Published online May 7, 2019. doi: 10.3748/wjg.v25.i17.2110
- This article has been retracted.
- Retraction in: World J Gastroenterol. Jan 7, 2023; 29(1): 221-222 See also: Errata, Retraction, Duplicate Publication and Comment Policy
Peer-review started: January 11, 2019
First decision: January 30, 2019
Revised: February 15, 2019
Accepted: February 22, 2019
Article in press: February 23, 2019
Published online: May 7, 2019
Reflux esophagitis (RE) is a common digestive disorder, and its frequent recurrences cause significant physical pain and are financially burdensome to patients. However, studies on the natural history of treated RE are few. Although proton pump inhibitors (PPIs) as the first-line treatment provide notable symptomatic relief, disordered gut microbiota has been observed among PPI users. Probiotics are commonly administered to patients to regulate the disordered intestinal flora.
To evaluate the therapeutic effects in RE patients treated with a combination of esomeprazole and probiotics [Bacillus subtilis (B. subtilis) and Enterococcus faecium (E. faecium)].
One hundred and thirty-four RE patients were randomized into two groups of 67 subjects each. The probiotics group was administered with esomeprazole 20 mg b.i.d. and live combined B. subtilis and E. faecium enteric-coated capsules 500 mg t.i.d. for eight weeks; the placebo group was administered with esomeprazole 20 mg b.i.d. and placebo for eight weeks. Subsequently, 12-wk follow-up was carried out on patients who achieved both endoscopic and clinical cure. Endoscopy, reflux diagnostic questionnaire (RDQ), gastrointestinal symptom rating scale (GSRS), and lactulose hydrogen breath test were performed to evaluate the therapeutic effects. A difference of P < 0.05 was considered statistically significant.
Sixty-six patients in the probiotics group and 64 patients in the placebo group completed the 8-wk treatment. The healing rate and RDQ score had no significant difference between the two groups (P > 0.05). However, the GSRS diarrhea syndrome score was decreased significantly in the probiotics group (P = 0.002), and the small intestinal bacterial overgrowth negative rate in the probiotics group was significantly higher than that in the placebo group (P = 0.002). Of 114 endoscopically and clinically cured patients, 96 completed the follow-up. The log-rank test showed that the time to relapse was shorter in the placebo group than in the probiotics group (P = 0.041). Furthermore, the therapy had a significant influence on relapse time, and the risk of relapse in the probiotics group was lower than that in the placebo group at any time point during the 12-wk follow-up (hazard ratio = 0.52, P = 0.033).
Esomeprazole combined with probiotics (B. subtilis and E. faecium) have a beneficial effect on RE treatment and patient management.
Core tip: Reflux esophagitis (RE) recurrences cause significant physical pain and financial burden to patients. Proton pump inhibitors (PPIs) are the first-line treatment for RE. Although PPIs provide notable symptomatic relief, their effects on the gut microbiota have drawn attention. In the present study, we evaluated the effectiveness of combining esomeprazole with probiotics [Bacillus subtilis (B. subtilis) and Enterococcus faecium (E. faecium)]. We found that the combined administration could reduce the incidence of small intestinal bacterial overgrowth and improve abdominal symptoms in patients with RE. It may also prolong the time to relapse, showing the potential of probiotics (B. subtilis and E. faecium) for the treatment and management of RE.
- Citation: Sun QH, Wang HY, Sun SD, Zhang X, Zhang H. Beneficial effect of probiotics supplements in reflux esophagitis treated with esomeprazole: A randomized controlled trial. World J Gastroenterol 2019; 25(17): 2110-2121
- URL: https://www.wjgnet.com/1007-9327/full/v25/i17/2110.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i17.2110
Reflux esophagitis (RE) is a common digestive disorder that occurs when gastric/duodenal contents flow pathologically into the esophagus, leading to inflammation, erosion, and ulceration of the esophageal mucosa. Frequent relapses are common with RE, resulting in significant physical pain and financial burden on patients. Studies on the treatment of RE are scarce[1,2]. The first-line treatment for RE is administration of proton pump inhibitors (PPIs)[3], which are the most commonly prescribed drugs worldwide. Some studies have reported complete responses in approximately 70%-80% of patients after eight weeks of PPI treatment[4].
Although PPIs provide notable symptomatic relief, their effects on the gut microbiota have gained recent attention. A large population-based cohort study showed a significant reduction in the abundance of gut flora and microbial diversity and an associated significant increase in the amount of oral and upper gastrointestinal (GI) tract bacteria among PPI users[5]. Profound changes have been observed in the gastric and intestinal microbiota of PPI users[6-9].
Small intestinal bacterial overgrowth (SIBO) refers to an elevated bacterial count that reflects changes in the composition and structure of the small intestine[5]. Many studies have reported an increased incidence of SIBO during PPI therapy[10]. SIBO presents with a variety of GI symptoms, such as diarrhea, abdominal distension, and constipation[11]. Many recent studies have shown that PPIs can cause symptoms of GI discomfort similar to those associated with SIBO[12-15].
Probiotics comprise microorganisms that enhance the integrity of the intestinal mucosal barrier and balance the microbial ecosystem. This is achieved via probiotic competition with harmful bacteria and the production of metabolites that inhibit the growth of the harmful bacteria. Probiotics are commonly administered to patients with intestinal flora abnormalities.
This clinical trial aimed to evaluate the effectiveness of combining esomeprazole with probiotics [live combined Bacillus subtilis (B. subtilis) and Enterococcus faecium (E. faecium)] for the treatment of patients with RE by comparing the outcomes after eight weeks of treatment in a treatment group and a placebo group.
