INTRODUCTION
Gastric cancer remains the fifth most common cancer worldwide. While the majority of cases are sporadic, 1-3% of gastric cancers are related to hereditary cancer syn-dromes, including hereditary diffuse gastric cancer syndrome (HDGC). Pa-thogenic germline CDH1 variants have been associated with HDGC, although some families fulfilling HDGC clinical criteria do not have detectable germline variants[1]. CDH1 encodes for the tumor suppressor E-cadherin, which serves as a critical cell adhesion molecule[2]. The connection of CDH1 mutations to HDGC syndrome was first described in New Zealand, when Jones et al[1,3-5] suspected genetic predisposition as the cause of a high rate of gastric cancer in three Maori families. Since 1998, more than 120 different pathogenic variants of CDH1 have been described, and carrying a germline CDH1 pathogenic variant has been shown to portend a high risk of diffuse gastric cancer, characterized by signet ring cell carcinoma (SRCC) on histopathology, as well as lobular breast cancer in women[6-8].
Genetic testing for CDH1 variants is recommended for those who meet clinical criteria for HDGC[1]. Criteria (considering first- and second-degree relatives) include having two or more family members with gastric cancer (including one confirmed diffuse gastric cancer), having one case of diffuse gastric cancer prior to age 40, or having both diffuse gastric cancer and lobular breast cancer in a family (with one diagnosed before age 50). Genetic testing can also be considered in those with cleft lip or palate and diffuse gastric cancer, in the presence of bilateral lobular breast cancer, or in families with two or more cases of lobular breast cancer before age 50. Testing for CDH1 should include both sequencing and deletion/duplication analysis, and is now commonly performed by numerous commercial laboratories[9]. Germline CDH1 pathogenic variants are found in approximately 25%-50% of families meeting HDGC criteria, though rates vary by ethnic background and country[9-12]. Those who meet testing criteria but do not have an identified CDH1 pathogenic variant pose their own challenges in management and risk stra-tification. Recent studies have suggested CTNNA1 and MAP3K6 as other potential causative genes responsible for HDGC, however further work to confirm these associations is required[10,13]. Additionally, other cancer susceptibility genes associated with a spectrum of cancers outside of gastric cancer, such as BRCA2, STK11, and PALB2 have also been identified in families meeting HDGC criteria, suggesting some clinically-defined HDGC families may have a genetic basis related to another hereditary syndrome[9].
The lifetime risk of diffuse gastric cancer in individuals with a germline CDH1 pathogenic variant is reported to be up to 80%[1,14,15]. However, this high cumulative lifetime risk of diffuse gastric cancer may be an over-estimate, as the advent of multi-gene panel testing has identified a notable number of CDH1 pathogenic variants in families without a history of diffuse gastric cancer[9,14-16], suggesting reduced pe-netrance in some families. Currently, individuals with a germline CDH1 pathogenic variant are recommended to undergo prophylactic total gastrectomy, typically between the ages of 20-30[17,18]. However, given the major implications for quality of life and nutritional status after gastrectomy, especially in younger patients, some patients opt to delay or defer prophylactic total gastrectomy[19-22].
The role of endoscopy in the management of patients with HDGC has been studied extensively and plays an important role for diagnosis, surveillance, and risk stratification. Herein, we highlight the role of endoscopy in individuals with HDGC, and review the recent research and advances in the field.
Guidelines for the use of endoscopy in HDGC with a known pathogenic CDH1 variant
For patients with a pathogenic germline CDH1 variant who undergo prophylactic total gastrectomy, baseline upper endoscopy is recommended prior to surgery to evaluate for gross tumor or other concomitant pathology that may alter the surgical approach[1]. For those electing to defer risk reducing gastrectomy, annual surveillance endoscopy following the Cambridge protocol is advised. If microscopic or macro-scopic disease is detected on surveillance, prompt gastrectomy referral is required[1,17]. White light exam is recommended for surveillance, since other endo-scopic modalities have not proven efficacious, as discussed below.
