Published online Oct 7, 2019. doi: 10.3748/wjg.v25.i37.5630
Peer-review started: June 17, 2019
First decision: July 21, 2019
Revised: August 22, 2019
Accepted: September 9, 2019
Article in press: September 9, 2019
Published online: October 7, 2019
Tumor recurrence after orthotopic liver transplantation (OLT) remains a serious threat for long-term survival of the recipients with hepatocellular carcinoma (HCC), since very few factors or measures have shown impact on overcoming HCC recurrence after OLT. Postoperative infection suppresses tumor recurrence and improves patient survival in lung cancer and malignant glioma probably via stimulating the immune system. Post-transplant infection (PTI), a common complication, is deemed to be harmful for the liver transplant recipients from a short-term perspective. Nevertheless, whether PTI inhibits HCC recurrence after OLT and prolongs the long-term survival of HCC patients needs to be clarified.
To investigate the potential influence of PTI on the survival and tumor recurrence of patients with HCC after OLT.
A total of 238 patients with HCC who underwent OLT between August 2002 and July 2016 at our center were retrospectively included and accordingly subdivided into a PTI group (53 patients) and a non-PTI group (185 patients). Univariate analyses, including the differences of overall survival (OS), recurrence-free survival (RFS), and post-recurrence survival (PRS), between the PTI and non-PTI subgroups as well as survival curve analysis were performed by the Kaplan-Meier method, and the differences were compared using the log rank test. The variables with a P-value < 0.1 in univariate analyses were included in the multivariate survival analysis by using a Cox proportional-hazards model.
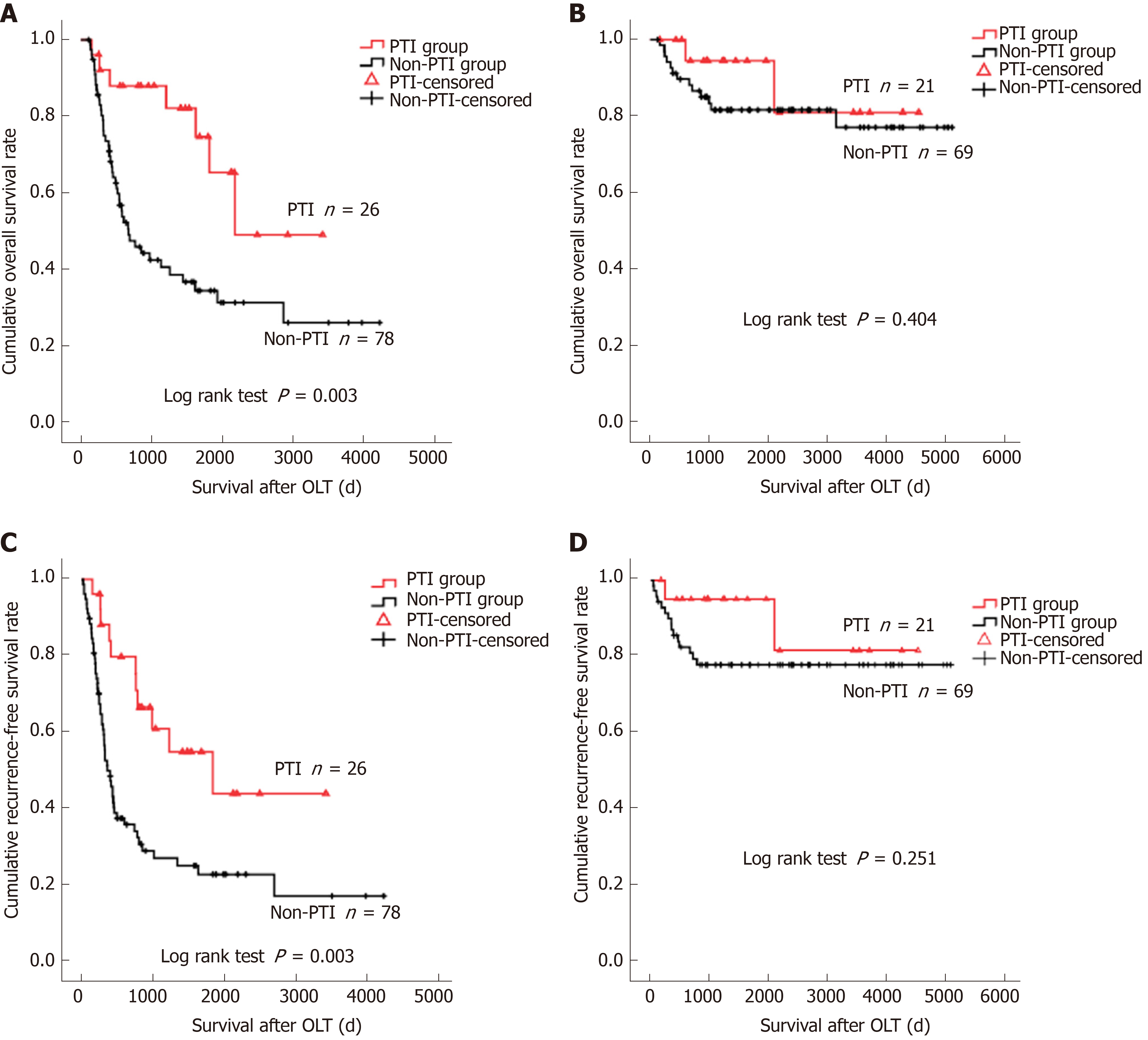
