Published online Jan 15, 2003. doi: 10.3748/wjg.v9.i1.165
Revised: June 23, 2002
Accepted: July 3, 2002
Published online: January 15, 2003
AIM: To investigate the effects of oxytocin (OT) on isolated rabbit proximal colon and its mechanism.
METHODS: Both longitudinal muscle (LM) and circular muscle (CM) were suspended in a tissue chamber containing 5 mL Krebs solution (37 °C), bubbled continuously with 950 mL·L-1 O2 and 50 mL·L-1 CO2. Isometric spontaneous contractile responses to oxytocin or other drugs were recorded in circular and longitudinal muscle strips.
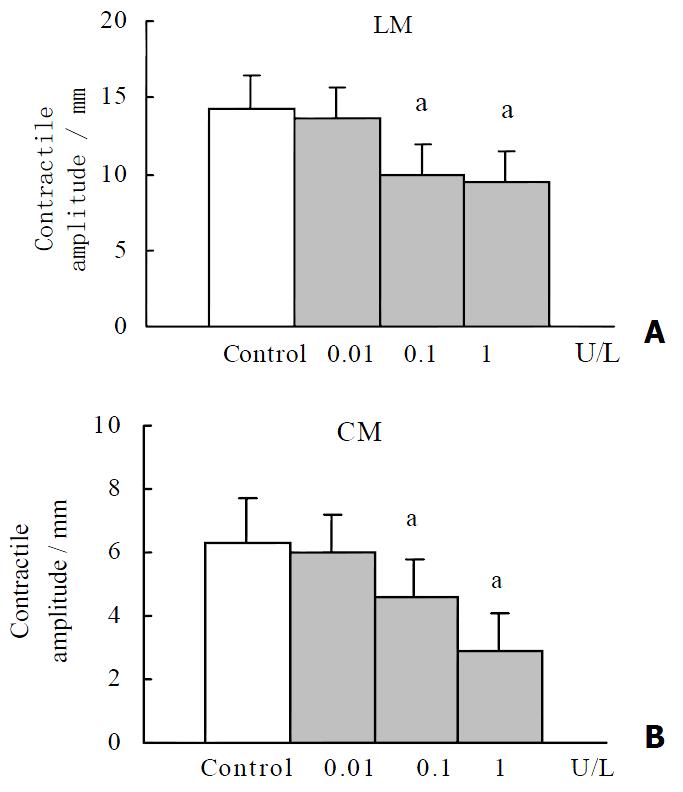
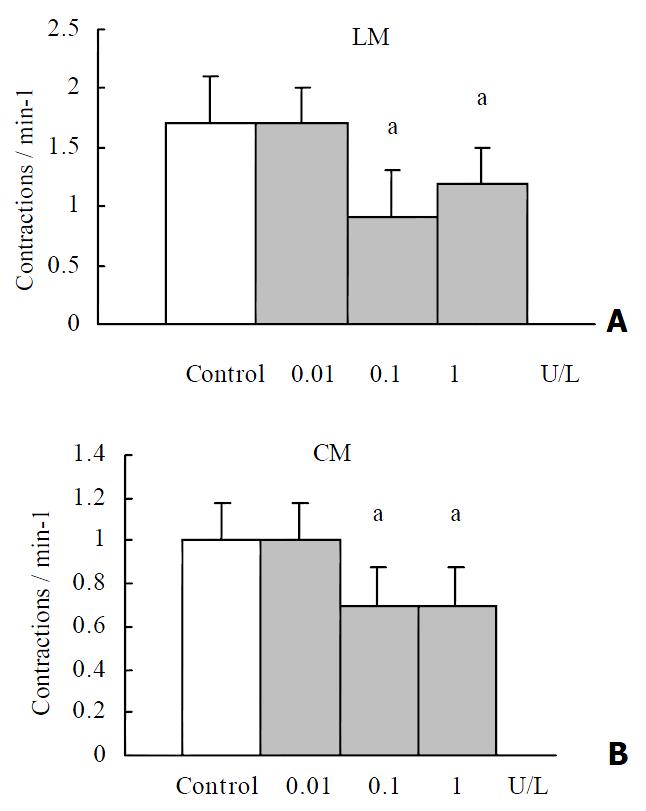
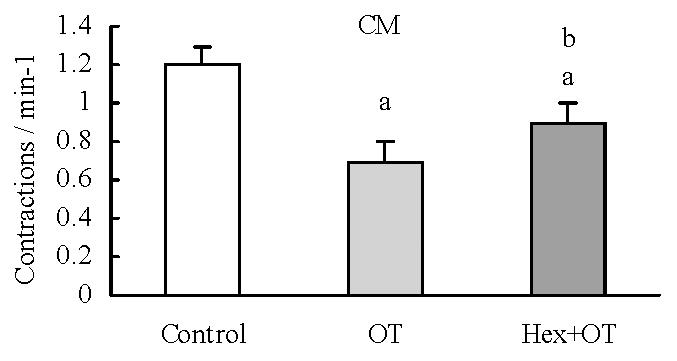
RESULTS: OT (0.1 U·L-1) failed to elicit significant effects on the contractile activity of proximal colonic smooth muscle strips (P > 0.05). OT (1 to 10 U·L-1) decreased the mean contractile amplitude and the contractile frequency of CM and LM. Hexamethonium (10 μmol·L-1) partly blocked the inhibition of oxytocin (1 U·L-1) on the contractile frenquency of CM. Nω-nitro-L-arginine-methylester (L-NAME, 1 μmol·L-1), progesterone (32 μmol·L-1) and estrogen (2.6 μmol·L-1) had no effects on OT-induced responses.
