P.; Schibli, R.; Müller, C. Preclinical comparison of albumin-binding radiofolates: Impact of linker entities on the in vitro and in vivo properties. Mol. Pharm. 2017, 14, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Reber, J.; Haller, S.; Dorrer, H.; Bernhardt, P.; Zhernosekov, K.; Türler, A.; Schibli, R. Direct in vitro and in vivo comparison of 161Tb and 177Lu using a tumour-targeting folate conjugate. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Schmitt, A.; Bernhardt, P.; Nilsson, O.; Ahlman, H.; Kolby, L.; Schmitt, J.; Forssel-Aronsson, E. Biodistribution and dosimetry of 177Lu-labeled [DOTA0,Tyr3]octreotate in male nude mice with human small cell lung cancer. Cancer Biother. Radiopharm. 2003, 18, 593–599. [Google Scholar] [CrossRef]
- Uusijärvi, H.; Bernhardt, P.; Ericsson, T.; Forssell-Aronsson, E. Dosimetric characterization of radionuclides for systemic tumor therapy: Influence of particle range, photon emission, and subcellular distribution. Med. Phys. 2006, 33, 3260–3269. [Google Scholar] [CrossRef]
- Svensson, J.; Molne, J.; Forssell-Aronsson, E.; Konijnenberg, M.; Bernhardt, P. Nephrotoxicity profiles and threshold dose values for [177Lu]-DOTATATE in nude mice. Nucl. Med. Biol. 2012, 39, 756–762. [Google Scholar] [CrossRef]
- Müller, C.; Struthers, H.; Winiger, C.; Zhernosekov, K.; Schibli, R. DOTA conjugate with an albumin-binding entity enables the first folic acid-targeted 177Lu-radionuclide tumor therapy in mice. J. Nucl. Med. 2013, 54, 124–131. [Google Scholar] [CrossRef]
- Sanceau, J.; Poupon, M.F.; Delattre, O.; Sastre-Garau, X.; Wietzerbin, J. Strong inhibition of ewing tumor xenograft growth by combination of human interferon-alpha or interferon-beta with ifosfamide. Oncogene 2002, 21, 7700–7709. [Google Scholar] [CrossRef] [PubMed]
- Haller, S.; Pellegrini, G.; Vermeulen, C.; van der Meulen, N.P.; Köster, U.; Bernhardt, P.; Schibli, R.; Müller, C. Contribution of Auger/conversion electrons to renal side effects after radionuclide therapy: Preclinical comparison of 161Tb-folate and 177Lu-folate. EJNMMI Res. 2016, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Umbricht, C.A.; Benesova, M.; Schmid, R.M.; Türler, A.; Schibli, R.; van der Meulen, N.P.; Müller, C. 44Sc-PSMA-617 for radiotheragnostics in tandem with 177Lu-PSMA-617-preclinical investigations in comparison with 68Ga-PSMA-11 and 68Ga-PSMA-617. EJNMMI Res. 2017, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Kolsky, K.L.; Joshi, V.; Mausner, L.F.; Srivastava, S.C. Radiochemical purification of no-carrier-added scandium-47 for radioimmunotherapy. Appl. Radiat. Isot. 1998, 49, 1541–1549. [Google Scholar] [CrossRef]
- Misiak, R.; Walczak, R.; Was, B.; Bartyzel, M.; Mietelski, J.W.; Bilewicz, A. 47Sc production development by cyclotron irradiation of 48Ca. J. Radioanal. Nucl. Chem. 2017, 313, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Loveless, C.S.; Radford, L.L.; Ferran, S.J.; Queern, S.L.; Shepherd, M.R.; Lapi, S.E. Photonuclear production, chemistry, and in vitro evaluation of the theranostic radionuclide 47Sc. EJNMMI Res. 2019, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Bodei, L.; Cremonesi, M.; Ferrari, M.; Pacifici, M.; Grana, C.M.; Bartolomei, M.; Baio, S.M.; Sansovini, M.; Paganelli, G. Long-term evaluation of renal toxicity after peptide receptor radionuclide therapy with 90y-dotatoc and 177Lu-DOTATATE: The role of associated risk factors. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1847–1856. [Google Scholar] [CrossRef]
- Haller, S.; Reber, J.; Brandt, S.; Bernhardt, P.; Groehn, V.; Schibli, R.; Müller, C. Folate receptor-targeted radionuclide therapy: Preclinical investigation of anti-tumor effects and potential radionephropathy. Nucl. Med. Biol. 2015, 42, 770–779. [Google Scholar] [CrossRef]




| Radionuclide | Half-Life | Eβ− Average | Eγ (Intensity) 2 | Availability |
|---|---|---|---|---|
| 47Sc | 3.35 d | 162 keV | 159 keV (68%) | produced as part of this research project |
| 177Lu | 6.65 d | 134 keV | 113 keV (6%) 208 keV (10%) | commercially available |
| 90Y | 2.67 d | 934 keV | none | commercially available |
| Radioligand | Activity [MBq] | Tumor D [Gy] | Kidney Dose [Gy] | Euthanasia of the First Mouse | Median Survival [days] | TGI [%] | TGDI2 | TGDI5 |
|---|---|---|---|---|---|---|---|---|
| Saline | - | - | Day 18 | 26 | - | 1.0 ± 0.3 | 1.0 ± 0.2 | |
| 47Sc-folate | 12.5 | 21.3 | 22.5 | Day 32 | 39 | 69 ± 17 1 | 1.5 ± 0.3 1 | 1.3 ± 0.2 1 |
| 177Lu-folate | 10 | 20.8 | 23.0 | Day 29 | 43 | 67 ± 28 1 | 1.5 ± 0.5 1 | 1.5 ± 0.3 1 |
| 90Y-folate | 5 | 21.5 | 22.0 | Day 36 | 41 | 76 ± 15 1 | 1.7 ± 0.2 1 | 1.6 ± 0.1 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siwowska, K.; Guzik, P.; Domnanich, K.A.; Monné Rodríguez, J.M.; Bernhardt, P.; Ponsard, B.; Hasler, R.; Borgna, F.; Schibli, R.; Köster, U.; et al. Therapeutic Potential of 47Sc in Comparison to 177Lu and 90Y: Preclinical Investigations. Pharmaceutics 2019, 11, 424. https://doi.org/10.3390/pharmaceutics11080424
Siwowska K, Guzik P, Domnanich KA, Monné Rodríguez JM, Bernhardt P, Ponsard B, Hasler R, Borgna F, Schibli R, Köster U, et al. Therapeutic Potential of 47Sc in Comparison to 177Lu and 90Y: Preclinical Investigations. Pharmaceutics. 2019; 11(8):424. https://doi.org/10.3390/pharmaceutics11080424
Chicago/Turabian StyleSiwowska, Klaudia, Patrycja Guzik, Katharina A. Domnanich, Josep M. Monné Rodríguez, Peter Bernhardt, Bernard Ponsard, Roger Hasler, Francesca Borgna, Roger Schibli, Ulli Köster, and et al. 2019. "Therapeutic Potential of 47Sc in Comparison to 177Lu and 90Y: Preclinical Investigations" Pharmaceutics 11, no. 8: 424. https://doi.org/10.3390/pharmaceutics11080424