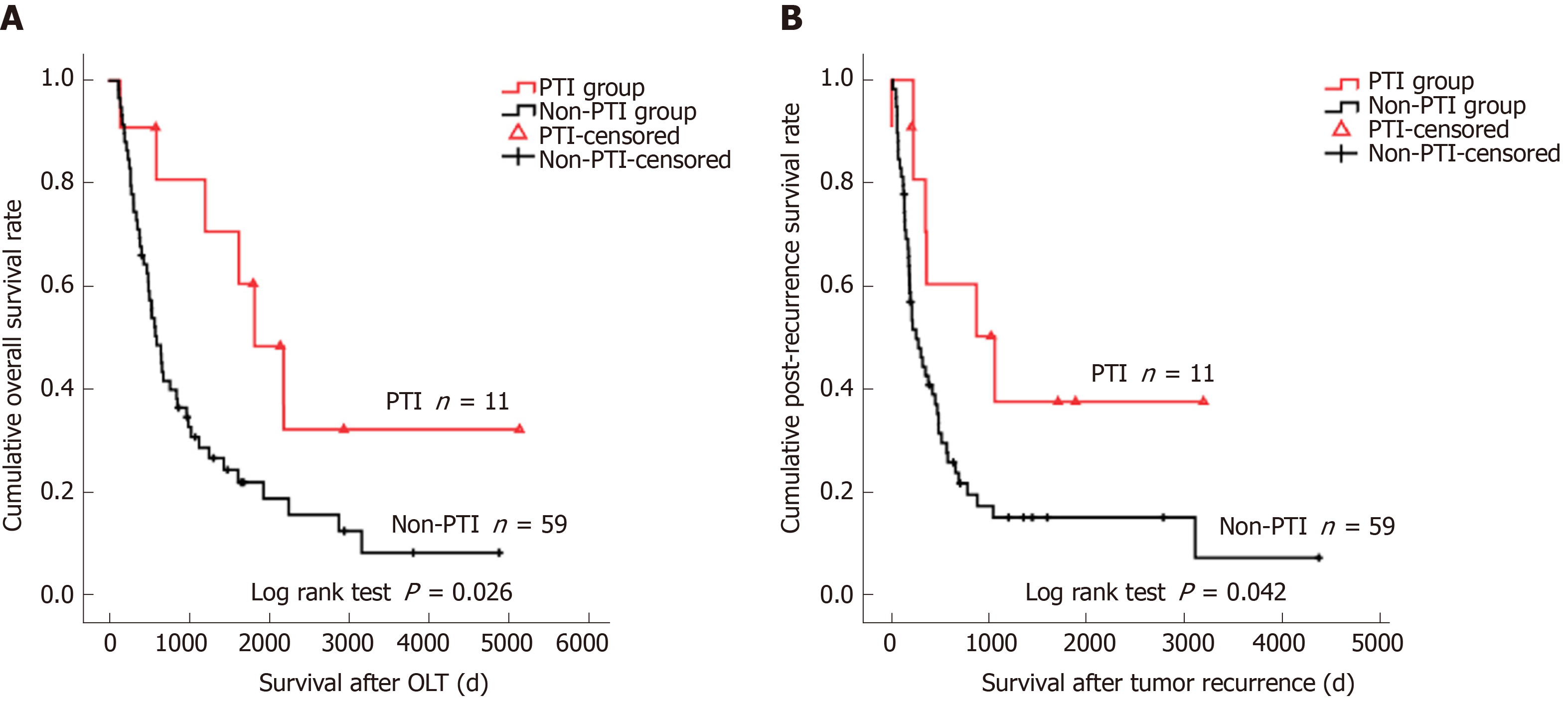
The 1-, 3-, and 5-year OS and RFS rates of the whole cohort were 86.6%, 69.0%, and 63.6%, and 75.7%, 60.0%, and 57.3%, respectively. The 1-, 3-, and 5-year OS rates for the PTI patient group (96.0%, 89.3%, and 74.0%) were significantly higher than those for the non-PTI group (84.0%, 63.4%, and 60.2%) (P = 0.033). The absence of PTI was an independent risk factor for dismal OS (relative risk [RR] = 2.584, 95%CI: 1.226-5.449) and unfavorable RFS (RR = 2.683, 95%CI: 1.335-5.390). Subgroup analyses revealed that PTI remarkably improved OS (P = 0.003) and RFS (P = 0.003) rates of HCC patients with vascular invasion (IV), but did not impact on OS (P = 0.404) and RFS (P = 0.304) of patients without VI. Among the patients who suffered post-transplant tumor recurrence, patients with PTI showed significantly better OS (P = 0.026) and PRS (P = 0.042) rates than those without PTI.
PTI improves OS and RFS of the transplant HCC patients at a high risk for post-transplant death and tumor recurrence, which is attributed to suppressive effect of PTI on HCC recurrence.
Core tip: Post-transplant infection (PTI) is routinely deemed as a harmful event for liver transplant recipients from a short-term perspective. Nevertheless, the present study demonstrated that PTI prolonged overall and recurrence-free survival of the patients with hepatocellular carcinoma (HCC) at a high risk for post-transplant death and tumor recurrence, which may be attributed to the persistent tumor suppressive effect of PTI on HCC recurrence after orthotopic liver transplantation (OLT). Further prospective multicenter investigations and experimental studies regarding the mechanism by which PTI inhibits HCC progression may contribute to future successful prophylaxis of HCC recurrence after OLT.