CONCLUSION: OT inhibits the motility of proximal colon in rabbits. The action is partly relevant with N receptor, but irrelevant with that of NO, progesterone or estrogen.
- Citation: Xie DP, Chen LB, Liu CY, Liu JZ, Liu KJ. Effect of oxytocin on contraction of rabbit proximal colon in vitro. World J Gastroenterol 2003; 9(1): 165-168
- URL: https://www.wjgnet.com/1007-9327/full/v9/i1/165.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i1.165
Oxytocin (OT) is a very abundant neuropeptide. The structure of the OT gene was elucidated in 1984[1], and the sequence of the OT receptor was reported in 1992[2]. OT exerted a wide spectrum of central and peripheral effects[3-5]. It was reported that hypothalamic paraventricular nucleus is a site of controlling gastric function[6], oxytocin facilitated the manifestation of inhibitory effects of hypothalamus on the motor function of gastrointestinal tract[7]. The experiments on rats had shown that gastric motility was inhibited by microinjection of oxytocin into the dorsal motor nucleus of the vagus (DMN), and that the inhibition of gastric motility after electrical stimulation of the hypothalamic paraventricular nucleus was blocked by microinjection of an oxytocin receptor antagonist directly into the DMN. These results suggested that oxytocin acted on the gastric motility via DMN[8]. The reports on peripheral action of oxytocin to influence gastrointestinal (GI) motility were controversial: OT decreases the contractions of the guinea pig stomach in vitro, and inhibits the tone and peristaltic contractions of stomach and small intestines in fasting dogs in vivo[9]; but increases the gastric emptying of semisolid food in normal human and the contractions of gastric smooth muscle strips in rats[10]. The effect of oxytocin on colonic motility is still unknown.
OTR is functionally coupled to GTP binding proteins, stimulates the activity of phospholipase C-β isoforms. Finally, a variety of cellular events are initiated. For example, the forming Ca2+-calmodulin complexes triggers the activation of neuronal and endothelial isoforms of nitric oxide (NO) synthase[11]. Steroid hormones were reported to control oxytocin receptor (OTR) activity via both genomic and nongenomic pathways[12]. Estrogen induces the OTR mRNA expression, and then increases the OTR density on the membrane of the uterus smooth muscle and central nervous system[13,14]; on the other hand, progesterone inhibits the nuclear OTR mRNA expression, and decreases the sensitivity of the target cell on OT stimulation[15,16]; progesterone was also reported to bind to OTR with high affinity and inhibit the receptor function[17]. In this study, we investigated the effect of OT on proximal colonic motility of rabbits; We also investigated if the OT-induced responses were relevant with NO, steroid hormones or N receptor.
Rabbits of both sexes, weighing 1.5-2 kg, were fasted for 24-hour and sacrificed. The proximal colon (1 cm from the cecocolonic junction) was removed. The segment of the colon was opened along the mesentery. Muscle strips (8 × 2 mm) were cut, parallel to either the circular or the longitudinal fibers, and named circular muscle (CM) and longitudinal muscle (LM). The mucosa on each strip was carefully removed.
The muscle strip was suspended in a tissue chamber containing 5 mL Krebs solution (37 °C) and bubbled continuously with 950 mL·L-1 O2 and 50 mL·L-1 CO2[18]. One end of the strip was fixed to a hook on the bottom of the chamber. The other end was connected to an external isometric force transducer (JZ-BK, BK). Motility of colonic strips (under an initial tension of 1 g) in 2 tissue chambers were simultaneously recorded on ink-writing recorders (LMS-ZB, Cheng-Du). After 1 h equilibration, OT (0.1, 1, 10 U·L-1) was added in the tissue chamber to observe their effects on proximal colon; Nω-nitro-L-arginine-methylester (L-NAME, 1 µmol·L-1), hexamethonium (10 µmol·L-1, progesterone (32 µmol·L-1) or estrogen (2.6 µmol·L-1), given 3 min before the administration of OT (1 U L-1), was added separately to investigate whether the actions of OT were relevant with NO, N receptor or steroids. The resting tension, the contractile frequency, and the mean contractile amplitude of LM and CM were measured.
The following agents were used: oxytocin (Biochemical Pharmaceutical Company, Shanghai, China), Nω-nitro-L-arginine-methylester (L-NAME) and hexamethonium (Sigma Chemical Company), progesterone and estrogen (The Ninth Pharmaceutical Factory in Shanghai).
The results were presented as -χ±s, and statistically analyzed by paired t test, P < 0.05 was considered to be significant.
OT (0.1 U·L-1) failed to elicit significant effects on the contractile activity of proximal colonic smooth muscle strips (P > 0.05). OT (1 to 10 U·L-1) decreased the mean contractile amplitude and the contractile frequency of CM and LM (Figure 1, Figure 2). It had no significant effects on the resting tension of CM and LM.
Hexamethonium (10 μmol·L-1) had no significant effect on the contractile activity of each colonic smooth muscle strip. Hexamethonium given 3 minute before administration of OT (1 U·L-1) decreased OT-induced inhibition on the contractile frenquency of CM (Figure 3). It had no significant effects on the other action of OT.
L-NAME (1 μmol·L-1) itself had no significant effects on proximal colon in rabbits. When given 3 min before the administration of OT (1 U·L-1), It had no significant effects on OT-induced responses.
Progesterone (32 μmol·L-1) and estrogen (2.6 μmol·L-1) had no significant effects on proximal colonic motility. When given 3 min before the administration of OT (1 U·L-1), neither progesterone nor estrogen had significant effects on OT-induced responses.
The present study revealed that oxytocin 1 U·L-1 to 10 U·L-1 inhibited the spontaneous contractile motility of proximal colonic smooth muscle in rabbits. Petring et al[10] reported that veneinjection of oxytocin could increase the gastric emptying of semisolid food in normal human. Oxytocin10-100 U·L-1 stimulated the spontaneous contractile motility of gastric body and gastric antrum in rats in vitro. But oxytocin (10 pmol·L-1-10 nmol·L-1) suppressed the spontaneous contractions of circular smooth muscle from guinea-pig gastric antrum and gastric emptying in male rats[19,20]. Therefore, it seems that there are species, region and OT concentration differences in OT-induced contractions in gastrointestinal tract.
Our results showed that hexamethonium partly blocked the decreasing action of oxytocin on the contractile frequency of colonic strips, but not that of the contractile amplitude of the strips. These results suggested that oxytocin partly inhibited the contractile frequency of colonic strips via ganglion N receptor.
NO is found to be an inhibitory neurotransmitter of enteric neurons. NOS neurons has been identified in the myenteric plexus[21-23]. The action of OT has been reported to relevent with NO[24] We observed the effect of L-NAME (inhibitor of NOS activity) on the inhibitory action of oxytocin to decide weather oxytocin inhibits the colonic contraction via NO synthetic pathway. The results showed that L-NAME had no effect on the inhibitory action of oxytocin, which suggested that the inhibitory action of oxytocin on colonic motility was irrelevant with NO.
OT has been identified to exert the actions via OT receptors in many tissues, including the hypothalamus, uterus, kidney, pancreas, heart, vasculature, and thymus[25-30]. Oxytocin receptor are suggested to be present on the members of guinea-pig antral smooth muscle cells[19]. OT might act on the proximal colonic smooth muscle via selective oxytocin receptor, which still need to be further studied.
The function and physiological regulation of the OT system has been reported to be strongly steroid dependent[31,32]. But our results showed that both progesterone and estrogen had no significant effects on OT-induced responses.
Effects on the gastrointestinal tract muscle during pregnancy are caused primarily by hormonal changes, such as progesterone, estrogen, and other hormones[33]. Motility changes occur throughout the gastrointestinal tract during pregnancy, including colonic transit manifested primarily as abdominal bloating and constipation[34]. Both progesterone and estrogen have been shown to inhibit colonic motility and transit in rats[35]. But Hinds and associates[36] reported that no significant differences in colon transit were found between phase of the menstrual cycle or between women and men. Our results also showed that neither progesterone nor estrogen affected the proximal colonic motility in rabbits. OT was another hormone relate to pregnancy, and its plasma concentration was higher in late pregnancy[37,38]. Our results showed that OT inhibited the colonic motility. Results from these studies provided new insight into the mechanisms controlling colonic motility during pregnancy and may form the basis for drug treatments of colonic motility disorders.
In conclusion, oxytocin inhibit the contractile activity of proximal colonic smooth muscle of rabbits in vitro. The action is partly relevant with N receptor, but irrelevant with that of NO, progesterone or estrogen.
Edited by Wu XN
| 1. | Ivell R, Richter D. Structure and comparison of the oxytocin and vasopressin genes from rat. Proc Natl Acad Sci USA. 1984;81:2006-2010. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 253] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 2. | Kimura T, Tanizawa O, Mori K, Brownstein MJ, Okayama H. Structure and expression of a human oxytocin receptor. Nature. 1992;356:526-529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 420] [Cited by in F6Publishing: 448] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 3. | Arletti R, Bertolini A. Oxytocin acts as an antidepressant in two animal models of depression. Life Sci. 1987;41:1725-1730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 147] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Arletti R, Benelli A, Bertolini A. Influence of oxytocin on feeding behavior in the rat. Peptides. 1989;10:89-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 245] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 5. | Ackerman AE, Lange GM, Clemens LG. Effects of paraventricular lesions on sex behavior and seminal emission in male rats. Physiol Behav. 1997;63:49-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Flanagan LM, Dohanics J, Verbalis JG, Stricker EM. Gastric motility and food intake in rats after lesions of hypothalamic paraventricular nucleus. Am J Physiol. 1992;263:R39-R44. [PubMed] [Cited in This Article: ] |
| 7. | Dobrovol'skaia ZA, Kosenko AF. [The role of oxytocin in realizing hypothalamic effects on the motor function of the digestive tract]. Fiziol Zh SSSR Im I M Sechenova. 1989;75:734-737. [PubMed] [Cited in This Article: ] |
| 8. | Flanagan LM, Olson BR, Sved AF, Verbalis JG, Stricker EM. Gastric motility in conscious rats given oxytocin and an oxytocin antagonist centrally. Brain Res. 1992;578:256-260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 68] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Milenov K, Barth T, Jost K, Kasakov L. Effect of deamino-dicarba-oxytocin and oxytocin on myoelectrical and mechanical activity of uterus, stomach and small intestine in dog. Endocrinol Exp. 1979;13:177-183. [PubMed] [Cited in This Article: ] |
| 10. | Petring OU. The effect of oxytocin on basal and pethidine-induced delayed gastric emptying. Br J Clin Pharmacol. 1989;28:329-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Melis MR, Argiolas A. Reduction of drug-induced yawning and penile erection and of noncontact erections in male rats by the activation of GABAA receptors in the paraventricular nucleus: involvement of nitric oxide. Eur J Neurosci. 2002;15:852-860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Zingg HH, Grazzini E, Breton C, Larcher A, Rozen F, Russo C, Guillon G, Mouillac B. Genomic and non-genomic mechanisms of oxytocin receptor regulation. Adv Exp Med Biol. 1998;449:287-295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Engstrom T, Bratholm P, Christensen NJ, Vilhardt H. Up-regulation of oxytocin receptors in non-pregnant rat myometrium by isoproterenol: effects of steroids. J Endocrinol. 1999;161:403-411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Terenzi MG, Jiang QB, Cree SJ, Wakerley JB, Ingram CD. Effect of gonadal steroids on the oxytocin-induced excitation of neurons in the bed nuclei of the stria terminalis at parturition in the rat. Neuroscience. 1999;91:1117-1127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Behrendt-Adam CY, Adams MH, Simpson KS, McDowell KJ. Oxytocin-neurophysin I mRNA abundance in equine uterine endometrium. Domest Anim Endocrinol. 1999;16:183-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Murata T, Murata E, Liu CX, Narita K, Honda K, Higuchi T. Oxytocin receptor gene expression in rat uterus: regulation by ovarian steroids. J Endocrinol. 2000;166:45-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Grazzini E, Guillon G, Mouillac B, Zingg HH. Inhibition of oxytocin receptor function by direct binding of progesterone. Nature. 1998;392:509-512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 330] [Cited by in F6Publishing: 299] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 18. | Xie DP, Li W, Qu SY, Zheng TZ, Yang YL, Ding YH, Wei YL, Chen LB. Effect of areca on contraction of colonic muscle strips in rats. World J Gastroenterol. 2002;8:350-352. [PubMed] [Cited in This Article: ] |
| 19. | Duridanova DB, Nedelcheva MD, Gagov HS. Oxytocin-induced changes in single cell K+ currents and smooth muscle contraction of guinea-pig gastric antrum. Eur J Endocrinol. 1997;136:531-538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Liu CY, Chen LB, Liu PY, Xie DP, Wang PS. Effects of progesterone on gastric emptying and intestinal transit in male rats. World J Gastroenterol. 2002;8:338-341. [PubMed] [Cited in This Article: ] |
| 21. | Peng X, Feng JB, Yan H, Zhao Y, Wang SL. Distribution of nitric oxide synthase in stomach myenteric plexus of rats. World J Gastroenterol. 2001;7:852-854. [PubMed] [Cited in This Article: ] |
| 22. | Venkova K, Krier J. A nitric oxide and prostaglandin-dependent component of NANC off-contractions in cat colon. Am J Physiol. 1994;266:G40-G47. [PubMed] [Cited in This Article: ] |
| 23. | Powell AK, Bywater RA. Endogenous nitric oxide release modulates the direction and frequency of colonic migrating motor complexes in the isolated mouse colon. Neurogastroenterol Motil. 2001;13:221-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Haraldsen L, Söderström-Lauritzsen V, Nilsson GE. Oxytocin stimulates cerebral blood flow in rainbow trout (Oncorhynchus mykiss) through a nitric oxide dependent mechanism. Brain Res. 2002;929:10-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Adan RA, Van Leeuwen FW, Sonnemans MA, Brouns M, Hoffman G, Verbalis JG, Burbach JP. Rat oxytocin receptor in brain, pituitary, mammary gland, and uterus: partial sequence and immunocytochemical localization. Endocrinology. 1995;136:4022-4028. [PubMed] [Cited in This Article: ] |
| 26. | Jasper JR, Harrell CM, O'Brien JA, Pettibone DJ. Characterization of the human oxytocin receptor stably expressed in 293 human embryonic kidney cells. Life Sci. 1995;57:2253-2261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Jeng YJ, Lolait SJ, Strakova Z, Chen C, Copland JA, Mellman D, Hellmich MR, Soloff MS. Molecular cloning and functional characterization of the oxytocin receptor from a rat pancreatic cell line (RINm5F). Neuropeptides. 1996;30:557-565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Jankowski M, Hajjar F, Kawas SA, Mukaddam-Daher S, Hoffman G, McCann SM, Gutkowska J. Rat heart: a site of oxytocin production and action. Proc Natl Acad Sci USA. 1998;95:14558-14563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 144] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 29. | Jankowski M, Wang D, Hajjar F, Mukaddam-Daher S, McCann SM, Gutkowska J. Oxytocin and its receptors are synthesized in the rat vasculature. Proc Natl Acad Sci USA. 2000;97:6207-6211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 121] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Elands J, Resink A, De Kloet ER. Neurohypophyseal hormone receptors in the rat thymus, spleen, and lymphocytes. Endocrinology. 1990;126:2703-2710. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 86] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629-683. [PubMed] [Cited in This Article: ] |
| 32. | Soloff MS, Grzonka Z. Binding studies with rat myometrial and mammary gland membranes on effects of manganese on relative affinities of receptors for oxytocin analogs. Endocrinology. 1986;119:1564-1569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Everson GT. Gastrointestinal motility in pregnancy. Gastroenterol Clin North Am. 1992;21:751-776. [PubMed] [Cited in This Article: ] |
| 34. | Baron TH, Ramirez B, Richter JE. Gastrointestinal motility disorders during pregnancy. Ann Intern Med. 1993;118:366-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 129] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 35. | Ryan JP, Bhojwani A. Colonic transit in rats: effect of ovariectomy, sex steroid hormones, and pregnancy. Am J Physiol. 1986;251:G46-G50. [PubMed] [Cited in This Article: ] |
| 36. | Hinds JP, Stoney B, Wald A. Does gender or the menstrual cycle affect colonic transit. Am J Gastroenterol. 1989;84:123-126. [PubMed] [Cited in This Article: ] |
| 37. | Mizutani S, Hayakawa H, Akiyama H, Sakura H, Yoshino M, Oya M, Kawashima Y. Simultaneous determinations of plasma oxytocin and serum placental leucine aminopeptidase (P-LAP) during late pregnancy. Clin Biochem. 1982;15:141-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Kumaresan P, Subramanian M, Anandarangam PB, Kumaresan M. Radioimmunoassay of plasma and pituitary oxytocin in pregnant rats during various stages of pregnancy and parturition. J Endocrinol Invest. 1979;2:65-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 0.3] [Reference Citation Analysis (0)] |