Surveillance endoscopy via the Cambridge protocol
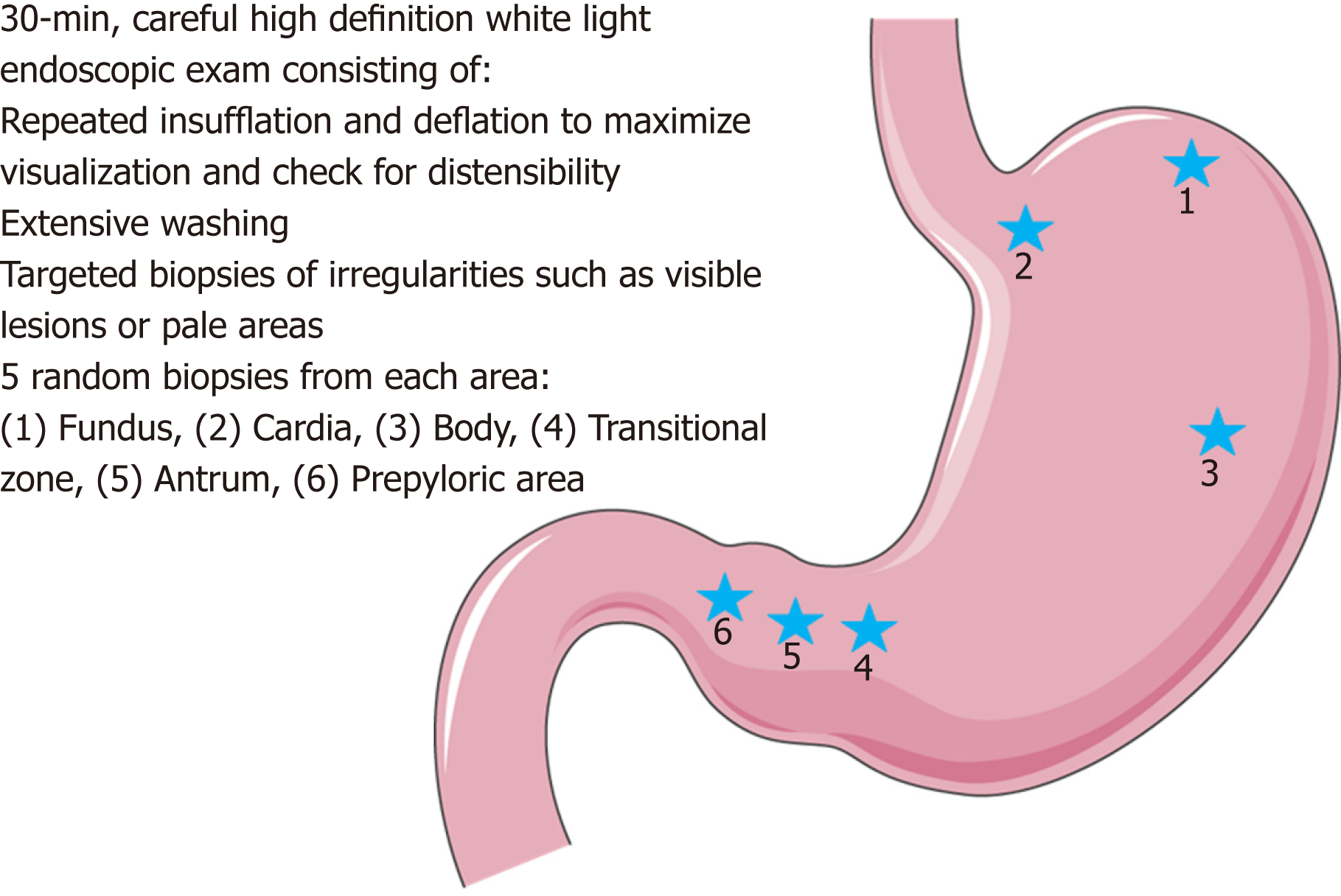
The Cambridge protocol was developed to help guide endoscopic surveillance in HDGC[1]. Surveillance via the Cambridge protocol ideally should be performed in a high-volume center with a multi-disciplinary team having expertise in HDGC. Given the large number of biopsies performed, it is recommended to stop anticoagulation if possible prior to the procedure. Endoscopy should include a careful high definition white light (HDWL) exam in a session of at least 30 min. The endoscopic exam should include repeated insufflation and deflation to maximize visualization of the entire gastric mucosa and check for distensibility (the lack of which should raise concern for an infiltrative process such as linitis plastica). Additionally, the exam should utilize extensive washing with the assistance of mucolytic and anti-foaming agents, to permit careful examination and documentation of the entire gastric mucosa. If there is concern for poor distensibility, further evaluation should include computed tomography scan and endoscopic ultrasonography (EUS).
Prior to obtaining random gastric biopsies, all visible lesions, pale areas (considered more likely to have abnormal signet ring cells), and other gastric abnormalities should be separately biopsied and sent for pathologic evaluation[5,23]. After sampling of all visible lesions, five random biopsies should then be taken from each of 6 anatomic regions: prepyloric, antrum, transitional zone, body, fundus, and cardia, with these groups of biopsies each being sent separately for pathologic analysis (see Figure 1). Despite no known association between Helicobacter pylori (H. pylori) and HDGC, baseline H. pylori testing on the gastric biopsy specimens is recommended given that H. pylori is considered a class I carcinogen by the World Health Organization. Subsequent treatment and confirmation of eradication of individuals who are H. pylori positive is advised[1,24].
Figure 1 Illustrative representation of the Cambridge protocol for hereditary diffuse gastric cancer syndrome surveillance, full details in text and on image.
Figure modified with text, markings (stars), and annotation after adaptation of “Stomach” from Servier Medical Art by Servier, licensed under a Creative Commons Attribution 3.0 Unported License. Original photo adapted from https://smart.servier.com/smart_image/stomach-3/.
EFFICACY OF ENDOSCOPY IN CDH1 FAMILIES
Endoscopic detection of SRCC foci in CDH1 carriers is poor, and evaluation of surgical pathology demonstrates cancer foci in 45%-60% of those with negative endoscopic evaluations[5,11,21,25]. The reason for this poor performance is due to the occult and difficult to predict nature of HDGC. HDGC is rarely restricted to one location in the stomach[6,26]. Independent studies have found vastly different results when evaluating the regions of the stomach where the HDGC-related SRCC foci are located[27-29]. This heterogeneity in location, as well as the inability to reliably visualize the SRCC foci on visual mucosal examination, both play a large role in the inability to reliably detect SRCC foci endoscopically. A model developed by Fujita et al[28] eva-luated yield of cancer foci in a topographic pattern on 10 gastrectomy specimens from individuals with pathogenic germline CDH1 variants. This model estimated that for a 90% detection of cancer foci, the theoretical number of biopsies necessary to capture at least a single cancer focus was estimated to be 1768 per patient, which is clearly not clinically feasible. However, the yield of endoscopically detecting SRCC foci pre-operatively is both correlated with the number of biopsies taken and the number of SRCC lesions in the gastrectomy specimen[30]. As described by de Almeida et al[6], there are also practical issues beyond the number of biopsies required, including scarring from repeated biopsies, which may mimic pale areas concerning for malignancy and may cause confusion and repeated re-biopsy of the same areas of mucosa.
Unfortunately, the specific CDH1 variant itself does not aid in determining the potential location of SRCC or the probability of finding SRCC, especially given the vast number of CDH1 pathogenic variants that have been described[9,31,32]. There is also limited data of the benefit of continued surveillance in CDH1 carriers. Most cancer foci are detected on the index endoscopy, which is likely due to detecting prevalent rather than incident cases[11]. This is likely due to the fact that multiple foci of SRCC develop before age 30 and have a long indolent course[33]. It is further unknown how long these foci remain indolent, and what environmental or genetic predispositions can lead to more extensive disease.
In addition to the imperfect sensitivity of endoscopic surveillance, there are possible complications from endoscopy, as well as potential psychological hurdles in those individuals undergoing serial endoscopic evaluation, including concerns of lifelong endoscopy and the chances of false negatives[9,11,19,34]. The concern of false reassurance in those with a negative endoscopy is not to be underestimated, either.
Chromoendoscopy
In an attempt to improve the efficiency of SRCC detection in CDH1 carriers, several advanced endoscopic techniques have been studied, including the use of chro-moendoscopy. Based on a Japanese study, Shaw et al[5] published a series in 2005 describing annual chromoendoscopic surveillance in 33 patients with a pathogenic CDH1 variant over a 5-year period. The patients initially drank a mucolytic (N-acetylcysteine) then changed position every 5 min for 30 min to ensure complete gastric coverage. The endoscopist then performed a HDWL endoscopic exam, and the patient received intravenous pentagastrin for gastric acid secretion, followed by spraying of methylene blue onto the mucosa. After absorption and suction, Congo red dye was sprayed and the mucosa was irrigated. Only pale areas were biopsied, and no random biopsies were performed. Of 56 total pale areas, 23 lesions (41%) showed SRCC (in 10 patients). When comparing these results to total gastrectomy pathology, it was shown that there was a positive correlation for foci 4-10 mm in size, but not for foci < 4 mm.
While initially promising, due to concerns about dye toxicity, chromoendoscopic exam is currently not recommended as standard of care for HDGC[1,6]. Additionally, there were further concerns regarding the learning curve in chromoendoscopy as well as pale area detection and interpretation, as the number of pale areas detected by endoscopists increased annually, despite no change in relative number of annual surveillance procedures[5,11].
Electronic enhanced imaging
Electronic enhanced imaging takes advantage of pre- and/or post-processing light wavelengths and filtering to amplify surface, subsurface and vascular prominence and contours. Beyond careful white-light examination with targeted and random biopsies, electronic enhanced imaging techniques have not proven to be particularly valuable for diagnosis, screening or surveillance of patients with pathologic CDH1 gene mutations[5,25,35]. Lim et al[25] report that auto-fluorescence imaging (AFI) and narrow band imaging (NBI) were of limited utility, failing to reliably identify ab-normal appearing mucosa for targeted tissue sampling. While AFI did not prove to aid in the detection of early foci of signet ring cell neoplasia, NBI was a useful imaging adjunct to delineate pale areas and, in conjunction with zoom magnification, allowed careful assessment of vascular and mucosal patterns, with a high negative predictive value in their study. Emerging electronic enhanced imaging techniques with depth to the lamina propria layer will be tested in this condition owing to the unpredictable and patchy distribution of SRCC characteristic of early stage disease.
Endoscopic ultrasonography (EUS)
There is currently no recommendation for routine use of EUS in the management of HDGC. However, EUS can be considered for further investigation of abnormalities seen on standard HDWL endoscopic examination, such as poor distensibility of the stomach[1]. To evaluate the utility of EUS in patients with HDGC due to a CDH1 pathogenic variant, a retrospective analysis of 13 patients who underwent radial scanning endosonography in addition to guideline-recommended upper endoscopy before gastrectomy found no benefit in performing endoscopic ultrasound to improve detection of cancer foci[34]. In that study, the sensitivity for identifying SRCC foci by HDWL endoscopy was 45%, similar to other descriptive series.
Confocal endoscopic microscopy
There is an ongoing NIH clinical trial evaluating the use of confocal endoscopic microscopy, which provides histologic imaging of gastric mucosa during upper endoscopy, and could improve endoscopic surveillance sensitivity (ClinicalTrials.gov Identifier: NCT03648879)[36]. However, this technique remains experimental at this time, and should only be pursued in HDGC through a clinical trial.
Colorectal cancer screening
Although the majority of research on HDGC has focused on the role of upper endoscopic examination, there is limited evidence that there may be an association of colorectal SRCC in patients with germline CDH1 pathogenic variants[1]. More recent work has also speculated that the CDH1 variant location may be an important predictor of colorectal cancer risk[37]. Although based on limited data, enhanced colorectal cancer screening by colonoscopy is recommended for CDH1 carriers with a family history of colon cancer, especially when there is a presence of signet ring cells and/or mucinous features of the cancer[1,17]. In these individuals, screening should start at age 40 or 10 years prior to the youngest diagnosis in the family, and repeated every 3-5 years. In all other CDH1 carriers, standard colorectal cancer screening guidelines should be followed based on the individual’s personal and/or family history.
ROLE OF ENDOSCOPY IN PATIENTS WITH A PATHOGENIC CDH1 VARIANT AND NO FAMILY HISTORY OF GASTRIC CANCER
Individuals with a pathogenic variant of CDH1, but without family history of gastric cancer, pose a unique challenge. There is limited evidence on endoscopic surveillance of these patients, and one small series found that despite no family history of gastric cancer and negative endoscopic evaluation, 50% of patients had SRCC on surgical pathology[38]. It remains to be determined if these patients truly have decreased gastric cancer risk and reduced prevalence compared to CDH1 carriers with a strong family history of gastric cancer[9,14-16]. Another possibility is that in small or poorly reported-on families, family history may be inaccurate, as even in the best circumstances, family history collection can be poor[39-41]. As such, these patients may have a true family history of gastric cancer that simply goes unrecognized.
A similar dilemma arises in CDH1 carriers with lobular breast cancer and without a family history of diffuse gastric cancer. Recent studies regarding the management of lobular breast cancer, to which a pathogenic variant of CDH1 increased risk, in the absence of gastric cancer family history have been small, though informative[42-46]. These studies demonstrate that though rare, even in the absence of familial gastric cancer, lobular breast cancer may develop, and may even represent a separate cancer syndrome[44,45,47]. This has clinical consequences for the screening of breast cancer in those with a pathogenic variant of CDH1, though the need for gastrectomy in these individuals is similarly cloudy[42,43,47]. There have not been large reports of endoscopic utility in these patients.
An advantage of endoscopic surveillance in these groups would be the finding of SRCC on endoscopic biopsy, as this would justify the need for gastrectomy in the physician and patient alike. However, we would expect the sensitivity of endoscopy to be lacking, as it is in those with pathogenic variants of CDH1 and HDGC, and therefore a negative endoscopic examination should not be overly reassuring regarding gastric cancer risk. This is an area with much uncertainty that requires fur-ther research, especially in light of the growing preponderance of multi-gene panel testing with its resulting identification of incidental CDH1 variants in families without a history of CDH1-associated cancers.
ROLE OF ENDOSCOPY IN HDGC FAMILIES WITHOUT A PATHOGENIC CDH1 VARIANT
Individuals from families meeting HDGC criteria who lack a germline CDH1 pathogenic variant present a significant challenge as prophylactic total gastrectomy is typically not recommended for these individuals. In these cases, endoscopic surveillance is recommended on a regular basis in a high-volume specialty center, as the identification of SRCC foci on HDWL endoscopy in these individuals can be helpful for guiding future surgical management[1].
However, the yield of endoscopy for identifying malignant lesions is lower in those from families meeting HDGC criteria without a detectable CDH1 pathogenic variant. This was demonstrated in a United Kingdom-based prospective cohort study of endoscopic surveillance in families with and without CDH1 pathogenic variants; patients with CDH1 pathogenic variants had significantly higher rates of SRCC on endoscopy than their CDH1 negative counterparts[11]. In this study, 85 individuals underwent endoscopic surveillance, including 54 patients (63.5%) with a CDH1 pathogenic variant opting to delay gastrectomy and 31 patients undergoing surveillance who were CDH1 negative. Endoscopic surveillance included 30 random biopsies as well as targeted biopsy of mucosal abnormalities. Of those with CDH1 pathogenic variants, 33 (61.1%) had foci of SRCC detected during surveillance, the majority of which occurred at the index endoscopy. Those without a CDH1 pathogenic variant had a much lower detection rate of SRCC, 3 of 31 (9.7%) patients. This difference was statistically significant (P < 0.0005), and no other factors, including age, sex, proton pump inhibitor use, or H. pylori infection were associated with the differential detection of cancer foci.
It has been considered that there may be a different pathogenesis for diffuse gastric cancer in CDH1-negative HDGC individuals, i.e., that SRCC may not be the preceding lesion; however, this remains unclear and further investigation is required[11]. Given the number of unanswered questions, including pathogenesis, yield of endoscopy, and rate and frequency of progression to cancer, the management of individuals from CDH1-negative HDGC families remains challenging. Mi et al[11] suggested that risk stratification using allelic expression imbalance (as a marker for progression to neoplasia) may be helpful to better determine the risk for these individuals. Ideally in the future, enhanced understanding of the genetic basis for HDGC families currently without detectable CDH1 pathogenic variants (such as via improved CDH1 variant detection or identification of additional gastric cancer risk genes) may enable better risk stratification in this population.
Currently, endoscopic surveillance at a referral center is suggested for individuals from families meeting HDGC criteria and who lack a CDH1 pathogenic variant. It is clear in this high-risk group that endoscopic detection of a gastric malignancy, including foci of SRCC, should prompt a referral for consideration of total gastrectomy, yet counseling these patients on negative endoscopies presents its own challenge. Additionally, the optimal frequency of surveillance in this population also remains to be determined[18].
CONCLUSION
Management of patients with HDGC, both with and without pathogenic variants in CDH1, remains challenging given our lack of understanding about the critical drivers of penetrance, differential anatomical location of SRCC, and the imperfect sensitivity of endoscopy to detect early foci of SRCC. However, given the uncertainties in the field, the use of endoscopy in these individuals is an area that is ripe for future research, as improvement in endoscopic surveillance in HDGC would undoubtedly improve risk stratification, surgical management, and overall patient well-being in families with HDGC.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen SQ, Cheng H, Xin Q S-Editor: Yan JP L-Editor: A E-Editor: Ma YJ