- Citation: Chao JS, Zhao SL, Ou-yang SW, Qian YB, Liu AQ, Tang HM, Zhong L, Peng ZH, Xu JM, Sun HC. Post-transplant infection improves outcome of hepatocellular carcinoma patients after orthotopic liver transplantation. World J Gastroenterol 2019; 25(37): 5630-5640
- URL: https://www.wjgnet.com/1007-9327/full/v25/i37/5630.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i37.5630
Orthotopic liver transplantation (OLT) is a potentially curative treatment option for selected patients with early-stage hepatocellular carcinoma (HCC)[1]. Albeit careful pre-transplant selection, cancer recurrence after OLT inevitably oppressed a significant portion of HCC patients, and finally led to treatment failure[2]. Recent evidence demonstrated that post-transplant HCC recurrence could be accurately prognosticated by comprehensive evaluation of the explanted liver and tumor biomarkers[3-6]. Unfortunately, up to date, very few factors or prophylactic measures have shown impact on overcoming HCC recurrence and improving patient survival after OLT.
More than a century ago, Dr. William Coley reported that artificial infection of bacterial toxins could elicit significant remission and even cure the inoperable soft tissue sarcomas[7]. Coley’s fascinating findings gave rise to the notion that the host immune response against bacterial infection might suppress malignant tumors, thus improving patient survival[8]. Later study documented an improvement regarding the five-year survival rate in patients who developed empyema after surgical resection for lung cancer in comparison to those without infection[9]. More recent evidence confirmed a significant survival advantage in patients with malignant glioma conferred by postoperative infection[10]. Nevertheless, the exact impact of postoperative infection on survival of HCC patients is still unknown.
Sustained immunosuppressive therapy, poor nutritional status, imbalanced metabolic conditions, and severe surgical trauma render liver transplant recipients extremely vulnerable to pathogens. As such, infection is one of the most common post-transplant complications for the recipients[11-13]. Post-transplant infection (PTI) is deemed as a harmful event from a short-term perspective, because morbidity and even mortality caused by PTI remain a problem for the recipients[12-14]. However, from a long-term perspective, the exact influence of PTI on the survival and tumor recurrence of transplant HCC patients has not been evaluated.
The data of the patients with pathologically diagnosed HCC who underwent OLT at Shanghai General Hospital between August 2002 and July 2016 were collected from China Liver Transplant Registry (https://http://www.cltr.org/) and retrospectively analyzed. Experienced pathologists provided detailed pathological diagnosis and staging of the tumors according to AJCC Cancer Staging Manual sixth edition[15]. Infection-related information, including general and site-specific clinical indices of PTI (Supplementary Table 1), was extracted from the chart record and/or electronic medical record system of our hospital. Definitions of infection regarding the six most frequent causes of PTI (pneumonia, intra-abdominal infection, urinary tract infection, surgical wound infection, intravascular catheter-related infection, and bloodstream infection) were briefly explained in the Attachment 1 and described in detail in the previous literature[16]. Conference was held to reach consensus on the diagnosis of PTI for each patient by a panel of experts including the physicians of intensive care unit (ICU), surgeons, and radiologists. In the present study, we chose to evaluate PTI that occurred within 30 days following OLT mostly during ICU stay, because the recipients had the highest incidence of PTI during this period and the records of patient data were more reliable in the ICU settings[13,17,18].
Chest computed tomography (CT), abdominal lipiodol CT with three-dimensional angiography, and enhanced magnetic resonance imaging (MRI) were routinely performed for diagnosis and assessment of liver cirrhosis and tumor extent. High suspicion of distant tumor metastasis and major vascular invasion explored by imaging were contraindications for OLT. Patients with preoperative imaging diagnosis of liver tumor meeting the Milan criteria[19] (from August 2002 to November 2006), University of California, San Francisco (UCSF) criteria[20] (from December 2006 to May 2008), or Hangzhou Criteria[21] (from June 2008 to date) were included in the waiting list of OLT in our center. Pre-transplant regional therapies employed in our center included trans-catheter arterial chemoembolization, radiofrequency /microwave ablation, and liver resection. Patients with tumors down-staged by regional therapies and fulfilled the selection criteria were also subjected to OLT. Liver transplants at Shanghai General Hospital are performed using standard techniques without the use of venovenous bypass. All patients were admitted to the transplant ICU immediately after OLT.
Cefoperazone/sulbactam (3.0 g intravenous infusion, 2 to 3 doses per day) was used as antibacterial prophylaxis. Caspofungin or micafungin was used as antifungal prophylaxis when indicated by the protocol of our center (Attachment number 2). Ganciclovir or oral valganciclovir was used within three months after OLT as anti-cytomegalovirus (CMV) prophylaxis when proposed by our protocol (Attachment number 3).