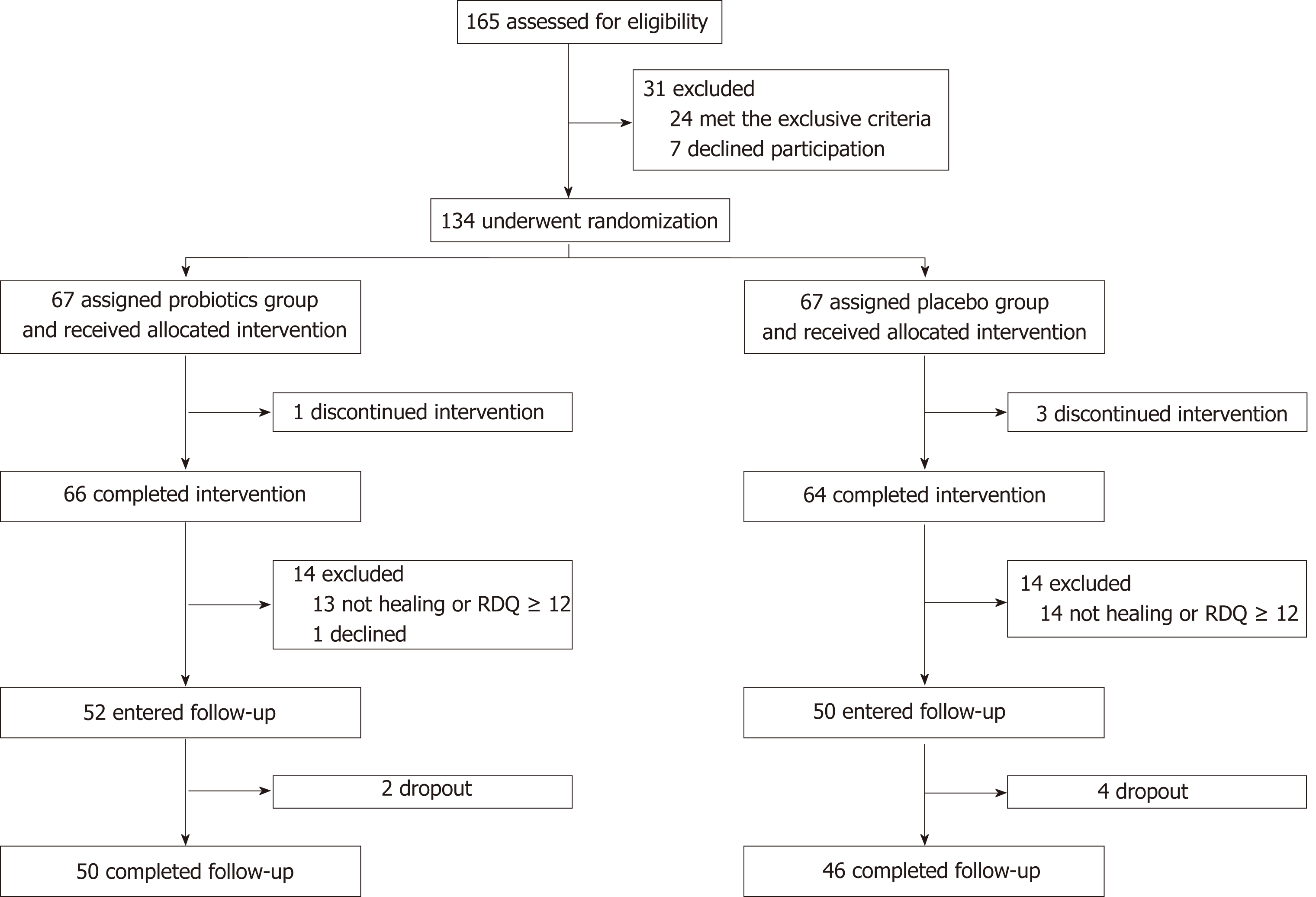
From June 2015 to December 2017, 134 RE outpatients or gastroenterology inpatients in the PKUCare Luzhong Hospital were recruited in this trial. RE was diagnosed based on the 2013 Guidelines for the Diagnosis and Management of Gastroesophageal Reflux Disease[4]. The inclusion criteria were: (1) patients who consented to undergo esomeprazole treatment, were not previously on PPI, or have stopped PPI treatment for at least 6 mo, and were aged 18-65 years; (2) patients who have not taken antibiotics, probiotics, lactulose, other antacids, or drugs that increase GI motility nor undergone an enema in the past 4 wk; (3) normal hepatic and renal function; and (4) SIBO negative on the lactulose hydrogen breath test (LHBT). The exclusion criteria were: (1) history of cirrhosis, renal impairment, tumors, thyroid disease, diabetes, Crohn’s disease, or ulcerative colitis; (2) comorbid hiatal hernia, peptic ulcer disease, esophageal stricture, diarrhea, malabsorption, and constipation due to liver, gallbladder, and pancreatic diseases; (3) history of GI or abdominal surgery; (4) pregnant or lactating women; (5) patients undergoing treatment with immune suppressants; and (6) patients who fulfilled the diagnosis of irritable bowel disease (IBS) according to the Rome III criteria, or patients who did not meet the diagnostic criteria but had persistent abdominal distension, diarrhea, or constipation for ≥ 3 mo. The enrollment flowchart is displayed in Figure 1.
All subjects signed an informed consent form. This study was reviewed and approved by the ethics committee of PKUCare Luzhong Hospital (2015-KY-003) and registered on the Chinese Clinical Trial Registry (No. ChiCTR1800018218).
Endoscopic findings were classified according to the Los Angeles Classification grading system (grade A: ≥1 mucosal break < 5 mm; grade B: ≥ 1 mucosal break > 5 mm; grade C: mucosal breaks extending between the tops of two mucosal folds, but < 75% of the circumference; grade D: mucosal breaks extending for > 75% of the circumference). Improvement in the endoscopic findings to grade N (normal) is defined as healing.
The EC60 Gastrolyzer 2 (United Kingdom) was used for the test. The subject first exhaled once to measure the baseline value before taking 200 mL of lukewarm water and 10 mL of lactulose (lactulose oral solution, Laiyang Jiangbo Pharmaceutical Co., Ltd., 10 mL/vial). After gargling, the patient exhaled once every 20 min for 3 h. A normal LHBT value was defined as baseline value < 20 ppm and a maximum peak value of < 20 ppm greater than the baseline value. A positive result was defined as classical double peak and (or) a fusion peak waveform.
The RDQ was used to assess the subjective reflux symptoms covering a 1-wk recall period. RDQ is categorized into four symptom clusters depicting heartburn, chest pain, acid reflux, and food reflux. The total RDQ scores (eight items) were calculated. Patients with RDQ ≥ 12 points were considered to have a relapse[16].
The GSRS is a disease-specific instrument, containing 15 items, each rated on a seven-point Likert scale from which one represents no discomfort and seven represents very severe discomfort[17]. The 15 GSRS items break down into the following five symptom clusters: abdominal pain (abdominal pain, hunger pain, and nausea); reflux syndrome (heartburn and acid regurgitation), diarrhea syndrome (diarrhea, loose stools, and urgent need for defecation), indigestion syndrome (borborygmus, abdominal distension, eructation, and increased flatus), and constipation syndrome (constipation, hard stools, and feeling of incomplete evacuation).
Phase 1: A random number table was used to divide the 134 RE patients into two groups of 67 subjects each. Esomeprazole is the first choice of PPI, having strong and lasting acid suppression effect. Medilac-s are live combined B. subtilis and E. faecium enteric-coated capsules. These two kinds of bacteria are regular members of the intestinal flora of healthy people. Taking this product can directly supplement normal physiological living bacteria, inhibit the excessive reproduction of harmful bacteria in the intestinal tract, and adjust the intestinal flora, which is applied widely in the clinic. The dosage of the medicine was determined by the published drug instructions[18]. The placebo was provided by the Pharmacy Department from the PKUCare Luzhong Hospital. The dosage form, appearance, size, and color of the placebo were completely identical with the drug. The drugs conform to China’s Good Manufacture Practice of Medical Products[19]. Patients in the placebo group took 20 mg of esomeprazole (Nexium, AstraZeneca PLC) orally twice a day and placebo (white starch capsules) thrice a day for eight weeks. Patients in the probiotics group took 20 mg of esomeprazole orally twice a day and 500 mg of live combined B. subtilis and E. faecium enteric-coated capsules (Hanmi Pharmaceutical Co., Ltd) thrice a day for 8 wk. The treatment was single blinded. Patients did not know their assigned groups. Observation for medication compliance (PPI and probiotic/placebo) was performed twice a week through phone, by asking the parents about compliance. Poor compliance was defined as missed doses for ≥ 3 d.
Phase 2: Patients who achieved endoscopic and clinical cure (RDQ < 12) during phase 1 entered the follow-up. The follow-up endpoint was defined as symptomatic recurrence (RDQ ≥ 12) or the end of the 12-wk follow-up (week 20).
Endoscopic evaluation was performed at baseline and repeated at the end of the treatment (week 8) to verify healing. GSRS was completed at baseline and week 8. RDQ and LHBT were completed at baseline before treatment, week 8, and the follow-up endpoint. The same physician performed an initial clinical evaluation and the following medical appointments. All subjects received telephone or outpatient follow-up once every two weeks. We assessed the therapeutic effect of treatments using the change in endoscopic evaluation and RDQ at the end of therapy and the end of follow-up (primary outcomes). Changes in GSRS and LHBT results were considered the secondary outcomes.
Adverse events were monitored throughout the study. Patients were not allowed to consume any other probiotics or prebiotics, and they were instructed to continue their usual eating and living habits. The use of antacids or motility-increasing drugs was stopped during the follow-up period unless the symptom relapsed. Concomitant use of medications was allowed, providing their registered medication intake.
