INTRODUCTION
Primary squamous cell carcinoma (SCC) of the liver is rare and reported sporadically. Only 24 such cases have been reported in the literature. It is found to be associated with hepatic teratoma[1], congenital cysts[2-14], solitary benign non-parasitic hepatic cysts (SBNHC)[2-6,8-11], hepatolithiasis/Caroli's disease[15,16] or cirrhosis[17]. The carcinogenesis seems to be related to the chronic inflammation of the biliary epithelium or the metaplastic and subsequent neoplastic transformation of cysts of the liver[15]. Although various therapeutic strategies, including systemic chemotherapy, radiotherapy, transarterial chemoembolization and hepatic resection, have been used, the prognosis of primary SCC of the liver is dismal with an overall survival less than one year.
We report a case of primary SCC of the liver occurring in association with hepatolithiasis. The patient underwent hepatectomy in January 2010 followed by radiotherapy, and has survived for over 19 mo without recurrence.
CASE REPORT
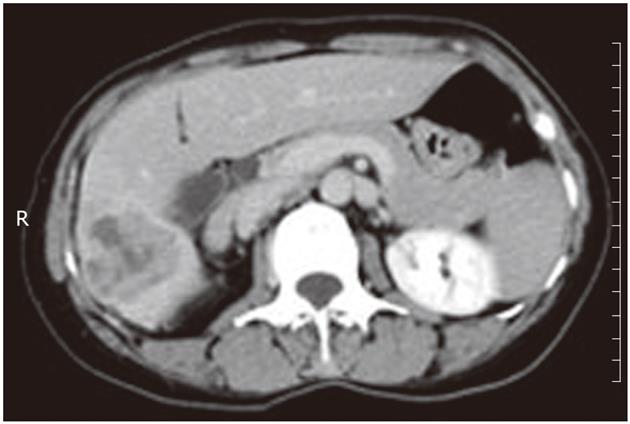
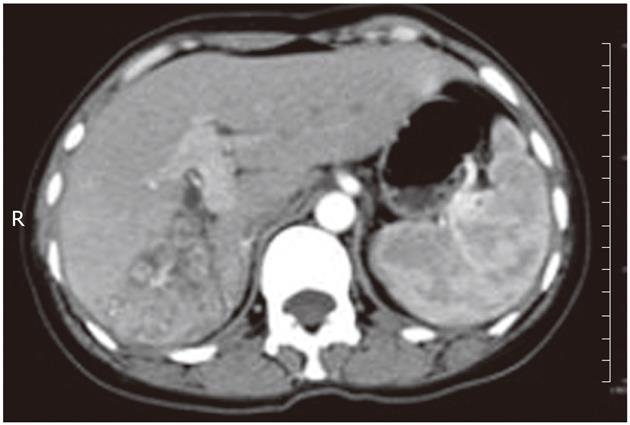
A 46-year-old woman presented with right upper quadrant pain and a 15-pound weight loss over the past five weeks before admission in January 2010. There was no radiating pain, vomiting, jaundice, fever, chill, cough or irregular vaginal bleeding. She had a history of hepatitis A, but without a history of alcohol drinking, smoking or surgery. On physical examination, the abdomen was soft, and no tenderness, rebound tenderness and palpable abdominal mass were found. The biochemical data were as follows: hemoglobin 110 g/L, white cell count 5.2 × 109/L with neutrophil 82.5%, platelet 334 × 109/L, alanine aminotransferase 28 μ/L, serum total protein 70 g/L, serum albumin 34.1 g/L, serum total bilirubin 15 μmol/L, serum direct bilirubin 9.5 μmol/L, alpha-fetal protein 7.33 ng/mL, and carcinoembryonic antigen (CEA) 8.5 ng/L, and serologic markers for the hepatitis-B virus were not detected. The plasma retention rate of indocyanine green at 15 min was 1.2%. Subsequent abdominal ultrasonography showed a mixed echoic mass measuring about 5.0 cm × 6.0 cm occupying the right lobe of the liver. The abdominal computed tomography (CT) showed a regular mass measuring about 5 cm × 6 cm at its greatest dimension with inhomogeneous density, mild delayed enhancement in the peripheral zone, necrosis in the central zone of the tumor and dilated secondary biliary ducts with intraductal lithiasis (Figures 1 and 2). Gastrointestinal endoscopy and colonoscopy showed negative findings. Chest CT revealed no mass over the lung.
Figure 1 Computed tomography scan showed a regular mass with inhomogeneous density, mild delayed enhancement in the peripheral zone, and necrosis in the central zone.
R: Right.
Figure 2 The bile ducts adjacent to the tumor were obviously dilated and full of biliary calculi.
R: Right.