Our institution’s postoperative immunosuppression regimen includes induction with basiliximab and a tapered dose of corticosteroids, and maintenance with combination or separate use of corticosteroids, calcineurin inhibitor (cyclosporine or tacrolimus), sirolimus, and mycophenolate mofetil. After discharge, the patients were followed at the outpatient clinic according to the standard protocol of our center as we described previously[5]. Briefly, tumor recurrence was monitored by measurements of plasma AFP and/or abnormal prothrombin and abdominal ultrasonography (every month the first half year, then every 3 months), lipiodol CT (every 3 months for the first year, every 6 months for the second year, and once per year thereafter). MRI, positron emission tomography, or radioisotope bone scan was taken when necessary. No prophylactic measures were taken to prevent tumor relapse after OLT. For the diagnosis of tumor recurrence, a consensus was reached by a panel of experts including the surgeons and radiologists. Treatment of recurrent HCC included trans-catheter arterial chemoembolization, radiofrequency/microwave ablation, systemic chemotherapy, surgical resection, and use of sorafenib. Recurrence-free survival (RFS) was defined as the period of patient survival without any evidence of tumor recurrence from the day of OLT, and detection of tumor relapse after OLT or death without tumor recurrence after OLT was considered as an event. Overall survival (OS) was defined as the period from the day of surgery till patient death, and death after OLT regardless of the cause was considered as an event. Post-recurrence survival (PRS) was calculated from the day when tumor relapse was diagnosed till the day when patient died, and death after tumor recurrence regardless of the cause was regarded as an event. Loss to follow-up or follow-up interruption at the end of the observation was treated as a censoring event.
SPSS software program (version 17; IBM Corporation, Armonk, NY, United States) was used to analyze the data. For continuous variables, data are expressed as medians in the interquartile range (IQR) and were compared using the Mann-Whitney U test. Categorical variables were assessed by the Chi-square test or Fisher’s exact test. Univariate analyses of OS, RFS, and PRS as well as survival curve analysis were performed by the Kaplan-Meier method, and the differences were compared using the log-rank test. The variables with a P-value < 0.1 in univariate analyses were included in the multivariate survival analysis by using a Cox proportional-hazards model. Statistical significance was established when the P-values were < 0.05.
A total of 238 patients with pathologically confirmed HCC who survived more than 30 days after OLT at our center between August 2002 and July 2016 were included in this study. Among these, 53 (22.3%) patients were identified to have PTI and included in the PTI group, and the remaining 185 (77.7%) patients were included in the non-PTI group. Baseline clinicopathological features of the two patient groups were compared and revealed no significant differences (Table 1).
| Clinicopathological parameter | Total (n = 238) | PTI group (n = 53) | Non-PTI group (n = 185) | P-value |
| Age, median (range), yr | 49.5 (43, 55) | 49 (43.5, 55.5) | 50 (44, 55) | 0.962 |
| Sex (male/female) | 209/29 | 44/9 | 165/20 | 0.226 |
| Liver cirrhosis (yes/no) | 214/24 | 46/7 | 168/17 | 0.392 |
| Pre-operative AFP level (≥ 200 ng/mL/< 200 ng/mL)1 | 96/137 | 20/31 | 76/106 | 0.744 |
| Child-Pugh Score (A/B/C) | 128/84/26 | 26/18/9 | 102/66/17 | 0.272 |
| HBsAg (positive/negative) | 214/24 | 48/5 | 166/19 | 0.895 |
| HBeAg (positive/negative) | 68/170 | 15/38 | 53/132 | 0.961 |
| Tumor number (single/multiple) | 135/103 | 32/21 | 103/82 | 0.613 |
| Tumor size (≤ 5 cm/> 5 cm) | 169/69 | 41/12 | 128/57 | 0.248 |
| Histological grade (G1-G2/G3-G4)1 | 190/30 | 46/4 | 144/26 | 0.186 |
| Vascular invasion (yes/no)1 | 105/92 | 26/22 | 79/70 | 0.890 |
| Lymph node involvement (yes/no) | 10/228 | 2/51 | 8/177 | 1.000 |
| Pathological TNM stage (I-II/III) | 197/41 | 43/10 | 154/31 | 0.720 |
Fifty-three patients had PTI, and the median duration of PTI was 12.0 days (IQR: 7.0 to 21.75 days). The common infection sites included the lungs, abdomen, urinary tract, intravascular catheter, incision, and bloodstream (Supplementary Table 2). The infectious microorganisms included bacterial (Klebsiella pneumoniae, Acinetobacter baumannii, enterococcus faecalis, stenotrophomonas maltophilia, etc.), fungal (Candida albicans, Candida prapsilosis, Aspergillus, etc.) and viral (cytomegalovirus) pathogens. Microbiological cultures were performed for clinical samples including sputum, drainage fluid, blood, catheter segment/tip, mid-stream urine, etc. At least one kind of pathogen was found by microbiological culture in 45 (84.9%) patients (Supplementary Table 3). Microbiological cultures were found negative in 8 (15.1%) patients, among whom 3 were diagnosed with cytomegalovirus infection (Supplementary Table 3). PTI was predominated by bacterial infections followed by fungal and viral infections.
To minimize interference, two patients in the PTI group who died of severe pneumonia at 35 and 55 days after OLT and two patients in the non-PTI group who died of surgical complication at 45 and 50 days after OLT were excluded from survival analyses. The median follow-up was 1117 days (IQR: 439.75 to 2309 days), 70 (29.9%) patients had tumor recurrence, and the median tumor recurrence time was 300.5 days (IQR: 138.25 to 633 days) after OLT. The 1-, 3-, and 5-year OS rates of the study cohort were 86.6%, 69.0%, and 63.6%, and the 1-, 3-, and 5-year RFS rates were 75.7%, 60.0%, and 57.3%, respectively. Frequent tumor recurrence sites were the liver, lungs, bone, brain, and adrenal glands. Among the 80 patients died after OLT, 54 (67.5%) died of tumor recurrence. The 1-, 3-, and 5-year OS rates for the PTI patient group (96.0%, 89.3%, and 74.0%) were significantly higher than those for the non-PTI group (84.0%, 63.4%, and 60.2%, P = 0.033) (Figure 1). Univariate analyses revealed that age (P = 0.093), cirrhotic background (P = 0.004), preoperative alpha fetoprotein (AFP) level (P = 0.002), tumor size (P < 0.001), histological grade (P < 0.001), vascular invasion (VI) (P < 0.001), lymph node involvement (P < 0.001), pathological TNM stage (P < 0.001), and PTI (P = 0.033) were the factors associated with OS, while cirrhotic background (P = 0.020), preoperative AFP level (P = 0.003), tumor size (P < 0.001), histological grade (P < 0.001), VI (P < 0.001), lymph node involvement (P < 0.001), pathological TNM stage (P < 0.001), and PTI (P = 0.063) were the factors associated with RFS (Table 2). Multivariate analysis identified high histological grade (G3-G4) [relative risk (RR) = 1.977, 95% confidential interval (CI): 1.073-3.642 for OS; RR = 1.964, 95%CI: 1.083-3.561 for RFS], VI (RR = 4.237, 95%CI: 2.293-7.827 for OS; RR = 3.702, 95%CI: 1.959-6.999 for RFS), and absence of PTI (RR = 2.584, 95%CI: 1.226-5.449 for OS; RR = 2.683, 95%CI: 1.335-5.390 for RFS) were the independent risk factors for dismal OS and RFS (Table 3). In subgroup analysis, patients with PTI showed significantly better OS than those without PTI in the VI (P = 0.003) subgroup (Figure 2A), whereas PTI did not impact the OS of patients without VI (P = 0.404) (Figure 2B). These findings highlighted that PTI conferred significant survival benefit on patients at a high risk factor for post-OLT death. Furthermore, patients with PTI showed significantly better RFS than those without PTI in the VI (P = 0.003) subgroup (Figure 2C). However, PTI showed no influence on the RFS of the patients without VI (P = 0.251) (Figure 2D). These results indicated that PTI improved the RFS of the patients at a high risk factor for tumor recurrence. Moreover, we evaluated the potential benefit of PTI on the OS and PRS of the patients who suffered post-transplant tumor relapse. As a result, the patients with PTI revealed significantly better OS (P = 0.026) and PRS (P = 0.042) than those without PTI (Figure 3). These data suggested that PTI holds persistent survival benefit on the transplant HCC patients even after tumor relapse.
| Variable | Overall survival | Recurrence-free survival | ||
| χ2 | P-value | χ2 | P-value | |
| Age (≤ 50 vs > 50) | 2.828 | 0.093 | 1.682 | 0.195 |
| Sex (male vs female) | 0.011a | 0.916 | -0.020 | 0.888 |
| Liver cirrhosis (no vs yes) | 8.361 | 0.004b | 5.429 | 0.020a |
| Pre-operative AFP level (≥ 200 ng/mL vs < 200 ng/mL) | 9.938 | 0.002b | 8.879 | 0.003b |
| Child-Pugh score (A/B/C) | 1.609 | 0.447 | 1.020 | 0.600 |
| HBsAg (positive vs negative) | 0.512 | 0.474 | 0.349 | 0.555 |
| HBeAg (positive vs negative) | 0.253 | 0.615 | 0.412 | 0.521 |
| Tumor number (multiple vs single)1 | 0.800 | 0.371 | 2.027 | 0.155 |
| Tumor size (> 5 cm vs ≤ 5 cm)1 | 37.554 | < 0.001c | 45.365 | < 0.001c |
| Histological grade (G3-G4 vs G1-G2)1 | 12.165 | < 0.001c | 14.790 | < 0.001c |
| Vascular invasion (yes vs no)1 | 32.767 | < 0.001c | 45.440 | < 0.001c |
| Lymphnode involvement (yes vs no)1 | 12.750 | < 0.001c | 25.258 | < 0.001c |
| Pathological TNM stage (III vs I/II)1 | 17.938 | < 0.001c | 30.984 | < 0.001c |
| PTI (without vs with) | 4.554 | 0.033a | 3.463 | 0.063 |
Infectious complication is commonly recognized as a negative event for the recipients from short-term perspective[12,13]. However, medical progress in the past decades has made PTI less fatal than ever before. In our cohort, 51 (96.2%) out of 53 recipients survived PTI. Interestingly, from long-term perspective, we demonstrated that the OS of the PTI group was significantly better than that of the non-PTI group after OLT (P = 0.033, Figure 1), and that absence of PTI was an independent risk factor (RR = 2.584, 95%CI: 1.226-5.449, Table 3) for dismal OS, indicating that PTI benefits OS of transplant HCC patients. In the present study, recurrent HCC accounted for 67.5% deaths, supporting the notion that tumor recurrence was the major stumbling block for long-term survival of HCC patients treated by OLT[22]. We therefore hypothesized that the benefit of PTI on OS of the patients mainly arose due to its inhibitory effect on post-transplant tumor relapse, which was supported by the findings that patients without PTI showed a significantly higher RR of 2.683 (95%CI: 1.335-5.390) for post-transplant tumor recurrence than those with PTI (Table 3). Subgroup analyses revealed that PTI remarkably improved OS and RFS of HCC patients with VI, a high risk factor for post-OLT death (RR = 4.237, 95%CI: 2.293-7.827, Table 3) and tumor recurrence (RR = 3.702, 95%CI: 1.959-6.999, Table 3), whereas PTI did not impact on the OS or RFS of those without VI. It can be speculated that PTI may exert potent suppressive effects on the residual circulating HCC cells after OLT in patients with VI, thus suppressing HCC recurrence and prolonging post-transplant survival. In patients without VI, excellent long-term survival free from tumor recurrence could be achieved by surgical procedure alone, because OLT offers the chance of complete removal of tumor cells. The insignificant impact of PTI on RFS of the whole cohort in univariate analysis (P = 0.063, Table 2) should be attributed to the insignificant impact of PTI on RFS of the subgroup without VI.