All data were processed and analyzed with the R Studio (version 3.4.3, R Studio Inc., Boston, United States), and the packages ‘survival’ (version 2.42-6), ‘survminer’ (version 0.4.3), and ‘dplyr’ (version 0.7.7) were used to run and visualize statistical tests. Statistical significance was defined as P < 0.05. Quantitative data that conformed to a normal distribution are expressed as the mean ± standard deviation, and t-test was used for intergroup comparison. Chi-squared test was applied to frequency data for intergroup comparison. Kaplan-Meier analysis was utilized to analyze the cumulative relapse rate of RE. Cox regression analysis was conducted considering the prognostic variables of clinical characteristics at entry and initial treatment therapy to explore the effect of other factors on the relative risk of relapse.
The statistical power calculation was carried out to estimate the sample size for the superiority trial. According to our review of studies, relapse rates of patients with healed lesions have been reported to be 54% to 66.2% at 12 wk after drug therapy was withdrawn[2,20,21], so our estimation of the average relapse rate for the placebo group was 60%. Also, cured RE patients who received an additional maintenance treatment had a relapse rate of 10% at 12 wk and 28.4% to 30% at 32 wk after drug therapy was stopped[1,22]. Given that the therapeutic effect of probiotics supplements on RE recurrence had never been studied and the probiotics are not antacid, we took 30% as our estimation of the relapse rate for the probiotics group. Hence, we estimated that the average relapse rate was 30%. With a two-tailed test of α = 0.05 and 1 - β = 0.80, the calculation indicated that a sample size of 40 for each group would be sufficient. To power our trial to be able to detect the difference between groups maximumly, we included as many patients as possible within our study budget rather than just meeting the minimum sample size requirement of 40 patients[23].
Clinical features at baseline: One and three patients discontinued the intervention in the probiotics and placebo groups, respectively. Finally, 130 patients completed the study, of which 66 and 64 patients were in the probiotics and placebo groups, respectively (Figure 1). Baseline characteristics and questionnaire scores are shown in Table 1. There were no statistically significant differences in age, sex, body mass index, smoking history, waist circumference, esophagitis grade, and GSRS and RDQ scores between the two groups at baseline (P > 0.05 for all). The general status of patients in both groups was balanced, and the experiment results were comparable.
| Characteristic | Probiotics group (n = 66) | Placebo group (n = 64) | P-value | |
| Age (yr) | 41.76 ± 9.38 | 41.89 ± 9.75 | 0.937 | |
| Male n (%) | 39 (59.1) | 40 (62.5) | 0.691 | |
| BMI (kg/m2) | 24.61 ± 3.51 | 23.90 ± 3.14 | 0.230 | |
| Smoking n (%) | 12 (18.2) | 10 (15.6) | 0.698 | |
| Waist circumference (cm) | 78.68 ± 5.03 | 78.84 ± 6.49 | 0.874 | |
| RDQ score | 19.41 ± 4.23 | 18.44 ± 5.17 | 0.244 | |
| GSRS score | Abdominal pain | 6.38 ± 2.64 | 6.48 ± 3.20 | 0.846 |
| Reflux | 10.35 ± 2.48 | 10.31 ± 2.68 | 0.937 | |
| Diarrhea | 6.44 ± 1.97 | 6.89 ± 2.39 | 0.242 | |
| Indigestion | 7.53 ± 2.67 | 7.03 ± 2.17 | 0.245 | |
| Constipation | 5.48 ± 1.28 | 5.34 ± 2.13 | 0.647 | |
| Esophagitis grade at baseline (n) | A | 26 | 29 | 0.495 |
| B | 22 | 21 | 0.950 | |
| C | 13 | 11 | 0.712 | |
| D | 5 | 3 | 0.493 | |
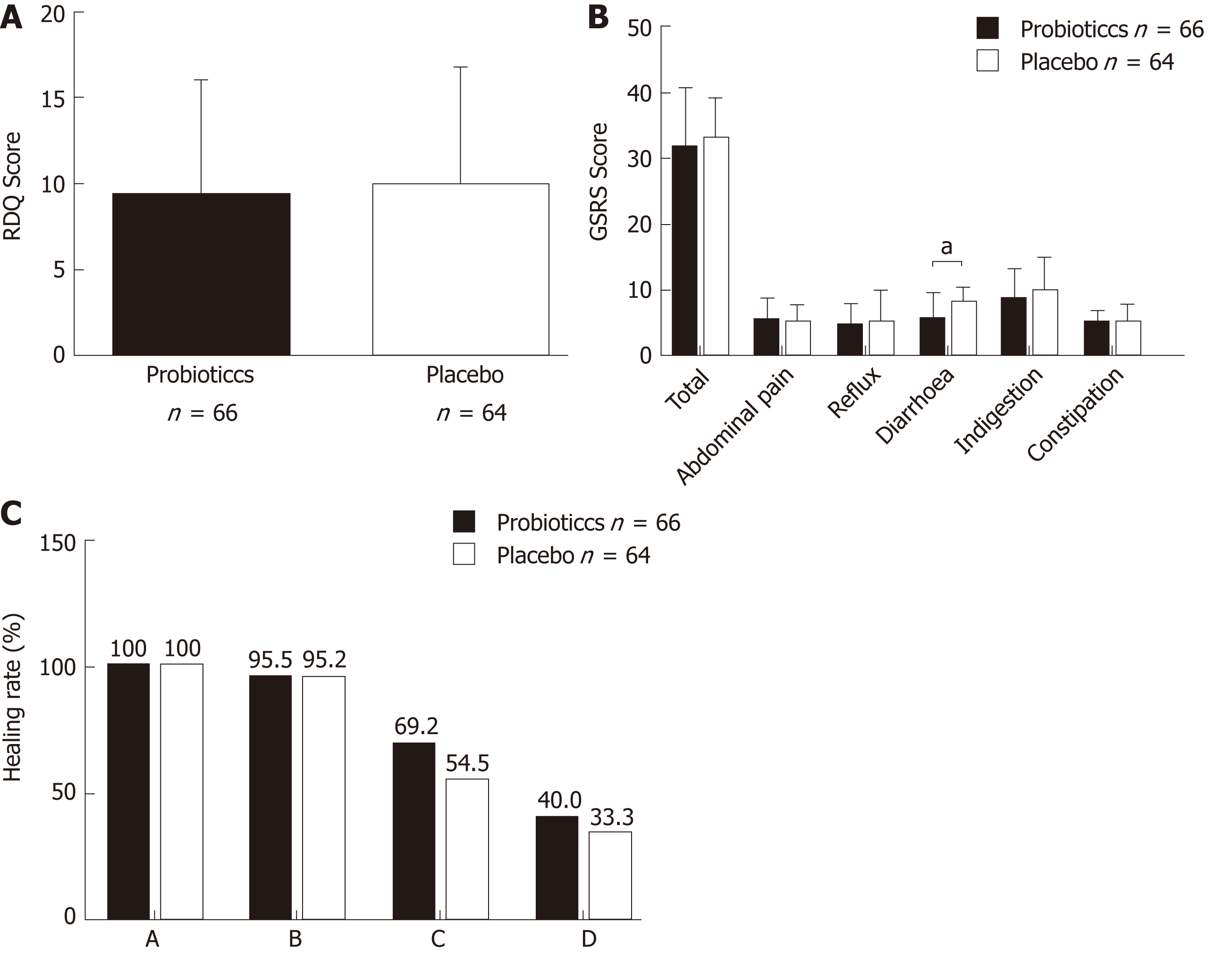
Intervention: Figure 2 shows the RDQ scores, GSRS scores, and endoscopic healing rates in the probiotics and placebo groups after eight weeks of treatment. In the probiotics group, total RDQ score was 9.29 ± 6.65, total GSRS score was 31.59 ± 8.95, GSRS abdominal pain score was 5.45 ± 3.39, GSRS reflux syndrome score was 4.71 ± 3.20, GSRS diarrhea syndrome score was 6.20 ± 3.88, GSRS indigestion syndrome score was 8.58 ± 4.57, and GSRS constipation syndrome score was 5.05 ± 1.83. In the placebo group, they were 9.86 ± 6.84, 32.94 ± 6.04, 5.11 ± 2.57, 5.16 ± 2.72, 7.94 ± 2.36, 9.82 ± 5.04, and 5.02 ± 2.72, respectively. There was no significant difference between the two groups in RDQ score (P = 0.631), total GSRS score (P = 0.317), GSRS abdominal pain score (P = 0.521), GSRS reflux syndrome score (P = 0.390), GSRS indigestion syndrome score (P = 0.144), and GSRS constipation syndrome score (P = 0.941). However, the GSRS diarrhea syndrome score was decreased significantly in the probiotics group (P = 0.002).
Endoscopic examinations were performed after 8-wk treatment. The endoscopic healing rates in the probiotics group at week 8 were 100% (26/26), 95.5% (21/22), 69.2% (9/13), and 40.0% (2/5) in patients with grades A, B, C, and D, respectively; in the placebo group, the healing rates were 100% (29/29), 95.2% (20/21), 54.5% (6/11), and 33.3% (1/3) in patients with grades A, B, C, and D, respectively. There was no significant difference in the healing rate between the probiotics and placebo groups in all grades (grade A: P > 0.05, grade B: P = 0.974; grade C: P = 0.495; grade D: P = 0.849).
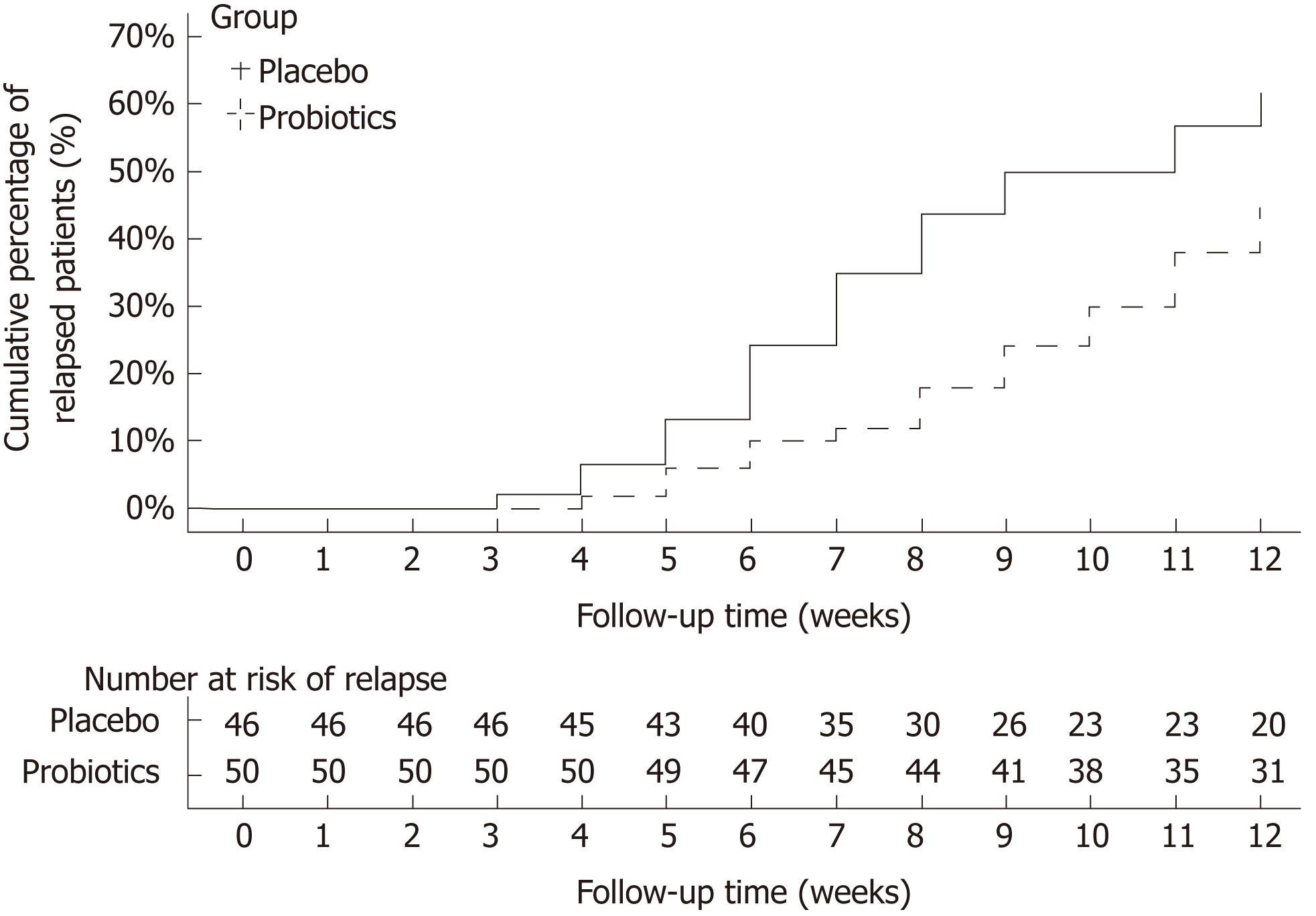
Of 114 eligible healed patients, 102 entered phase 2 (1 refused, 11 with RDQ ≥ 12), 96 completed the follow-up, 50 were from the probiotics groups, and 46 were from the placebo group. At the endpoint of the follow-up, 22 patients had a relapse in the probiotics group, whereas 28 patients had a relapse in the placebo group. Figure 3 shows the cumulative rate of symptomatic recurrence. The result of the log-rank test showed that the two curves differed significantly (P = 0.041), which means that the treatment therapy has a significant influence on relapse time, and the time to relapse is shorter in the placebo group than in the probiotics group. Among the recurrent patients, RDQ scores in the placebo group (17.11 ± 2.85) was higher than that in the probiotics group (15.40 ± 2.34). There was a significant difference in outcome between the two groups (P = 0.024).
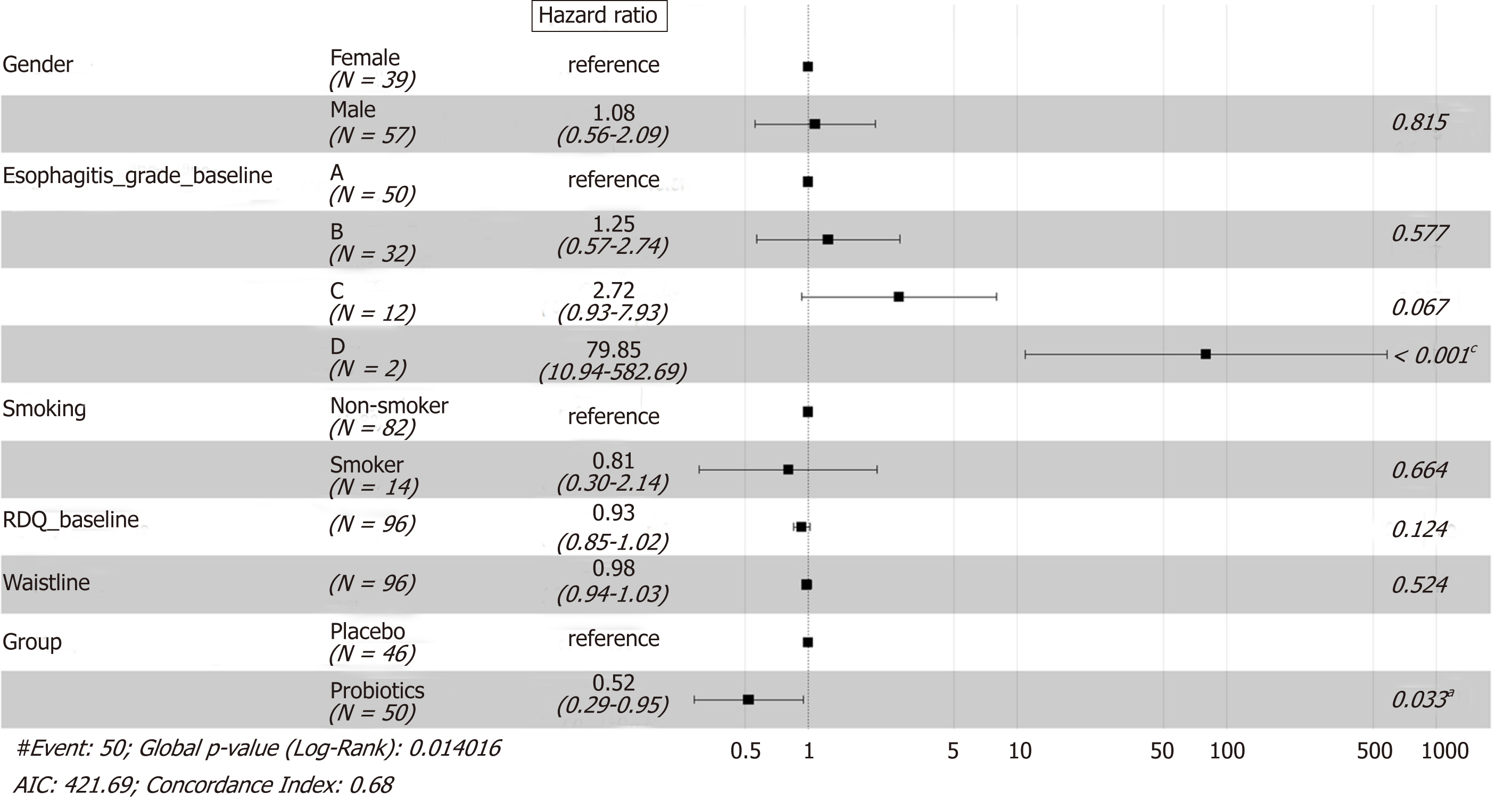
Cox regression analysis on the relapse data showed that the treatment therapy and esophagitis grade at entry had a significant effect on the recurrence. The risk of relapse in the probiotics group was lower than that in the placebo group at any time point during the 12-wk follow-up [hazard ratio (HR) = 0.52, P = 0.033]. Patients with esophagitis grade D had a higher risk of relapse than patients with esophagitis grade A at entry (HR = 79.85, P < 0.001). No other evidence was observed that gender, smoking, baseline RDQ score, or waistline would influence the rate of relapse significantly (Figure 4).
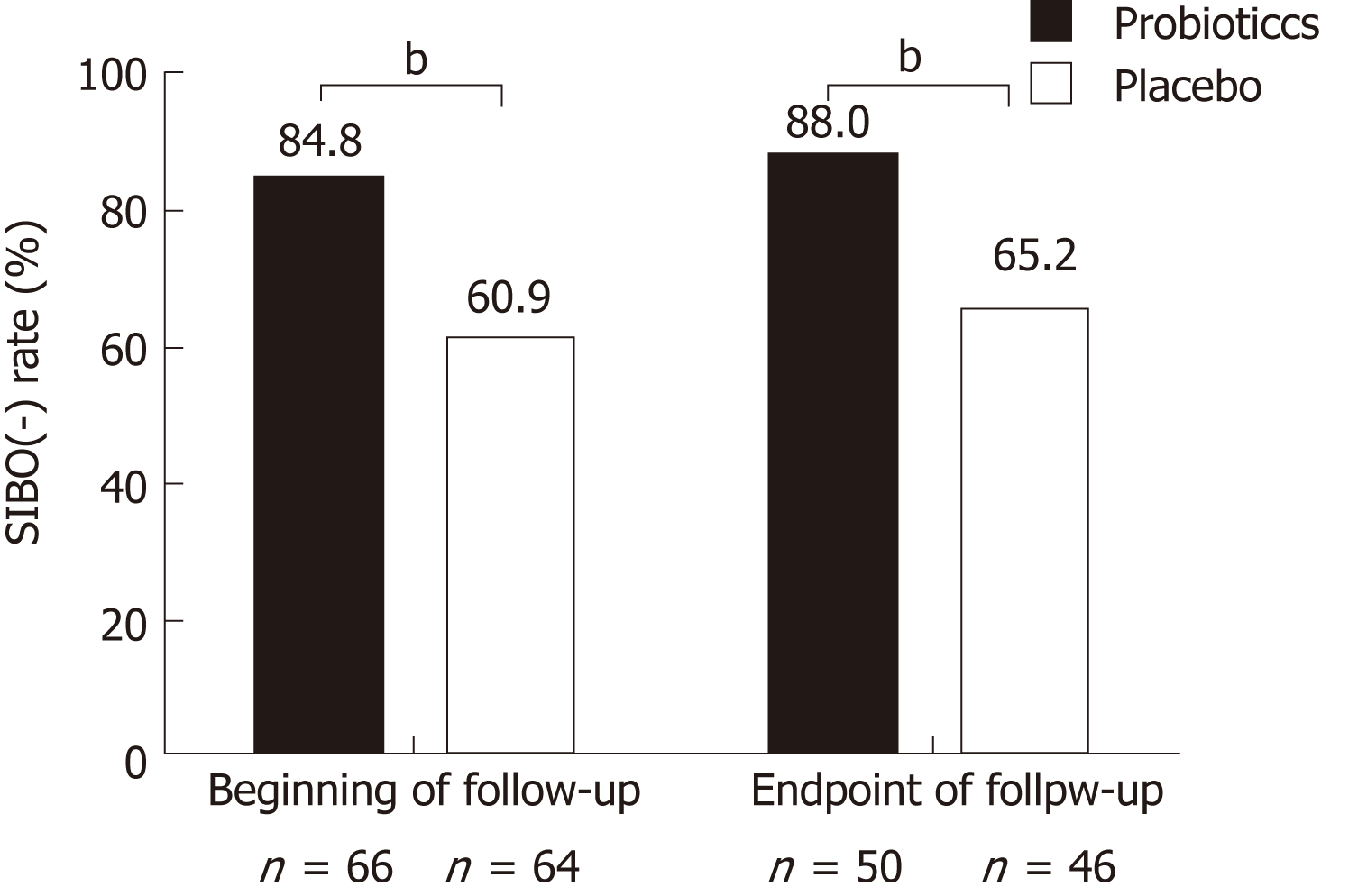
All the patients underwent LBHT testing at baseline, week 8, and the follow-up endpoint. At baseline, all the patients were SIBO negative. After the 8-wk treatment, the SIBO negative rate in the probiotics group (84.8%, 56/66) was higher than that in the placebo group (60.9%, 39/64); the difference between the two groups was statistically significant (P = 0.002). At the endpoint of follow-up, the SIBO negative rate was slightly increased in both groups, 88.0% (44/50) in the probiotics group and 65.2% (30/46) in the placebo group. The percentage of SIBO negative patients in both groups did not change significantly with time (Figure 5). The rate of relapse in SIBO positive patients (45.9%, 34/74) was higher than that in SIBO negative patients (72.7%, 16/22) at the endpoint of follow-up (P = 0.027).
Four patients suffered adverse events in phase 1 and discontinued the intervention. One in the probiotics group and two in the placebo group had nausea and vomiting. One in the placebo group had dermatitis. Minor adverse events were recorded and evaluated by GSRS. In the follow-up period, two patients in the probiotic group and two in the placebo group withdrew for taking drugs that may influence the gut microbiota (antibiotics and probiotics). Two in the placebo group were lost to follow-up.
To our knowledge, this is the first randomized controlled clinical trial to evaluate the impact of disordered gut microbiota on RE, as well as the therapeutic effects of probiotic supplements in patients with RE.
In our study, 8-wk treatment with esomeprazole (20 mg b.i.d.) and Medilac-s, live combined B. subtilis and E. faecium enteric-coated capsules (500 mg t.i.d.), reduced the incidence of SIBO and improved the diarrhea syndrome in RE patients. The endoscopic healing rates were higher in cases with low-grade esophagitis but lower in cases with more severe baseline esophagitis. The healing rates of RE patients in the probiotics and placebo groups were similar. The probiotics supplements may not influence the acid-suppression efficacy because esomeprazole is the most effective and long-lasting antacid PPI[24].
Acid suppression with PPIs has been suggested to be a precursor to the development of SIBO. In a clinical study on patients with functional dyspepsia, Tsuda et al[25] found that 4 wk of PPI use caused SIBO. Oana et al[26] conducted a clinical trial on pediatric gastroesophageal reflux disease (GERD) patients administered probiotics and PPI for 12 wk and found that probiotics administration decreased the rate of dysbiosis in children treated with PPI. Jacobs C et al[27] conducted a study focusing on the risk factors of SIBO. Studies showed that PPI use was an independent risk factor for SIBO. However, some other clinical trials showed different conclusions. In one prospective study, quantitative cultures of duodenal aspirates were performed to detect SIBO. Giamarellos-Bourboulis et al[28] found that PPI intake could not increase SIBO. A double-blind placebo-controlled randomized trial of the effect of probiotics on SIBO in children treated with omeprazole conducted by Badriul Hegar et al[24] found that probiotics did not decrease the risk of developing SIBO. However, it is notable that in this trial the subjects were children and they took PPIs for 4 wk. The dosage and duration of therapy in this study were lower and shorter than those in reports on adults[29,30]. The duration of PPI therapy was directly related to SIBO incidence[31]. Moreover, two meta-analyses reported that the use of PPI could increase the risk of SIBO[32,33].
Del Piano et al[34] found Escherichia coli (E. coli) in the gastric juice of patients who used PPI for more than 3 mo, and given that E. coli is extremely rare in the stomach of healthy people, this result indicated that reducing gastric juice pH would result in excessive growth of stomach-associated bacteria (such as E. coli) and increase the risk of infection and intestinal diseases. A recent study demonstrated that excessive bacterial growth might be due to reduced intragastric bacterial obliteration[35]. A cohort study by Ardatskaia et al[36] found no differences in the incidence of SIBO between patients with atrophic gastritis and patients with GERD following long-term PPI treatment; however, the rates in both groups were higher than in healthy populations, which also proved that a deficiency in gastric acid can result in reduced complexity of gut microbial communities. Long-term PPI use had been shown to decrease Bacteroides and increase Firmicutes in the gut, which may predispose an individual to the development of Clostridium difficile infection (CDI)[37]. A crossover trial conducted by Daniel et al[38] showed that significant changes during PPI use in taxa associated with CDI (increased Enterococcaceae and Streptococcaceae, and decreased Clostridiales) and taxa associated with GI bacterial overgrowth (increased Micrococcaceae and Staphylococcaceae) provided a mechanism by which PPIs predispose an individual to CDI. A study involving multiple methods of microbiota analysis, including quantitative RT-PCR, 16S rRNA sequencing analysis, and a metagenomic analysis, showed that bacteria such as Streptococcus, which are present in the human oral cavity, throat, and nasal cavity, increased in the intestine, implying that bacterial translocation, as well as enteric infections, may have occurred. This may be because PPIs reduced stomach acidity, and the barrier function is weakened[9]. The use of PPIs favors a relative excess of Streptococcus and Campylobacteriosis, and this might explain the persistence of dyspeptic and diarrhea symptoms in patients on PPI therapy[7,39,40].
On the other hand, a 2-wk course of Lactobacillus supplements in patients on long-term PPI treatment (>12 mo) has been shown to significantly reduce total bacterial count, proving the beneficial effects of probiotics in clinical treatment[34]. Del Piano et al believed that Lactobacillus and lactic acid bacteria had inhibitory effects on Coliforms. When patients on long-term PPI treatment were supplemented with probiotics, their Enterococcus faecalis, E. coli, mold, and yeast counts were all drastically reduced[31]. These findings proved that probiotics could regulate gut microbiota.
In our research, the addition of a probiotic combination (B. subtilis and E. faecium) to esomeprazole therapy led to a decrease in SIBO compared to that with the placebo, and the abdominal symptoms were also alleviated. This probiotic, Medilac-s, contains two live probiotics, combined B. subtilis and E. faecium, which can be stored at room temperature. They are constituents of normal intestinal flora in healthy people. They directly supplement normal intestinal flora, inhibit excessive proliferation of harmful bacteria in the gut, and regulate gut microbiota. We found that treatment with combined esomeprazole and live combined B. subtilis and E. faecium enteric-coated capsules had prophylactic effects on SIBO.
Although this combination of drugs did not increase the healing rate of esophagitis, the time to relapse was prolonged for 12 wk after PPI therapy withdrawal. Moreover, in the follow-up research, patients with SIBO had higher risks of symptomatic relapse than SIBO-negative patients. Cox regression analysis showed that the therapy administered (placebo or not) and esophagitis grade D were significant risk factors for recurrence of reflux symptoms. The possible explanation for this may be that a higher reflux recurrence rate is the result of changes in GI motility caused by SIBO. Akiho et al[41]carried out a study on IBS and found that Th2 cytokines could induce smooth muscle hypercontractility during intestinal infection. Th2 cytokines also induced transforming growth factor (TGF)-β1 expression and elevations in cyclooxygenase-2 and prostaglandin E2 levels in smooth muscle cells, resulting in intestinal motility disorder. German et al[42] employed a dog SIBO model and found that TGF-β1 and tumor necrosis factor (TNF)-α mRNA expression levels were decreased after SIBO treatment with antibiotics, i.e., SIBO resulted in enhanced duodenal mucosal immune responses in dogs. SIBO-induced mild chronic inflammatory reactions and immune responses persistently acted persistently on smooth muscles in the GI tract, resulting in functional impairment, which simultaneously caused GERD or IBS-like symptoms. A study by Tugtepe et al[43] found impaired smooth muscle activity in the esophagus in a rat model of chronic RE. Currently, peristaltic abnormalities are present in 40%-50% of GERD patients[44]. Changes in gut microbiota may result in varying effects on gut mucosa and activate the immune and inflammatory response systems in the GI tract, resulting in functional impairment in the digestive and nervous systems, as well as visceral hypersensitivity, and impaired GI peristalsis. The above studies may partially explain why SIBO is associated with a higher recurrence rate of reflux symptoms and how a probiotics supplement can reduce the risks of relapse up to 12 wk after PPI withdrawal. In the future, further studies are needed to examine the pathophysiological mechanisms. Our study provides corroborated clinical trial materials as a basis for these studies.
Furthermore, a correlation between the severity of esophageal erosions and symptom relapse has been demonstrated in our study. Patients with SIBO are more likely to relapse. However, there were only two patients who were followed, and both of them relapsed, resulting in a wide confidence interval. More patients with esophagitis grade D are needed to verify this conclusion.
The significant strength of the present study was the strict exclusion criteria, wherein patients with hiatal hernia, GERD-predisposition, or bowel disorder were not recruited in order to ensure a homogeneous study group. A limitation of this study was the fact that we did not use jejunal cultures for SIBO assessment. Culture of the jejunal aspirate is recognized as the most direct method for diagnosing SIBO[45]. However, obtaining and culturing of jejunal aspirates are time-consuming and costly. In patients with isolated distal SIBO, SIBO could remain undiagnosed despite using jejunal cultures. Because of all of these disadvantages, LHBT was used in this study as an indirect but reliable alternative test to assess SIBO. Another limitation is that this was a single-center study with a limited sample size. Furthermore, the dietary habits of the included patients may affect the morbidity of RE and SIBO, and the effects of only B. subtilis and E. faecium probiotics on gut microbiota were studied. Furthermore, we did not perform endoscopy on asymptomatic patients after primary healing was achieved, and as a result, we were not able to detect asymptomatic relapses of esophagitis erosions. Therefore, the actual rate of mucosal relapse could not be determined in our study.
The combined administration of probiotics (B. subtilis and E. faecium) and esomeprazole could reduce the incidence of SIBO and improve abdominal symptoms in patients with RE. It may also prolong the time to relapse, showing the potential of probiotics (B. subtilis and E. faecium) for the treatment and management of RE.
Profound changes have been observed in the gastric and intestinal microbiota of proton pump inhibitor users. Probiotics are commonly administered to patients with intestinal flora abnormalities. No prior studies have been conducted to evaluate the therapeutic effects of probiotics [Bacillus subtilis (B. subtilis) and Enterococcus faecium (E. faecium)] on patients with reflux esophagitis (RE).
We conducted a randomized controlled clinical trial to evaluate the impact of disordered gut microbiota on RE as well as the therapeutic effect of probiotics supplements on patients with RE.
This clinical trial aimed to study the RE patients treated with the combination of probiotic (B. subtilis and E. faecium) and esomeprazole.
This study included 134 patients with RE who met the criteria. In phase 1, patients were divided into two groups. The probiotics group was given esomeprazole and live combined B. subtilis and E. faecium enteric-coated capsules for eight weeks, and the placebo group was given esomeprazole and placebo for eight weeks. Endoscopic evaluation, gastrointestinal symptom rating scale (GSRS), reflux diagnostic questionnaire (RDQ), and lactulose hydrogen breath test (LHBT) were performed at the end of the treatment. In phase 2, patients who achieved endoscopic and clinical cure (RDQ < 12) entered the follow-up. RDQ and LHBT were completed at the follow-up endpoint.
After eight-week treatment, the GSRS diarrhea syndrome score was decreased significantly in the probiotics group, and the small intestinal bacterial overgrowth (SIBO) negative rate in the probiotics group was significantly higher than that in the placebo group. Furthermore, the therapy had a significant influence on relapse time, and the risk of relapse in the probiotics group was lower than that in the placebo group at any time point during the 12-wk follow-up (hazard ratio = 0.52). However, only B. subtilis and E. faecium as probiotics were studied on gut microbiota in our study. More kinds of probiotics should be studied.
The combined administration of probiotics (B. subtilis and E. faecium) and esomeprazole could reduce the incidence of SIBO and improve abdominal symptoms in patients with RE. It may also prolong the time to relapse, showing the potential of probiotics (B. subtilis and E. faecium) for the treatment and management of RE.
The limitation of this study is the fact that we did not use jejunal cultures for SIBO assessment and did not perform endoscopy on asymptomatic patients after primary healing was achieved. Additional randomized controlled trials are needed to study more probiotics and different dosages, and prolong the follow-up time to evaluate the long-term effect.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kositamongkol P, Kressel A, Lee S S-Editor: Ma RY L-Editor: Wang TQ E-Editor: Ma YJ
| 1. | Yamamoto E, Brito HS, Ogata SK, Machado RS, Kawakami E. High rate of clinical and endoscopic relapse after healing of erosive peptic esophagitis in children and adolescents. J Pediatr Gastroenterol Nutr. 2014;59:594-599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | López-Colombo A, Pacio-Quiterio MS, Jesús-Mejenes LY, Rodríguez-Aguilar JE, López-Guevara M, Montiel-Jarquín AJ, López-Alvarenga JC, Morales-Hernández ER, Ortiz-Juárez VR, Ávila-Jiménez L. Risk factors associated with gastroesophageal reflux disease relapse in primary care patients successfully treated with a proton pump inhibitor. Rev Gastroenterol Mex. 2017;82:106-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Sigterman KE, van Pinxteren B, Bonis PA, Lau J, Numans ME. Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database Syst Rev. 2013;CD002095. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 4. | Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308-28; quiz 329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1136] [Cited by in F6Publishing: 1023] [Article Influence: 93.0] [Reference Citation Analysis (0)] |
| 5. | Pimentel M. Breath Testing for Small Intestinal Bacterial Overgrowth: Should We Bother? Am J Gastroenterol. 2016;111:307-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Castellani C, Singer G, Kashofer K, Huber-Zeyringer A, Flucher C, Kaiser M, Till H. The Influence of Proton Pump Inhibitors on the Fecal Microbiome of Infants with Gastroesophageal Reflux-A Prospective Longitudinal Interventional Study. Front Cell Infect Microbiol. 2017;7:444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Minalyan A, Gabrielyan L, Scott D, Jacobs J, Pisegna JR. The Gastric and Intestinal Microbiome: Role of Proton Pump Inhibitors. Curr Gastroenterol Rep. 2017;19:42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 8. | Stark CM, Susi A, Emerick J, Nylund CM. Antibiotic and acid-suppression medications during early childhood are associated with obesity. Gut. 2019;68:62-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 9. | Hojo M, Asahara T, Nagahara A, Takeda T, Matsumoto K, Ueyama H, Matsumoto K, Asaoka D, Takahashi T, Nomoto K, Yamashiro Y, Watanabe S. Gut Microbiota Composition Before and After Use of Proton Pump Inhibitors. Dig Dis Sci. 2018;63:2940-2949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 10. | Singh A, Cresci GA, Kirby DF. Proton Pump Inhibitors: Risks and Rewards and Emerging Consequences to the Gut Microbiome. Nutr Clin Pract. 2018;33:614-624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Ghoshal UC, Ghoshal U. Small Intestinal Bacterial Overgrowth and Other Intestinal Disorders. Gastroenterol Clin North Am. 2017;46:103-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 12. | Fujimori S. What are the effects of proton pump inhibitors on the small intestine? World J Gastroenterol. 2015;21:6817-6819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 43] [Cited by in F6Publishing: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Corleto VD, Festa S, Di Giulio E, Annibale B. Proton pump inhibitor therapy and potential long-term harm. Curr Opin Endocrinol Diabetes Obes. 2014;21:3-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 14. | Cares K, Al-Ansari N, Macha S, Zoubi N, Zaghloul H, Thomas R, Lalinsky P, El-Baba M. Short article: Risk of small intestinal bacterial overgrowth with chronic use of proton pump inhibitors in children. Eur J Gastroenterol Hepatol. 2017;29:396-399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Naito Y, Kashiwagi K, Takagi T, Andoh A, Inoue R. Intestinal Dysbiosis Secondary to Proton-Pump Inhibitor Use. Digestion. 2018;97:195-204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 16. | China gastroesophageal reflux disease research collaboration group, China gastroesophageal reflux disease research collaboration group. Zhonghua Xiaohua Zazhi. 2003;23:651-654. [DOI] [Cited in This Article: ] |
| 17. | Revicki DA, Wood M, Wiklund I, Crawley J. Reliability and validity of the Gastrointestinal Symptom Rating Scale in patients with gastroesophageal reflux disease. Qual Life Res. 1998;7:75-83. [PubMed] [Cited in This Article: ] |
| 18. | Wilkins T, Sequoia J. Probiotics for Gastrointestinal Conditions: A Summary of the Evidence. Am Fam Physician. 2017;96:170-178. [PubMed] [Cited in This Article: ] |
| 19. | The ministry of health of the People's Republic of China, Good Manufacture Practice of Medical Products (2010 revision). . [Cited in This Article: ] |
| 20. | Hetzel DJ, Dent J, Reed WD, Narielvala FM, Mackinnon M, McCarthy JH, Mitchell B, Beveridge BR, Laurence BH, Gibson GG. Healing and relapse of severe peptic esophagitis after treatment with omeprazole. Gastroenterology. 1988;95:903-912. [PubMed] [Cited in This Article: ] |
| 21. | Carlsson R, Dent J, Watts R, Riley S, Sheikh R, Hatlebakk J, Haug K, de Groot G, van Oudvorst A, Dalväg A, Junghard O, Wiklund I. Gastro-oesophageal reflux disease in primary care: an international study of different treatment strategies with omeprazole. International GORD Study Group. Eur J Gastroenterol Hepatol. 1998;10:119-124. [PubMed] [Cited in This Article: ] |
| 22. | Kinoshita Y, Hongo M, Kusano M, Furuhata Y, Miyagishi H, Ikeuchi S; RPZ Study Group. Therapeutic Response to Twice-daily Rabeprazole on Health-related Quality of Life and Symptoms in Patients with Refractory Reflux Esophagitis: A Multicenter Observational Study. Intern Med. 2017;56:1131-1139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Sealed Envelope Ltd. Power calculator for binary outcome superiority trial. Available from: https://www.sealedenvelope.com/power/binary-superiority/. . [Cited in This Article: ] |
| 24. | Hegar B, Hutapea EI, Advani N, Vandenplas Y. A double-blind placebo-controlled randomized trial on probiotics in small bowel bacterial overgrowth in children treated with omeprazole. J Pediatr (Rio J). 2013;89:381-387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Tsuda A, Suda W, Morita H, Takanashi K, Takagi A, Koga Y, Hattori M. Influence of Proton-Pump Inhibitors on the Luminal Microbiota in the Gastrointestinal Tract. Clin Transl Gastroenterol. 2015;6:e89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 116] [Article Influence: 12.9] [Reference Citation Analysis (1)] |
| 26. | Belei O, Olariu L, Dobrescu A, Marcovici T, Marginean O. Is It Useful to Administer Probiotics Together With Proton Pump Inhibitors in Children With Gastroesophageal Reflux? J Neurogastroenterol Motil. 2018;24:51-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Jacobs C, Coss Adame E, Attaluri A, Valestin J, Rao SS. Dysmotility and proton pump inhibitor use are independent risk factors for small intestinal bacterial and/or fungal overgrowth. Aliment Pharmacol Ther. 2013;37:1103-1111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 28. | Giamarellos-Bourboulis EJ, Pyleris E, Barbatzas C, Pistiki A, Pimentel M. Small intestinal bacterial overgrowth is associated with irritable bowel syndrome and is independent of proton pump inhibitor usage. BMC Gastroenterol. 2016;16:67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Thorens J, Froehlich F, Schwizer W, Saraga E, Bille J, Gyr K, Duroux P, Nicolet M, Pignatelli B, Blum AL, Gonvers JJ, Fried M. Bacterial overgrowth during treatment with omeprazole compared with cimetidine: a prospective randomised double blind study. Gut. 1996;39:54-59. [PubMed] [Cited in This Article: ] |
| 30. | Hutchinson S, Logan R. The effect of long-term omeprazole on the glucose-hydrogen breath test in elderly patients. Age Ageing. 1997;26:87-89. [PubMed] [Cited in This Article: ] |
| 31. | Del Piano M, Anderloni A, Balzarini M, Ballarè M, Carmagnola S, Montino F, Orsello M, Pagliarulo M, Tari R, Soattini L, Sforza F, Mogna L, Mogna G. The innovative potential of Lactobacillus rhamnosus LR06, Lactobacillus pentosus LPS01, Lactobacillus plantarum LP01, and Lactobacillus delbrueckii Subsp. delbrueckii LDD01 to restore the "gastric barrier effect" in patients chronically treated with PPI: a pilot study. J Clin Gastroenterol. 2012;46 Suppl:S18-S26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Lo WK, Chan WW. Proton pump inhibitor use and the risk of small intestinal bacterial overgrowth: a meta-analysis. Clin Gastroenterol Hepatol. 2013;11:483-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 216] [Article Influence: 19.6] [Reference Citation Analysis (1)] |
| 33. | Su T, Lai S, Lee A, He X, Chen S. Meta-analysis: proton pump inhibitors moderately increase the risk of small intestinal bacterial overgrowth. J Gastroenterol. 2018;53:27-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 94] [Article Influence: 15.7] [Reference Citation Analysis (2)] |
| 34. | Del Piano M, Pagliarulo M, Tari R, Carmagnola S, Balzarini M, Lorenzini P, Pane M. Correlation between chronic treatment with proton pump inhibitors and bacterial overgrowth in the stomach: any possible beneficial role for selected lactobacilli? J Clin Gastroenterol. 2014;48 Suppl 1:S40-S46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Lombardo L, Foti M, Ruggia O, Chiecchio A. Increased incidence of small intestinal bacterial overgrowth during proton pump inhibitor therapy. Clin Gastroenterol Hepatol. 2010;8:504-508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 231] [Cited by in F6Publishing: 217] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 36. | Ardatskaia MD, Loginov VA, Minushkin ON. [Syndrome of bacterial overgrowth in patients with the reduced stomach acid secretion: some aspects of the diagnosis]. Eksp Klin Gastroenterol. 2014;30-36. [PubMed] [Cited in This Article: ] |
| 37. | Clooney AG, Bernstein CN, Leslie WD, Vagianos K, Sargent M, Laserna-Mendieta EJ, Claesson MJ, Targownik LE. A comparison of the gut microbiome between long-term users and non-users of proton pump inhibitors. Aliment Pharmacol Ther. 2016;43:974-984. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 38. | Gasbarrini A, Lauritano EC, Gabrielli M, Scarpellini E, Lupascu A, Ojetti V, Gasbarrini G. Small intestinal bacterial overgrowth: diagnosis and treatment. Dig Dis. 2007;25:237-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 39. | Leonard J, Marshall JK, Moayyedi P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am J Gastroenterol. 2007;102:2047-56; quiz 2057. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 423] [Cited by in F6Publishing: 396] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 40. | Bavishi C, Dupont HL. Systematic review: the use of proton pump inhibitors and increased susceptibility to enteric infection. Aliment Pharmacol Ther. 2011;34:1269-1281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 290] [Cited by in F6Publishing: 298] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 41. | Akiho H, Deng Y, Blennerhassett P, Kanbayashi H, Collins SM. Mechanisms underlying the maintenance of muscle hypercontractility in a model of postinfective gut dysfunction. Gastroenterology. 2005;129:131-141. [PubMed] [Cited in This Article: ] |
| 42. | German AJ, Helps CR, Hall EJ, Day MJ. Cytokine mRNA expression in mucosal biopsies from German shepherd dogs with small intestinal enteropathies. Dig Dis Sci. 2000;45:7-17. [PubMed] [Cited in This Article: ] |
| 43. | Tugtepe H, Tugay M, Bozkurt S, Yildiz F, Utkan T, Yegen BC, Dagli TE. Esophageal smooth muscle reactivity is impaired in chronic reflux esophagitis by both receptor- and nonreceptor-mediated mechanisms. J Pediatr Surg. 2007;42:641-646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 44. | Yi ZH, Feng L, Wen MY, Liu JR, Yang L. [Association between acid reflux and esophageal dysmotility in patients with gastroesophageal reflux disease]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2014;45:480-483. [PubMed] [Cited in This Article: ] |
| 45. | Chandra S, Dutta U, Noor MT, Taneja N, Kochhar R, Sharma M, Singh K. Endoscopic jejunal biopsy culture: a simple and effective method to study jejunal microflora. Indian J Gastroenterol. 2010;29:226-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |