Are Medication Swallowing Lubricants Suitable for Use in Dysphagia? Consistency, Viscosity, Texture, and Application of the International Dysphagia Diet Standardization Initiative (IDDSI) Framework
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. pH and Density
2.2.2. The International Dysphagia Diet Standardization Initiative (IDDSI)
2.2.3. Rheological Properties
2.2.4. Bostwick Thickness Consistency
2.2.5. Texture Properties
2.3. Statistical Analysis
3. Results
3.1. The International Dysphagia Diet Standardization Initiative (IDDSI)
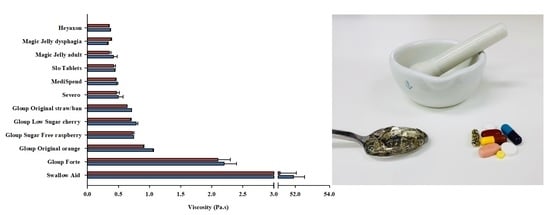
3.2. Rheological Properties
3.3. Bostwick Consistometer
3.4. Texture Characterisation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Punzalan, C.; Budnitz, D.S.; Chirtel, S.J.; Geller, A.I.; Jones, O.E.; Mozersky, R.P.; Wolpert, B. Swallowing problems and dietary supplements: Data from U.S. Food and Drug Administration Adverse Event Reports, 2006–2015. Ann. Intern. Med. 2019, 171, 771–773. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.; Lopez, J.; Meier, J. Antihypertensive medication adherence in the Department of Veterans Affairs. Am. J. Med. 2007, 120, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Forough, A.S.; Lau, E.T.; Steadman, K.J.; Cichero, J.A.Y.; Kyle, G.J.; Serrano Santos, J.M.; Nissen, L.M. A spoonful of sugar helps the medicine go down? A review of strategies for making pills easier to swallow. Patient Prefer. Adherence 2018, 12, 1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, E.T.; Steadman, K.J.; Cichero, J.A.; Nissen, L.M. Dosage form modification and oral drug delivery in older people. Adv. Drug Deliv. 2018, 135, 75–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sura, L.; Madhavan, A.; Carnaby, G.; Crary, M.A. Dysphagia in the elderly: Management and nutritional considerations. Clin. Interv. Aging 2012, 7, 287. [Google Scholar] [CrossRef] [Green Version]
- David Buchholz, M.D. Neurologic causes of dysphagia. Dysphagia 1987, 1, 152–156. [Google Scholar] [CrossRef]
- Barczi, S.R.; Sullivan, P.A.; Robbins, J.A. How should dysphagia care of older adults differ? Establishing optimal practice patterns. Semin. Speech Lang. 2000, 21, 0347–0364. [Google Scholar] [CrossRef]
- Martino, R.; Foley, N.; Bhogal, S.; Diamant, N.; Speechley, M.; Teasell, R. Dysphagia after stroke: Incidence, diagnosis, and pulmonary complications. Stroke 2005, 36, 2756–2763. [Google Scholar] [CrossRef] [Green Version]
- Rofes, L.; Arreola, V.; Mukherjee, R.; Swanson, J.; Clavé, P. The effects of a xanthan gum-based thickener on the swallowing function of patients with dysphagia. Aliment. Pharmacol. Ther. 2014, 39, 1169–1179. [Google Scholar] [CrossRef]
- Hinds, N.P.; Wiles, C.M. Assessment of swallowing and referral to speech and language therapists in acute stroke. QJM 1998, 91, 829–835. [Google Scholar] [CrossRef]
- Ramsey, D.J.; Smithard, D.G.; Kalra, L. Early assessments of dysphagia and aspiration risk in acute stroke patients. Stroke 2003, 34, 1252–1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozaki, K.; Kagaya, H.; Yokoyama, M.; Saitoh, E.; Okada, S.; González-Fernández, M.; Palmer, J.P.; Uematsu, H. The risk of penetration or aspiration during videofluoroscopic examination of swallowing varies depending on food types. Tohoku J. Exp. Med. 2010, 220, 41–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cichero, J.A.; Lam, P.; Steele, C.M.; Hanson, B.; Chen, J.; Dantas, R.O.; Duivestein, J.; Kayashita, J.; Lecko, C.; Murray, J.; et al. Development of international terminology and definitions for texture-modified foods and thickened fluids used in dysphagia management: The IDDSI framework. Dysphagia 2017, 32, 293–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cichero, J.A.; Steele, C.; Duivestein, J.; Clavé, P.; Chen, J.; Kayashita, J.; Dantas, R.; Lecko, C.; Speyer, R.; Lam, L.; et al. The need for international terminology and definitions for texture-modified foods and thickened liquids used in dysphagia management: Foundations of a global initiative. Curr. Phys. Med. Rehabil. Rep. 2013, 1, 280–291. [Google Scholar] [CrossRef] [Green Version]
- Su, M.; Zheng, G.; Chen, Y.; Xie, H.; Han, W.; Yang, Q.; Sun, J.; Lv, Z.; Chen, J. Clinical applications of IDDSI framework for texture recommendation for dysphagia patients. J. Texture Stud. 2018, 49, 2–10. [Google Scholar] [CrossRef] [Green Version]
- Lam, P.; Stanschus, S.; Zaman, R.; Cichero, J.A. The international dysphagia diet standardisation initiative (IDDSI) framework: The kempen pilot. Br. J. Neurosci. Nurs. 2017, 13, S18–S26. [Google Scholar] [CrossRef] [Green Version]
- Barbon, C.E.A.; Steele, C.M. Characterizing the flow of thickened barium and non-barium liquid recipes using the IDDSI flow test. Dysphagia 2019, 34, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Nissen, L.M.; Haywood, A.; Steadman, K.J. Solid medication dosage form modification at the bedside and in the pharmacy of Queensland hospitals. J. Pharm. Pract. Res. 2009, 39, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Jukes, S.; Cichero, J.A.; Haines, T.; Wilson, C.; Paul, K.; O’Rourke, M. Evaluation of the uptake of the Australian standardized terminology and definitions for texture modified foods and fluids. Int. J. Speech Lang. Pathol. 2012, 14, 214–225. [Google Scholar] [CrossRef]
- Cichero, J.A. Thickening agents used for dysphagia management: Effect on bioavailability of water, medication and feelings of satiety. Nutr. J. 2013, 12, 54. [Google Scholar] [CrossRef] [Green Version]
- Zargaraan, A.; Rastmanesh, R.; Fadavi, G.; Zayeri, F.; Mohammadifar, M.A. Rheological aspects of dysphagia-oriented food products: A mini review. Food Sci. Hum. Wellness 2013, 2, 173–178. [Google Scholar] [CrossRef] [Green Version]
- Hadde, E.K.; Cichero, J.A.; Nicholson, T.M. Viscosity of thickened fluids that relate to the Australian national standards. Int. J. Speech Lang. Pathol. 2016, 18, 402–410. [Google Scholar] [CrossRef]
- Côté, C.; Germain, I.; Dufresne, T.; Gagnon, C. Comparison of two methods to categorize thickened liquids for dysphagia management in a clinical care setting context: The Bostwick consistometer and the IDDSI flow test. Are we talking about the same concept? J. Texture Stud. 2019, 50, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Houjaij, N.; Dufresne, T.; Lachance, N.; Ramaswamy, H. Textural characterization of pureed cakes prepared for the therapeutic treatment of dysphagic patients. Int. J. Food Prop. 2009, 12, 45–54. [Google Scholar] [CrossRef]
- Steele, C.M.; Alsanei, W.A.; Ayanikalath, S.; Barbon, C.E.; Chen, J.; Cichero, J.A.; Coutts, K.; Dantas, R.O.; Duivestein, J.; Giosa, L.; et al. The influence of food texture and liquid consistency modification on swallowing physiology and function: A systematic review. Dysphagia 2015, 30, 2–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borwankar, R.P. Food texture and rheology: A tutorial review. J. Food Eng. 1992, 16, 1–16. [Google Scholar] [CrossRef]
- Owen, S.R.; Tung, M.A.; Paulson, A.T. Thermorheological studies of food polymer dispersions. J. Food Eng. 1992, 16, 39–53. [Google Scholar] [CrossRef]
- International Dysphagia Diet Standardisation Initiative (IDDSI). Complete IDDSI Framework (Detailed Definitions). 2019. Available online: http://bit.ly/2zn8OrZ (accessed on 22 June 2020).
- Szabó, P.; Kállai-Szabó, B.; Kállai-Szabó, N.; Sebe, I.; Zelkó, R. Preparation of hydroxypropyl cellulose microfibers by high-speed rotary spinning and prediction of the fiber-forming properties of hydroxypropyl cellulose gels by texture analysis. Cellulose 2014, 21, 4419–4427. [Google Scholar] [CrossRef]
- Kamboj, S.; Singh, K.; Tiwary, A.; Rana, V. Optimization of microwave assisted maillard reaction to fabricate and evaluate corn fiber gum-chitosan IPN films. Food Hydrocoll. 2015, 44, 260–276. [Google Scholar] [CrossRef]
- Paoletti, F.; Nardo, N.; Saleh, A.; Quaglia, G. Back extrusion test on emulsions stabilized with whey protein concentrates. LWT Food Sci. Technol. 1995, 28, 616–619. [Google Scholar] [CrossRef]
- Ong, J.J.X.; Steele, C.M.; Duizer, L.M. Sensory characteristics of liquids thickened with commercial thickeners to levels specified in the International Dysphagia Diet Standardization Initiative (IDDSI) framework. Food Hydrocoll. 2018, 79, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Diniz, P.B.; Vanin, G.; Xavier, R.; Parente, M.A. Reduced incidence of aspiration with spoon-thick consistency in stroke patients. Clin. Nutr. 2009, 24, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Clavé, P.; De kraa, M.; Girven, A.M.; Farre, M.; Palomera, E.; Serra-Prat, M. The effect of bolus viscosity on swallowing function in neurogenic dysphagia. Aliment. Pharmacol. Ther. 2006, 24, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Jeong, G.Y.; Yoo, B. Comparative study of IDDSI flow test and line-spread test of thickened water prepared with different dysphagia thickeners. J. Texture Stud. 2018, 49, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.M.; Chambers, E.; Matta, Z.; Clark, M. Serving temperature viscosity measurements of nectar-and honey-thick liquids. Dysphagia 2008, 23, 65–75. [Google Scholar] [CrossRef]
- Mann, L.L.; Wong, K. Development of an objective method for assessing viscosity of formulated foods and beverages for the dysphagic diet. J. Am. Diet. Assoc. 1996, 96, 585–588. [Google Scholar] [CrossRef]
- Engelen, L.; De Wijk, R.A.; Prinz, J.F.; Van Der Bilt, A.; Janssen, A.M.; Bosman, F. The effect of oral temperature on the temperature perception of liquids and semisolids in the mouth. Eur. J. Oral Sci. 2002, 110, 412–416. [Google Scholar] [CrossRef]
- Marcotte, M.; Hoshahili, A.R.T.; Ramaswamy, H. Rheological properties of selected hydrocolloids as a function of concentration and temperature. Food Res. Int. 2001, 34, 695–703. [Google Scholar] [CrossRef]
- Cichero, J.A.; Jackson, O.; Halley, P.J.; Murdoch, B.E. How thick is thick? Multicenter study of the rheological and material property characteristics of mealtime fluids and videofluoroscopy fluids. Dysphagia 2000, 15, 188–200. [Google Scholar] [CrossRef]
- Germain, I.; Dufresne, T.; Ramaswamy, H.S. Rheological characterization of thickened beverages used in the treatment of dysphagia. J. Food Eng. 2006, 73, 64–74. [Google Scholar] [CrossRef]
- Benchabane, A.; Bekkour, K. Rheological properties of carboxymethyl cellulose (CMC) solutions. Colloid Polym. Sci. 2008, 286, 1173. [Google Scholar] [CrossRef]
- Momosaki, R.; Abo, M.; Kobayashi, K. Swallowing analysis for semisolid food texture in poststroke dysphagic patients. J. Stroke Cerebrovasc. Dis. 2013, 22, 267–270. [Google Scholar] [CrossRef] [PubMed]


| Product Name | Manufacturer | Flavour | Composition | pH | Density (kg/m3) |
|---|---|---|---|---|---|
| Gloup Forte | Rushwood, Raamsdonksveer, The Netherlands | vanilla | Water, dried glucose syrup, sucrose, carrageenan, maltodextrin, potassium sorbate, citric acid, aroma | 5.16 | 1.1 |
| Gloup Low Sugar | Rushwood, Raamsdonksveer, The Netherlands | raspberry | Water, xylitol, carrageenan, maltodextrin, potassium sorbate, citric acid, colour, aroma | 5.36 | 1.04 |
| Gloup Original | Rushwood, Raamsdonksveer, The Netherlands | orange | Water, carrageenan, maltodextrin, potassium sorbate, sucrose, calcium chloride, citric acid, colour, aroma | 5.11 | 1.03 |
| Gloup Original | Rushwood, Raamsdonksveer, The Netherlands | strawberry/banana | Water, carrageenan, maltodextrin, potassium sorbate, sucrose, calcium chloride, citric acid, colour, aroma | 5.19 | 1.05 |
| Gloup Sugar Free | Rushwood, Raamsdonksveer, The Netherlands | cherry | Water, carrageenan, maltodextrin, potassium sorbate, aspartame, calcium chloride, citric acid, (natural) colour, (natural) aroma | 5.19 | 1.02 |
| Heyaxon | Jian An Pharmaceutical, Shenzhen, China | peach | Water, erythritol, xylitol, agar, citric acid, xanthan gum, sodium citrate, locust bean gum, pigment, peach perfumes, sucralose, crocine, glycerol fonostearate | 3.77 | 1.03 |
| Magic Jelly (adult) | Ryukakusan, Tokyo, Japan | lemon | Erythritol, hydrogenated maltose starch syrup, agar, gelling agents (polysaccharide thickeners, calcium acetate, sweetener (Stevia) | 3.65 | 1.04 |
| Magic Jelly (dysphagia) | Ryukakusan, Tokyo, Japan | lemon | Erythritol, hydrogenated maltose starch syrup, agar, gelling agents (polysaccharide thickeners, calcium acetate, sweetener (Stevia) | 3.64 | 1.05 |
| MediSpend | Fagron, Rotterdam, The Netherlands | lemon | Purified water, modified food starch, natural lemon flavour, sodium citrate, citric acid, sucralose, sodium benzoate | 4.2 | 1.01 |
| Severo | IMS Medical, Grootebroek, The Netherlands | anise | purified water, cellulose gum, flavour (anise), citric acid, potassium sorbate, aspartame, acesulfame K | 4.41 | 1 |
| Slo Tablets | Slo Drinks, Glossop, UK | cherry | Purified water, modified food starch, cherry flavour, sodium citrate, citric acid, sucralose, sodium benzoate, malic acid, simethicone | 4.28 | 1.01 |
| Swallow Aid | National Consumer Products Inc, USA | cherry | Malitol, Glycerine, Carboxymethyl cellulose, Acesulfame Potassium | - | - |
| Lubricants | Flow Test Validity | Flow Test # | Fork Drip Test * | Spoon Tilt Test ^ | Final IDDSI Classification | ||||
|---|---|---|---|---|---|---|---|---|---|
| Texture | Interpretation | Volume Remaining (mL) | Interpretation (IDDSI Classification) | Flow/Drip/No Drip | Interpretation (IDDSI Classification) | Pass/Fail | Interpretation (IDDSI Classification) | ||
| Heyaxon | Lumpy | Not valid | - | - | - | - | - | - | 7 |
| Magic Jelly dysphagia | Lumpy | Not valid | - | - | - | - | - | - | 7 |
| Magic Jelly adult | Lumpy | Not valid | - | - | - | - | - | - | 7 |
| Slo Tablets | Smooth | Valid | 8.7 | 3 | Flow | <3 | - | - | <3 |
| MediSpend | Smooth | Valid | 9.1 | 3 | Flow | <3 | - | - | <3 |
| Severo | Smooth | Valid | 7.5 | 2 | - | - | - | - | 2 |
| Gloup Original straw/ban | Smooth | Valid | 9.9 | 3 | Drip | 3 | - | - | 3 |
| Gloup Sugar Free | Smooth | Valid | 9.9 | 3 | Drip | 3 | - | - | 3 |
| Gloup Low Sugar | Smooth | Valid | 9.9 | 3 | Drip | 3 | - | - | 3 |
| Gloup Original orange | Smooth | Valid | 9.9 | 3 | Drip | 3 | - | - | 3 |
| Gloup Forte | Smooth | Valid | No flow | 4 | No drip | 4 | Pass | 4 | 4 |
| Swallow Aid | Smooth | Valid | No flow | 4 | No drip | 4 | Fail | 7 | 7 |
| Lubricants | Flow Test Validity | Flow Test # | Fork Drip Test * | Spoon Tilt Test ^ | Final IDDSI Classification | ||||
|---|---|---|---|---|---|---|---|---|---|
| Texture | Interpretation | Volume Remaining (mL) | Interpretation (IDDSI Classification) | Flow/Drip/No Drip | Interpretation (IDDSI Classification) | Pass/Fail | Interpretation (IDDSI Classification) | ||
| Heyaxon | Lumpy | Not valid | - | - | - | - | - | - | 7 |
| Magic Jelly dysphagia | Lumpy | Not valid | - | - | - | - | - | - | 7 |
| Magic Jelly adult | Lumpy | Not valid | - | - | - | - | - | - | 7 |
| Slo Tablets | Smooth | Valid | 9.5 | 3 | Flow | <3 | - | - | <3 |
| MediSpend | Smooth | Valid | 8.9 | 3 | Flow | <3 | - | - | <3 |
| Severo | Smooth | Valid | 7.8 | 2 | - | - | - | - | 2 |
| Gloup Original straw/ban | Smooth | Valid | 9.9 | 3 | No drip | 4 | Pass | 4 | 4 |
| Gloup Sugar Free | Smooth | Valid | No flow | 4 | No drip | 4 | Pass | 4 | 4 |
| Gloup Low Sugar | Smooth | Valid | No flow | 4 | No drip | 4 | Pass | 4 | 4 |
| Gloup Original orange | Smooth | Valid | No flow | 4 | No drip | 4 | Pass | 4 | 4 |
| Gloup Forte | Smooth | Valid | No flow | 4 | No drip | 4 | Pass | 4 | 4 |
| Swallow Aid | Smooth | Valid | No flow | 4 | No drip | 4 | Fail | 7 | 7 |
| Lubricants | Flow Test Validity | Flow Test # | Fork Drip Test * | Spoon Tilt Test ^ | Final IDDSI Classification | ||||
|---|---|---|---|---|---|---|---|---|---|
| Texture | Interpretation | Volume Remaining (mL) | Interpretation (IDDSI classification) | Flow/Drip/No Drip | Interpretation(IDDSI Classification) | Pass/Fail | Interpretation (IDDSI Classification) | ||
| Room temperature | |||||||||
| Gloup Original straw/ban | Smooth | Valid | 9.9 | 3 | No drip | 4 | Pass | 4 | 4 |
| Gloup Sugar Free cherry | Smooth | Valid | 9.9 | 3 | No drip | 4 | Pass | 4 | 4 |
| Gloup Low Sugar raspberry | Smooth | Valid | 9.9 | 3 | No drip | 4 | Pass | 4 | 4 |
| Gloup Original orange | Smooth | Valid | No flow | 4 | No drip | 4 | Pass | 4 | 4 |
| Gloup Forte | Smooth | Valid | No flow | 4 | No drip | 4 | Pass | 4 | 4 |
| Fridge temperature | |||||||||
| Gloup Original straw/ban | Smooth | Valid | No flow | 4 | No drip | 4 | Pass | 4 | 4 |
| Gloup Sugar Free cherry | Smooth | Valid | No flow | 4 | No drip | 4 | Pass | 4 | 4 |
| Gloup Low Sugar raspberry | Smooth | Valid | No flow | 4 | No drip | 4 | Pass | 4 | 4 |
| Gloup Original orange | Smooth | Valid | No flow | 4 | No drip | 4 | Pass | 4 | 4 |
| Gloup Forte | Smooth | Valid | No flow | 4 | No drip | 4 | Pass | 4 | 4 |
| Lubricants | Hardness (g) | Adhesiveness (mJ) | Cohesiveness | Gumminess (g) | Springiness (mm) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Room T | Fridge T | Room T | Fridge T | Room T | Fridge T | Room T | Fridge T | Room T | Fridge T | |
| Heyaxon | 4.38 ± 0.56 a | 3.67 ± 0.14 ab | 0.13 ± 0.00 a | 0.10 ± 0.03 a | 0.85 ± 0.01 ab | 0.88 ± 0.02 a | 3.7 ± 0.45 a | 3.20 ± 0.17 ab | 19.74 ± 0.05 a | 19.70 ± 0.02 a |
| Magic Jelly dysphagia | 4.17 ± 0.54 a | 5.17 ± 0.14 ac | 0.09 ± 0.01 a | 0.09 ± 0.03 a | 0.74 ± 0.02 ab | 0.84 ± 0.11 a | 3.1 ± 0.49 a | 4.30 ± 0.45 ac | 19.68 ± 0.05 a | 19.27 ± 0.34 a |
| Magic Jelly adult | 4.17 ± 0.36 a | 3.83 ± 0.49 ab | 0.07 ± 0.20 a | 0.24 ± 0.15 a | 0.94 ± 0.07 ab | 0.84 ± 0.04 a | 4.03 ± 0.24 a | 3.23 ± 0.28 ab | 19.16 ± 0.30 a | 19.64 ± 0.02 a |
| Slo Tablets | 3.67 ± 0.14 a | 3.17 ± 0.14 ab | 0.06 ± 0.01 a | 0.05 ± 0.01 a | 0.98 ± 0.02 a | 0.79 ± 0.05 a | 3.60 ± 0.08 a | 2.50 ± 0.24 ab | 19.02 ± 0.39 a | 14.88 ± 0.47 ab |
| MediSpend | 3.33 ± 0.36 a | 3.50 ± 0.00 ab | 0.07 ± 0.04 a | 0.07 ± 0.01 a | 0.85 ± 0.01 ab | 0.80 ± 0.04 a | 3.17 ± 0.14 a | 2.80 ± 0.12 ab | 19.37 ± 0.22 a | 19.08 ± 0.36 ab |
| Severo | 3.33 ± 0.14 a | 2.00 ± 0.24 b | 0.08 ± 0.02 a | 0.05 ± 0.01 a | 0.59 ± 0.13 b | 0.59 ± 0.09 a | 2.00 ± 0.50 a | 1.23 ± 0.33 a | 19.65 ± 0.04 a | 17.00 ± 0.43 ab |
| Gloup Original straw/ban | 4.33 ± 0.27 a | 5.50 ± 0.24 ac | 0.08 ± 0.02 a | 0.12 ± 0.01 a | 0.93 ± 0.03 ab | 0.80 ± 0.02 a | 4.03 ± 0.40 a | 4.4 ± 0.21 ac | 19.70 ± 0.06 a | 19.60 ± 0.19 a |
| Gloup Sugar Free | 4.67 ± 0.49 a | 6.50 ± 0.24 c | 0.09 ± 0.00 a | 0.13 ± 0.03 a | 0.83 ± 0.01 ab | 0.79 ± 0.02 a | 4.33 ± 0.10 a | 5.13 ± 0.23 bc | 16.90 ± 1.68 a | 18.32 ± 1.14 ab |
| Gloup Low Sugar | 4.83 ± 0.68 a | 6.83 ± 0.59 c | 0.12 ± 0.04 a | 0.15 ± 0.00 a | 0.75 ± 0.09 ab | 0.54 ± 0.05 a | 3.67 ± 0.73 a | 3.60 ± 0.66 ab | 16.79 ± 2.29 a | 12.55 ± 2.36 b |
| Gloup Original orange | 5.67 ± 0.27 a | 7.67 ± 0.14 c | 0.14 ± 0.02 a | 0.15 ± 0.01 a | 0.80 ± 0.04 ab | 0.60 ± 0.03 a | 4.60 ± 0.45 a | 4.63 ± 0.20 ac | 19.14 ± 0.29 a | 16.56 ± 1.10 ab |
| Gloup Forte | 9.17 ± 0.14 b | 11.00 ± 0.41 e | 0.33 ± 0.01 a | 0.36 ± 0.06 a | 0.83 ± 0.01 ab | 0.65 ± 0.05 a | 7.60 ± 0.19 b | 7.17 ± 0.43 c | 19.62 ± 0.06 a | 15.64 ± 1.67 ab |
| Swallow Aid | 16.50 ± 0.62 c | 18.80 ± 0.83 f | 1.95 ± 0.21 b | 1.50 ± 0.05 b | 0.99 ± 0.01 a | 1.62 ±0.15 b | 16.4 ± 0.41 c | 30.40 ± 1.37 d | 22.39 ± 2.02 a | 14.10 ± 0.58 ab |
| Lubricants | Back Extrusion | Forward Extrusion | ||||
|---|---|---|---|---|---|---|
| Hardness (g) | Consistency (mJ) | Adhesive Force (g) | Adhesiveness (mJ) | Hardness (g) | Consistency (mJ) | |
| Heyaxon | 62.17 ± 0.82 ab | 7.42 ± 0.27 ac | 28.50 ± 2.05 a | 4.85 ± 0.35 ab | 1150.8 ± 75.0 a | 68.2 ± 3.92 ab |
| Magic Jelly dysphagia | 63.33 ± 9.61 ab | 7.67 ± 1.11 abe | 26.50 ± 4.14 ab | 4.47 ± 0.44 ac | 1012.5 ± 40.9 ab | 145.1 ± 7.88 c |
| Magic Jelly adult | 51.67 ± 0.31 ac | 6.33 ± 0.07 cde | 19.67 ± 0.76 ab | 3.39 ± 0.01 bc | 936.0 ± 31.5 abc | 133.5 ± 6.18 c |
| Slo Tablets | 32.50 ± 0.20 c | 4.31 ± 0.01 d | 16.83 ± 0.14 b | 2.87 ± 0.02 c | 131.3 ± 1.7 d | 17.7 ± 0.22 e |
| Medispend | 36.67 ± 0.12 c | 5.00 ± 0.02 cd | 18.50 ± 0.24 ab | 3.32 ± 0.06 bc | 315.5 ± 31.1 ed | 22.7 ± 2.84 de |
| Severo | 40.17 ± 0.85 ac | 5.46 ± 0.08 cde | 22.83 ± 0.27 ab | 3.57 ± 0.09 bc | 405.2 ± 46.9 def | 42.8 ± 0.91 ae |
| Gloup Original straw/ban | 80.67 ± 1.55 b | 10.15 ± 0.15 b | 43.67 ± 0.54 c | 6.04 ± 0.10 a | 681.8 ± 11.9 bf | 86.1 ± 0.2 b |
| Gloup Sugar Free | 78.33 ± 1.83 b | 9.82 ± 0.12 ab | 41.50 ± 0.62 c | 5.71 ± 0.13 a | 472.5 ± 76.6 def | 50.4 ± 2.71 acf |
| Gloup Low Sugar | 80.83 ± 1.33 b | 9.87 ± 0.20 ab | 40.50 ± 1.65 c | 5.51 ± 0.09 a | 642.6 ± 70.0 cef | 68.4 ± 3.31 ab |
| Gloup Original orange | 106.50 ± 1.95 d | 13.57 ± 0.38 f | 59.67 ± 1.77 d | 7.84 ± 0.28 d | 665.3 ± 32.5 be | 77.7 ± 7.34 bf |
| Gloup Forte | 219.50 ± 1.89 e | 28.11 ± 0.40 g | 77.00 ± 0.85 e | 21.56 ± 0.56 e | 1258.5 ± 25.8 a | 164.8 ± 2.34 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malouh, M.A.; Cichero, J.A.Y.; Manrique, Y.J.; Crino, L.; Lau, E.T.L.; Nissen, L.M.; Steadman, K.J. Are Medication Swallowing Lubricants Suitable for Use in Dysphagia? Consistency, Viscosity, Texture, and Application of the International Dysphagia Diet Standardization Initiative (IDDSI) Framework. Pharmaceutics 2020, 12, 924. https://doi.org/10.3390/pharmaceutics12100924
Malouh MA, Cichero JAY, Manrique YJ, Crino L, Lau ETL, Nissen LM, Steadman KJ. Are Medication Swallowing Lubricants Suitable for Use in Dysphagia? Consistency, Viscosity, Texture, and Application of the International Dysphagia Diet Standardization Initiative (IDDSI) Framework. Pharmaceutics. 2020; 12(10):924. https://doi.org/10.3390/pharmaceutics12100924
Chicago/Turabian StyleMalouh, Marwa A., Julie A.Y. Cichero, Yady J. Manrique, Lucia Crino, Esther T. L. Lau, Lisa M. Nissen, and Kathryn J. Steadman. 2020. "Are Medication Swallowing Lubricants Suitable for Use in Dysphagia? Consistency, Viscosity, Texture, and Application of the International Dysphagia Diet Standardization Initiative (IDDSI) Framework" Pharmaceutics 12, no. 10: 924. https://doi.org/10.3390/pharmaceutics12100924