The initial diagnosis before operation was intrahepatic cholangiocarcinoma (ICC) according to the CT findings (a regular mass, periductal dilatation and intraductal lithiasis). Surgery remains the exclusive choice of curative therapy for ICC. The patients underwent right hepatectomy and cholecystectomy in February 2011. A hard and yellowish white tumor mass was found, measuring about 5 cm × 6 cm at the greatest dimension with central necrosis occupying mainly S5 of a non-cirrhotic liver and numerous biliary calculi in the dilated intrahepatic bile duct adjacent to the tumor. The specimen appeared with a 1-cm negative margin without cysts on its cut surface.
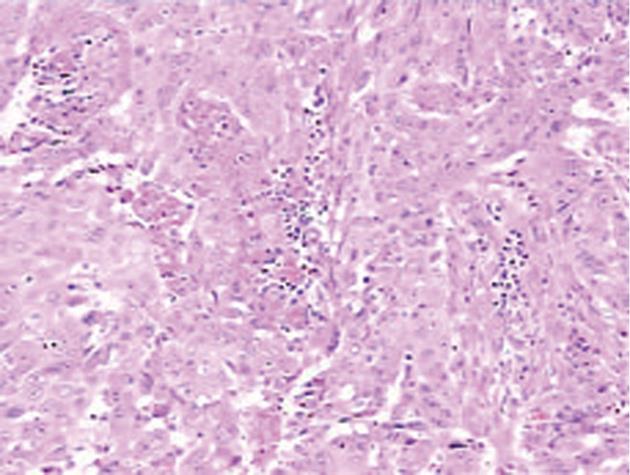
Histopathological examination showed a poorly-moderately differentiated SCC composed of squamous cells with keratinization and large areas of necrosis (Figure 3). The secondary bile ducts adjacent to the tumor were obviously dilated and full of biliary calculi. Chronic inflammatory lesions were found in the biliary epithelial and gallbladder mucosa without evidence of squamous metaplasia. Immunohistochemical tests were positive for cytokeratin (CK) 14, CK19 and CEA, but negative for CK 18 and thyroid transcription factor 1 (TTF-1). CK 14 positive indicated that basal cells of keratinized squamous epithelium were originated from the cancer cells. Metastatic lung or thyroid cancer to the liver could be ruled out, as TTF-1, an indicator of small cell carcinoma of the lung or thyroid, was negative. Subsequently a thorough search for a primary tumor showed a negative finding in the oral cavity and nasopharynx and a mild inflammatory change in cervical smear. Taken together, the final diagnosis of primary SCC of the liver was made.
Figure 3 Histology of the tumor: Squamous cells with keratinization and areas of necrosis.
Hematoxylin-eosin stain, × 10.
The post-operative course was uneventful and the patient was discharged on the 14th day after operation. The patient was started on radiotherapy 2 mo after operation. Routine follow-up was continued in the hepatobiliary clinic. No tumor recurrence and distant metastasis were found during the 19-mo follow-up period.
DISCUSSION
Primary SCC of the liver is very rare, and only 25 cases have been reported from 1934 till now (including the present one) in the literature. Among 13 (52%) of these cases, SCC arose from congenital hepatic cysts[2-14] or SBNHC[2-6,8-11]. There were only two cases of SCC associated with hepatolithiasis/Caroli’s disease[15,16]. The other cases were associated with cirrhosis and teratoma[1,17]. The precise steps leading to the carcinomas are unclear. Positive CK14 and negative CK18 indicate that basal cells of keratinized squamous epithelium are originated from the cancer. Positive CK19 indicates that the tumors are transformed from chronic inflammation of the biliary epithelium. Thus, chronic inflammation of the biliary epithelium might be the most reasonable mechanism of carcinogenesis.
Clinically, patient presents with the abdominal pain and weight loss as initial symptoms. CT scan of the abdomen is helpful for evaluating the primary SCC, such as the number of lesions, location, extension, and whether the tumor invades surrounding structures. If the mass is suspected to be malignant, biopsy or even surgical resection should be conducted if patient's condition permits.
Although several management strategies have been proposed, ranging from hepatectomy, systemic chemotherapy and radiotherapy to hepatic arterial injection of low-dose anti-cancer drugs, the prognosis of primary SCC of the liver is dismal with an overall survival less than one year. Naik et al[18] described a patient with SCC who survived for over 6 year after preoperative radiotherapy and surgical resection. Boscolo et al[19] reported a case of complete remission of poorly differentiated SCC after systemic chemotherapy (cisplatin + 5-fluorouracil) and surgery. Spaggiari et al[16] reported a case with a 7-year survival after right hepatectomy. In our case, SCC occurred in association with hepatolithiasis. The patient was successfully treated by hepatectomy followed by radiotheraphy. The postoperative course was smooth, and she is still alive 19 mo after the operation without recurrence.
In conclusion, we described the first case of primary SCC of the liver associated with hepatolithiasis. The patient was successfully treated by surgery followed by radiotherapy. She has survived for 19 mo with no tumor recurrence and distant metastasis.