Clinical studies have demonstrated controversial results regarding the relationship between postoperative infection and prognosis in patients with different types of malignancies. Ruckdeschel et al[9] have documented improved survival rates in patients who developed empyema after surgical resection for lung carcinoma. The survival benefit of intrapleural infection was principally found in cancer patients limited to the lung and its drainage of lymph nodes, and the protection from recurrent cancer conferred on these patients by postoperative empyema may have been mediated by the activation of regional cellular immune mechanisms[9]. On the contrary, a recent report has demonstrated a negative impact of postoperative intra-abdominal infection on disease-free and disease-specific survival in patients with stage II colon cancer[23]. These findings suggested that the influence of postoperative infection on tumor recurrence and patient survival might be dependent on the tumor type. Intriguingly, there were inconsistent findings in the previous literature regarding the relationship between postoperative infection and the survival of patients with glioblastoma[10,24]. After comparing 17 glioblastoma patients who suffered postoperative infection with 51 matched patients without infection, Bohman et al[24] failed to find a surmised correlation between postoperative infection and prolonged lifetime, although subgroup analysis of patients with deep infection showed a longer survival trend. In another study, Bonis et al[10] analyzed 197 glioblastoma patients treated by surgical resection, among whom 10 experienced postoperative infection, and 8 had a deep infection. However, the results showed a significant association between postoperative infection and prolonged survival. The discrepancy between these two studies may be attributed to differential diagnostic criteria for postoperative infection, because infection occurred less frequently and more severely in the later study. Thus, it can be deduced that probably only serious infection exerted potent anti-tumor effects and improved patient outcomes. In the present study, PTI was diagnosed when the patients showed septic symptoms, suggesting that the infection was relatively severe. The rationale may reside in whether the severity of microbial infection reaches the level or degree to stimulate systematic immune response to destroy the residual tumor cells. In our cohort, the infection sites of 53 patients with PTI included the lungs, abdomen, urinary tract, intravascular catheter, incision, and bloodstream (Supplementary Table 2). Considering that the origination (infection sits) of PTI is distant from the primary tumor location (the liver) and different from the post-transplant tumor recurrence sites, we speculated that the tumor suppressive function of PTI might be mediated by activating the whole body immune system, not by regional immune mechanism. Our data demonstrated that the pathogens of PTI were predominated by bacteria, followed by fungi and virus (Supplementary Table 3), and PTI improved OS and RFS of HCC patients with VI regardless of the type of pathogen. It can be deduced that both virus and bacteria could stimulate the immune system and then amplify the desired anti-tumor responses, clearing distant tumor cells and preventing recurrence of the cancer[25]. Nevertheless, due to the relative small sample size of the PTI group, it is difficult to subdivide the patients according to the infection site or the pathogen type and ascertain their respective prognostic influence. Further multicentre studies (to increase the number of patients) are needed to clarify whether infection site or pathogen type differentially impacts on post-transplant patient prognosis. Our study further showed that the impacts of PTI lasted even after tumor recurrence, because the PRS of the patients with PTI was significantly improved. These findings suggested a sustained anti-cancer effect of PTI on the patients, which could not be explained by simple reasons, e.g., high fever and transient reduction or withdrawal of immunosuppressant during the PTI period. We speculate that PTI eliminates the residual HCC cells probably via early reactions of innate immunity and later responses of adaptive immunity that has characteristics of specific killing ability and immunological memory even when tumor recurrence occurs. Further investigation to elucidate the underlying mechanisms is urgently needed.
To date, the clinical management of tumor recurrence in transplant HCC patients remains challenging and is associated with a poor prognosis independent of the type of treatment[26]. In fact, the innate chemoresistant property of HCC renders the prevention and treatment of post-transplant HCC recurrence with drugs in vain in the current clinical practice[27]. In this context, albeit the retrospective nature of this study, our results hopefully provided new insights into improving outcome of transplant HCC patients, especially those at a high risk for post-OLT death and tumor recurrence. Over a century ago, Dr Coley and others demonstrated successful treatment for malignant tumors by administration of killed bacterial vaccines[7,8]. Thanks to the ever-developing biotechnology, the development of bioengineered organisms towards specific therapeutic agents that target tumor cells via activation of immune system may present an attractive strategy to suppress HCC progression and prolong the patient survival after OLT with minimum side effects in the future. Nevertheless, further validity of PTI as a potent tumor suppressor factor for human HCC is critically needed in prospective cohorts at other transplant centers, as well as in well-designed experimental animal models.
In conclusion, the present study provided a novel conception that PTI was not simply a harmful event for liver transplant recipients, but a factor that could exert significant OS and RFS benefits for the HCC recipients at a high risk for post-OLT death and tumor recurrence, which may be attributed to the tumor suppressive effect conferred by PTI. Moreover, improved OS and PRS in the patients who suffered HCC recurrence with PTI compared with those without PTI suggested persistent tumor suppressive effect of PTI even after tumor relapse. However, before moving forward, our data need to be verified prospectively in larger cohorts and the mechanism by which PTI inhibits HCC progression should be investigated in well-designed studies. Our findings may stimulate successful management of post-transplant HCC recurrence in the future.
Tumor recurrence after orthotopic liver transplantation (OLT) is the stumbling block for long-term survival of the recipients with hepatocellular carcinoma (HCC). Identification of factors or measurements that have influence on overcoming HCC recurrence after OLT is of particular importance to innovate effective treatment strategy. Previous literature demonstrated that postoperative infection suppresses tumor recurrence and improves patient survival in lung cancer and malignant glioma probably via stimulating the immune system. Post-transplant infection (PTI), a common complication after OLT, is deemed to be harmful for the liver transplant recipients from a short-term perspective. Nevertheless, whether PTI inhibits HCC recurrence and prolongs the long-term survival of transplant HCC patients needs to be clarified.
The management of tumor recurrence in transplant HCC patients remains challenging. The results of the present study indicated new insights regarding how to improve outcome of transplant HCC patients, especially those at a high risk for post-OLT death and tumor recurrence.
To investigate the potential influence of PTI on the survival and tumor recurrence of patients with HCC after OLT.
A total of 238 patients with HCC who underwent OLT between August 2002 and July 2016 at our center were retrospectively included and accordingly subdivided into a PTI group (53 patients) and a non-PTI group (185 patients). Univariate analyses, including the differences of overall survival (OS), recurrence-free survival (RFS), and post-recurrence survival (PRS), between the PTI and non-PTI subgroups as well as survival curve analysis were performed by the Kaplan-Meier method, and the differences were compared using the log rank test. The variables with a P-value < 0.1 in univariate analyses were included in the multivariate survival analysis by using a Cox proportional-hazards model.
The 1-, 3-, and 5-year OS and RFS rates of the whole cohort were 86.6%, 69.0%, and 63.6%, and 75.7%, 60.0%, and 57.3%, respectively. The 1-, 3-, and 5-year OS rates for the PTI patient group (96.0%, 89.3%, and 74.0%) were significantly higher than those for the non-PTI group (84.0%, 63.4%, and 60.2%) (P = 0.033). Absence of PTI was an independent risk factor for dismal OS (RR = 2.584, 95%CI: 1.226-5.449) and unfavorable RFS (RR = 2.683, 95%CI: 1.335-5.390). Subgroup analyses revealed that PTI remarkably improved OS (P = 0.003) and RFS (P = 0.003) rates of HCC patients with vascular invasion (IV), but did not impact on OS (P = 0.404) and RFS (P = 0.304) of patients without VI. Among the patients who suffered post-transplant tumor recurrence, the patients with PTI showed significantly better OS (P = 0.026) and PRS (P = 0.042) rates than those without PTI.
PTI improves OS and RFS of transplant HCC patients at a high risk for post-transplant death and tumor recurrence, which may be attributed to suppressive effect of PTI on HCC recurrence.
Future development of bioengineered organisms that could potently activate the innate and adaptive immune system targeting tumor cells may present an attractive strategy to suppress HCC progression and prolong the patient survival after OLT with minimum side effects. Nevertheless, further validity of PTI as a potent tumor suppressor for HCC patients is critically needed in prospective cohorts at other transplant centers, as well as in well-designed experimental animal models.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Bekheit M, Chiu KW, Delladetsima I, Grassi A, Isaji S, Mihaila RG S-Editor: Yan JP L-Editor: Wang TQ E-Editor: Ma YJ
| 1. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2800] [Cited by in F6Publishing: 3438] [Article Influence: 573.0] [Reference Citation Analysis (3)] |
| 2. | Mehta N, Heimbach J, Harnois DM, Sapisochin G, Dodge JL, Lee D, Burns JM, Sanchez W, Greig PD, Grant DR, Roberts JP, Yao FY. Validation of a Risk Estimation of Tumor Recurrence After Transplant (RETREAT) Score for Hepatocellular Carcinoma Recurrence After Liver Transplant. JAMA Oncol. 2017;3:493-500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 218] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 3. | Agopian VG, Harlander-Locke M, Zarrinpar A, Kaldas FM, Farmer DG, Yersiz H, Finn RS, Tong M, Hiatt JR, Busuttil RW. A novel prognostic nomogram accurately predicts hepatocellular carcinoma recurrence after liver transplantation: Analysis of 865 consecutive liver transplant recipients. J Am Coll Surg. 2015;220:416-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 180] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 4. | Mazzaferro V, Sposito C, Zhou J, Pinna AD, De Carlis L, Fan J, Cescon M, Di Sandro S, Yi-Feng H, Lauterio A, Bongini M, Cucchetti A. Metroticket 2.0 Model for Analysis of Competing Risks of Death After Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology. 2018;154:128-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 301] [Cited by in F6Publishing: 381] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 5. | Sun H, Teng M, Liu J, Jin D, Wu J, Yan D, Fan J, Qin X, Tang H, Peng Z. FOXM1 expression predicts the prognosis in hepatocellular carcinoma patients after orthotopic liver transplantation combined with the Milan criteria. Cancer Lett. 2011;306:214-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Sun H, Tang H, Xie D, Jia Z, Ma Z, Wei D, Mishra L, Gao Y, Zheng S, Xie K, Peng Z. Krüppel-like Factor 4 Blocks Hepatocellular Carcinoma Dedifferentiation and Progression through Activation of Hepatocyte Nuclear Factor-6. Clin Cancer Res. 2016;22:502-512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Coley WB. The Treatment of Inoperable Sarcoma by Bacterial Toxins (the Mixed Toxins of the Streptococcus erysipelas and the Bacillus prodigiosus). Proc R Soc Med. 1910;3:1-48. [PubMed] [Cited in This Article: ] |
| 8. | DeWeerdt S. Bacteriology: a caring culture. Nature. 2013;504:S4-S5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Ruckdeschel JC, Codish SD, Stranahan A, McKneally MF. Postoperative empyema improves survival in lung cancer. Documentation and analysis of a natural experiment. N Engl J Med. 1972;287:1013-1017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 153] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | De Bonis P, Albanese A, Lofrese G, de Waure C, Mangiola A, Pettorini BL, Pompucci A, Balducci M, Fiorentino A, Lauriola L, Anile C, Maira G. Postoperative infection may influence survival in patients with glioblastoma: Simply a myth? Neurosurgery. 2011;69:864-8; discussion 868-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Green M. Introduction: Infections in solid organ transplantation. Am J. Transplant. 2013;13 Suppl 4:3-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 12. | Kim SI. Bacterial infection after liver transplantation. World J Gastroenterol. 2014;20:6211-6220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 103] [Cited by in F6Publishing: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 13. | Righi E. Management of bacterial and fungal infections in end stage liver disease and liver transplantation: Current options and future directions. World J Gastroenterol. 2018;24:4311-4329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 30] [Cited by in F6Publishing: 27] [Article Influence: 4.5] [Reference Citation Analysis (2)] |
| 14. | Martin-Gandul C, Mueller NJ, Pascual M, Manuel O. The Impact of Infection on Chronic Allograft Dysfunction and Allograft Survival After Solid Organ Transplantation. Am J Transplant. 2015;15:3024-3040. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 110] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 15. | AJCC; Greene FL PD, Fleming ID, Fritz AG, Balch CM, Haller DG. Liver. Greene FL PD, Fleming ID, Fritz AG, Balch CM, Haller DG. 2002;131-136. [Cited in This Article: ] |
| 16. | Calandra T, Cohen J; International Sepsis Forum Definition of Infection in the ICU Consensus Conference. The international sepsis forum consensus conference on definitions of infection in the intensive care unit. Crit Care Med. 2005;33:1538-1548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 554] [Cited by in F6Publishing: 569] [Article Influence: 29.9] [Reference Citation Analysis (1)] |
| 17. | Kawecki D, Pacholczyk M, Lagiewska B, Sawicka-Grzelak A, Durlik M, Mlynarczyk G, Chmura A. Bacterial and fungal infections in the early post-transplantation period after liver transplantation: Etiologic agents and their susceptibility. Transplant Proc. 2014;46:2777-2781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Laici C, Gamberini L, Bardi T, Siniscalchi A, Reggiani MLB, Faenza S. Early infections in the intensive care unit after liver transplantation-etiology and risk factors: A single-center experience. Transpl Infect Dis. 2018;20:e12834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5110] [Cited by in F6Publishing: 5017] [Article Influence: 179.2] [Reference Citation Analysis (0)] |
| 20. | Yao FY, Ferrell L, Bass NM, Bacchetti P, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: comparison of the proposed UCSF criteria with the Milan criteria and the Pittsburgh modified TNM criteria. Liver Transpl. 2002;8:765-774. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 319] [Cited by in F6Publishing: 341] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 21. | Zheng SS, Xu X, Wu J, Chen J, Wang WL, Zhang M, Liang TB, Wu LM. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation. 2008;85:1726-1732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 321] [Cited by in F6Publishing: 368] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 22. | Mazzola A, Costantino A, Petta S, Bartolotta TV, Raineri M, Sacco R, Brancatelli G, Cammà C, Cabibbo G. Recurrence of hepatocellular carcinoma after liver transplantation: An update. Future Oncol. 2015;11:2923-2936. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Sánchez-Velázquez P, Pera M, Jiménez-Toscano M, Mayol X, Rogés X, Lorente L, Iglesias M, Gallén M. Postoperative intra-abdominal infection is an independent prognostic factor of disease-free survival and disease-specific survival in patients with stage II colon cancer. Clin Transl Oncol. 2018;20:1321-1328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Bohman LE, Gallardo J, Hankinson TC, Waziri AE, Mandigo CE, McKhann GM 2nd, Sisti MB, Canoll P, Bruce JN. The survival impact of postoperative infection in patients with glioblastoma multiforme. Neurosurgery. 2009;64:828-34; discussion 834-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Forbes NS, Coffin RS, Deng L, Evgin L, Fiering S, Giacalone M, Gravekamp C, Gulley JL, Gunn H, Hoffman RM, Kaur B, Liu K, Lyerly HK, Marciscano AE, Moradian E, Ruppel S, Saltzman DA, Tattersall PJ, Thorne S, Vile RG, Zhang HH, Zhou S, McFadden G. White paper on microbial anti-cancer therapy and prevention. J Immunother Cancer. 2018;6:78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 26. | de'Angelis N, Landi F, Carra MC, Azoulay D. Managements of recurrent hepatocellular carcinoma after liver transplantation: A systematic review. World J Gastroenterol. 2015;21:11185-11198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 111] [Cited by in F6Publishing: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 27. | Lee JO, Kim DY, Lim JH, Seo MD, Yi HG, Oh DY, Im SA, Kim TY, Bang YJ. Palliative chemotherapy for patients with recurrent hepatocellular carcinoma after liver transplantation. J Gastroenterol Hepatol. 2009;24:800-805. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |