Published online May 28, 2008. doi: 10.3748/wjg.14.3201
Revised: April 12, 2008
Accepted: April 19, 2008
Published online: May 28, 2008
AIM: To evaluate the prevalence of hepatitis B virus (HBV) infection in inflammatory bowel disease (IBD) patients that followed up in our hospital and try to identify the possible risk factors involved in this infection transmission.
METHODS: This was a cross-sectional study for which 176 patients were selected according to their arrival for the medical interview. All these patients had already IBD diagnosis. The patient was interviewed and a questionnaire was filled out.
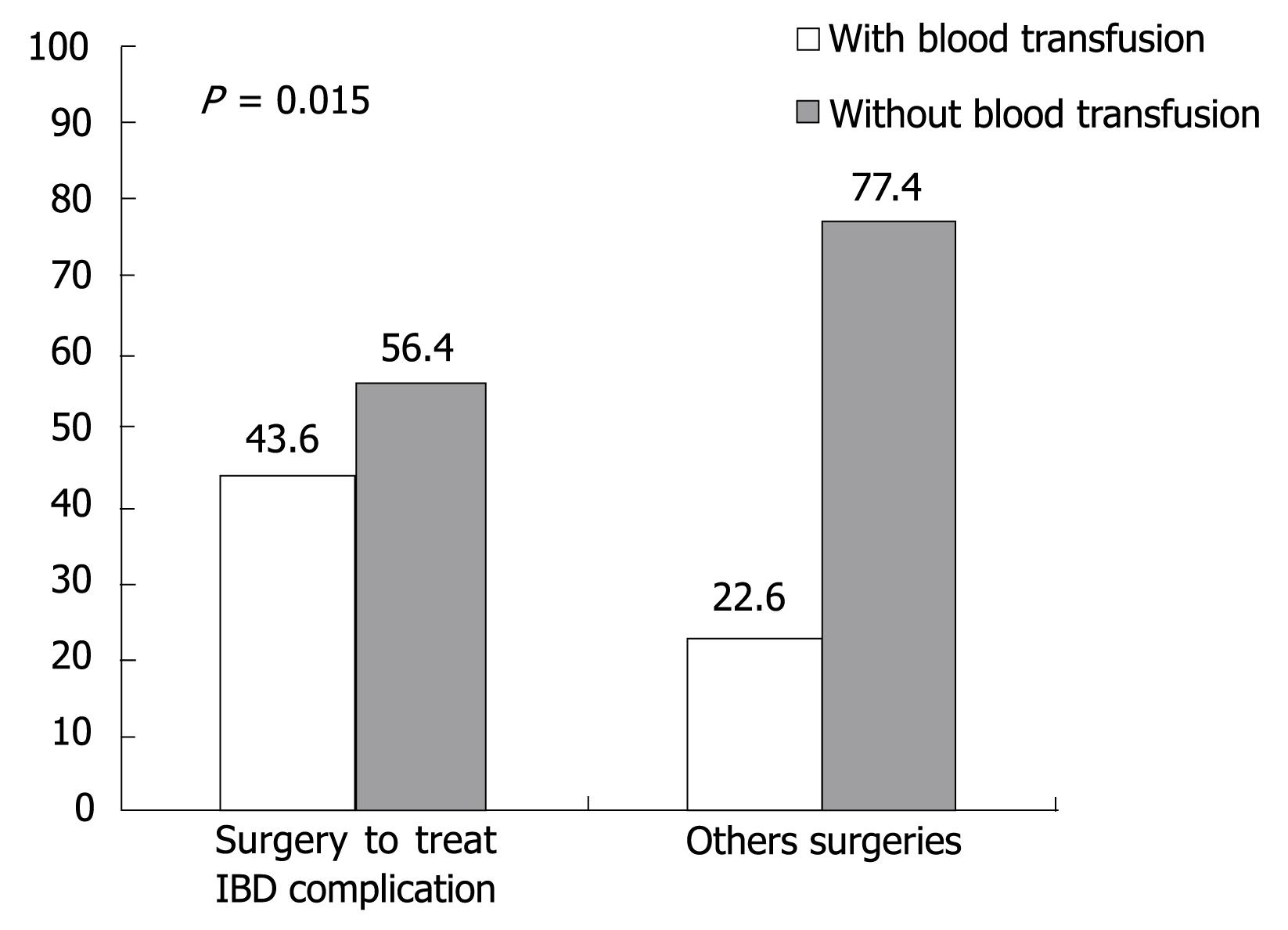
RESULTS: In the group of 176 patients whom we examined, we found that 17% (30) were anti-HBc positive. Out of 30 patients with positive anti-HBc, 2.3% (4) had positive HBsAg and negative HBV-DNA. In an attempt to identify the possible HBV infection transmission risk factors in IBD patients, it was observed that 117 patients had been submitted to some kind of surgical procedure, but only 24 patients had positive anti-HBc (P = 0.085). It was also observed that surgery to treat IBD complications was not a risk factor for HBV infection transmission, since we did not get a statically significant P value. However, IBD patients that have been submitted to surgery to treat IBD complications received more blood transfusions then patients submitted to other surgical interventions (P = 0.015).
CONCLUSION: There was a high incidence of positive anti-HBc (17%) and positive HBsAg (2.3%) in IBD patient when compared with the overall population (7.9%).
- Citation: Tolentino YFM, Fogaça HS, Zaltman C, Ximenes LLL, Coelho HSM. Hepatitis B virus prevalence and transmission risk factors in inflammatory bowel disease patients at Clementino Fraga Filho university hospital. World J Gastroenterol 2008; 14(20): 3201-3206
- URL: https://www.wjgnet.com/1007-9327/full/v14/i20/3201.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.3201
The hepatitis B virus (HBV) infection is a worldwide public health problem. There are two billion people infected by HBV, and among these more than 350 million have chronic infection. Patients with chronic infection have a high death risk for hepatic cirrhosis or liver cancer. These two diseases are responsible for about 1 million people dying every year, in spite of the infection incidence falling recently[1–4].
In developed countries the sexual route is responsible for 30% of infections and is the main route of HBV transmission[3–5].
Health professionals, such as surgeons, pathologists, dialysis and chemotherapy technicians, have a high risk of acquiring HBV infections through small skin lesions or through accident with instruments that cut or perforate[6].
Patients with inflammatory bowel disease (IBD) have high risk of infection by hepatitis viruses B or C[7] because during the course of their disease, they need blood transfusions, and sometimes surgical and endoscopic procedures for diagnosis and treatment[8–10]. Biancone et al observed that in Crohn’s disease (CD), 2/3 of the patients will need an intestinal resection and almost 50% will need multiple surgeries[11]. It is important to confirm this data to alert health professionals about prevention and early diagnosis of HBV infection, because the steroids and immunosuppressant drugs used in IBD treatment worsen the HBV liver disease. Few studies exist to verify if these drugs influence HBV infection in IBD patients[12–15].
The Clementino Fraga Filho University Hospital is a reference center for IBD diagnosis and treatment. As it is not known exactly what the HBV infection rate in this group of patients in this institution, we decided to do this study.
The first aim of this study was to evaluate the prevalence of HBV infection in IBD patients that followed up in the hospital. The second aim is to evaluate the possible risk factors involved in HBV infection transmission in this patients group.
This study was carried out between May 2002 and November 2004, for which 176 patients were recruited. All these patients had clinical, laboratory, radiological, endoscopic and histopathological IBD diagnosis. Included were patients of both sexes, at least 18 years old, for whom medical records were kept by the hospital and who live in Rio de Janeiro State. Patients with infectious, ischemic, actinic, and uncertain colitis were excluded.
The patients were selected, weekly, according to their order of arrival for the medical interview in the hospital IBD ambulatory. After, if the patient allowed us to include him/her in the study, he/she signed an informed consent term. Next, the patient was interviewed and during this interview, a questionnaire was filled out to obtain identification data such as age, sex and IBD type.
In order to identify possible risk factors for HBV infection transmission in this population, the patients were questioned about blood transfusion histories, surgical and endoscopic interventions, dialysis[16], use of endovenous illicit drugs[17], acupuncture treatment, the presence of tattoos[7] or “piercings” and if they engaged in promiscuous sex (defined as more than 3 sexual partners in a year or sexual intercourse with prostitutes)[18].
After the interview, 25 mL of blood were obtained from the patient and the material was submitted to the following analyses: qualitative test for total core antibodies; anti-HBc (Kit Diasorin S.p.A.-Italy); qualitative test for HBV antigen; HBsAg (Kit ELISA-Diasorin S.p.A.-Italy) and qualitative PCR-DNA for HBV (which can detect up to 10 particles/serum milliliter), this last analysis being only for patients with positive anti-HBc, patients with positive HBsAg, and for 14 (8%) patients with negative anti-HBc and HBsAg chosen at random.
Statistical analysis was processed by the SAS® software system. Differences were considered significant for an alpha risk of 5%.
Our objective was to verify if there is a significant association between a positive anti-HBc result and any of the risk factors analyzed. For this purpose the following methods were applied: for proportions comparison (qualitative variables) the chi-square test was used (χ2) or the exact Fisher test. For numeric variables comparison (quantitative) between two groups, the t-test was used for independent samples or the Mann-Whitney test, when the variable did not present normal distribution due to great dispersion or for the ordinal nature of the data.
In our sample there were 68 (38.6%) men and 108 (61.4%) women. There were 102 (58.0%) CD patients and 74 (42.0%) UC patients.
There were surgical procedure histories in 117 patients (66.5%). Blood transfusion was reported by 47 patients (26.7%). Eight patients (4.5%) confirmed the use of endovenous illicit drugs. None of the patient had undergone dialysis treatment and only 3 patients affirmed having a promiscuous sexual life (Table 1).
| Risk factors (n = 176) | n (%) |
| Blood transfusion | 47 (26.7) |
| Surgery | 117 (66.5) |
| Dialysis | 0 |
| Endovenous drug use | 8 (4.5) |
| Tatoo | 4 (2.3) |
| Acupuntura | 7 (4.0) |
| “Piercings” | 1 (0.6) |
| SPL | 3 (1.7) |
| Digestive endoscopes | 175 (99.4) |
Forty-nine patients were without treatment; 7 patients used immunosuppressant drugs; 74 used steroid drugs; while 46 patients used both.
Table 2 shows that among the 176 patients, 30 patients (17%) had positive anti-HBc: 17 (56.7%) with UC and 13 (43.3%) with CD.
| Anti-HBc | HBsAg | |
| Positive | 17 UC (56.7%) | 4 (2.3%) |
| 13 CD (43.3%) | ||
| Negative | 146 (83%) | 172 (97.7%) |
Among the 30 patients with positive anti-HBc, 4 had positive HBsAg.
The 30 patients with positive anti-HBc and the 14 patients with negative anti-HBc randomly selected were submitted to the PCR HBV-DNA qualitative test. All of these patients had negative PCR HBV-DNA results. The four patients with positive HBsAg were also submitted to qualitative PCR HBV-DNA tests and they also had negative results. Among these patients, those with positive anti-HBc and HBsAg tests are considered inactive HBV bearers.
Table 3 supplies the frequency (n) and the risk factor percentile (%) according to anti-HBC results and the corresponding P value. The statistical analysis was accomplished by the χ2 test or by the exact Fisher test.
| Anti-HBc | ||||||
| Positive | Negative | |||||
| Risk factors | n | % | n | % | P | |
| Sex | Men | 12 | 40.0 | 56 | 38.0 | 0.86 |
| Women | 18 | 60.0 | 90 | 61.6 | ||
| Digestive endoscopy | Yes | 16 | 53.3 | 91 | 62.3 | 0.35 |
| No | 14 | 46.7 | 55 | 37.7 | ||
| Retosigmoidoscopy | Yes | 13 | 43.3 | 50 | 34.0 | 0.34 |
| No | 17 | 56.7 | 96 | 65.8 | ||
| Blood transfusion | Yes | 10 | 33.3 | 37 | 25.3 | 0.36 |
| No | 20 | 66.7 | 109 | 74.7 | ||
| Surgery | Yes | 24 | 80.0 | 93 | 63.7 | 0.085 |
| No | 6 | 20.0 | 53 | 36.3 | ||
| Surgery to treat IBD complications | Yes | 8 | 33.3 | 47 | 50.5 | 0.13 |
| No | 16 | 66.7 | 46 | 49.5 | ||
It was observed that sex, digestive endoscopy, retosigmoidoscopy, and blood transfusions were not considered probable risk factors in HBV infection transmission.
When we calculated surgery only, we observed that 24 patients (80%) with positive anti-HBc were submitted to some type of surgical intervention while the other 20% (6 patients) with positive anti-HBc did not undergo any surgical intervention (P = 0.085). We also tried to stratify patients submitted to surgery into two groups: those who underwent surgery to treat IBD complications and those who underwent other surgeries. The P value was not significant (P = 0.13). Dialysis, endovenous illicit drug use, tattoos, acupuncture, piercing and a sexual promiscuous life style, were not analyzed due to low frequency of observed cases.
Table 4 shows that patients with positive anti-HBc have an average age significantly older (P = 0.001) than patients with negative anti-HBc. A significant difference was not observed for disease diagnosis time (P = 0.37) neither for number of colonoscopies (P = 0.52) among both positive and negative anti-HBc groups.
| Variable | Anti-HBc | n | Mean | SE | Minimum | Maximum | P |
| Age (yr) | Positive | 30 | 47.7 | 11.9 | 26 | 78 | 0.001 |
| Negative | 146 | 39.0 | 13.9 | 18 | 84 | ||
| Diagnose time (mo) | Positive | 30 | 114.1 | 109.3 | 8 | 372 | 0.37 |
| Negative | 146 | 89.9 | 90.4 | 1 | 600 | ||
| Colonoscopy number | Positive | 30 | 2.3 | 1.9 | 0 | 10 | 0.52 |
| Negative | 146 | 2.1 | 1.6 | 1 | 8 |
One hundred and seventeen patients were submitted to some form of surgery. We analyzed the relationship between blood transfusion and surgery carried out to treat IBD complications, and it was observed that patients submitted to surgeries to treat IBD complications needed more transfusions (P = 0.015) than patients submitted to other types of surgery, as illustrated in Figure 1.
In this study we observed that positive anti-HBc pre-valence was 17% (30 patients) in a sample of 176 patients. This data shows that positive anti-HBc prevalence in IBD patient groups is larger when compared with the overall Brazil population (7.9%) and with the Rio de Janeiro state population (2.5%) figures[18].
In the literature, we found only one case-control study that evaluated the HBV prevalence in IBD patients. In that study, the anti-HBc prevalence was larger in CD (10.9%) and ulcerative colitis patients (11.5%) when compared with control group individuals (5.1%)[11]. Our study had very similar results for CD and ulcerative colitis for positive anti-HBc prevalence. These results are probably because IBD patients are frequently exposed to surgical interventions and/or endoscopies as well as necessary blood transfusion that can be a means of transmitting HBV[1920].
Among 30 patients with positive anti-HBc result, 2.3% (4) had positive HBsAg with negative HBV-PCR DNA, patients that are considered HBV inactive bearers. This prevalence is considered high when we compare it with a Brazil Health Ministry study in 2006 in the central west, Northeast and Brasília regions that shows an HBsAg prevalence of 0.5%.
In addition to identifying HBV prevalence, we also tried to identify the possible risk factors for HBV transmission that could increase HBV infection prevalence among IBD patients. Considering 5% to be a significant threshold, we found that such factors as: (1) Sexual activity; (2) Digestive endoscopy; (3) Retosigmoidoscopy and (4) Blood transfusion were ruled out as possible risk factors for HBV infection of IBD patients.
Despite the fact that our sample contained 108 female patients corresponding to 61.4% of the total sample, when we compared the positive anti-HBc percentile in women and in men, we did not obtain P value with statistical significance (P = 0.86), even when we separated the male and female group according to IBD type, both groups being very similar in this respect. Biancone had a different result in his study. He demonstrated that female status was an important factor to be considered in HBV infection in CD patients[11].
Studies in the last 5 years have verified the possibility of HCV and HBV transmission mainly through endo-scopic procedures during therapeutic interventions. Studies demonstrated the presence of HBV-DNA in endo-scopic channels that were not submitted to appropriate disinfection processes[20]. In our sample procedures, such as digestive endoscopy and retosigmoidoscopy, we did not discover any evidence of HBV transmission risk factors (digestive endoscopy P = 0.35 and retosigmoidoscopy P = 0.34).
Considering blood transfusion is an important viral hepatitis transmission route[721], it has already been demonstrated by Long et al in 2000 and Biancone et al in 2001 that blood transfusion was an important risk factor in HCV transmission among IBD patients[1122]. However, we were not able to demonstrate that blood transfusion was a risk factor for HBV infection transmission in our group because in our sample only 10 out of 47 patients with positive anti-HBc received blood transfusions while the other 20 with positive anti-HBc did not have blood transfusion histories (P = 0.36).
When we analyzed surgery as a possible risk factor, despite of the fact of not having a P value smaller than 0.05, we observed that 80% (24) of patients with positive anti-HBc had been submitted to some surgical procedure while the other 20% (6) did not undergo any surgical procedures (P = 0.085).
Biancone et al showed that surgery, and mainly surgical procedures to treat IBD complications, were an important risk factor in HCV transmission among IBD patients[11]. We can try to explain the Biancone et al discoveries if we take into consideration that gastrointestinal surgeries to treat IBD complications are high complexity operations[10] and probably need blood transfusions during surgical procedure, which could cause a bias in the statistical analysis because the surgery itself was not the cause of transmission but the transfusion. The possible risk factor for HCV infection transmission in these cases was blood transfusion that patients received during these procedures. In our study we separated patients according to surgery type: group 1-patients submitted to surgery to treat IBD complications, and group 2-patients submitted to other surgical interventions; we did not find a significant P value (P = 0.13). However, as can be seen in Figure 1, our hypothesis that patients submitted to surgeries to treat IBD complications received more blood transfusions than patients submitted to other surgical interventions was confirmed (P = 0.015).
When we compare our results with Spijkerman et al’s study, our data is divergent because according to that study high complexity surgeries (i.e. surgeries with more than one hour of duration, surgeries with a larger incidence of postoperative complications and those with a higher risk of complication requiring further surgery or more blood transfusions) are associated with a higher risk of HBV infection transmission[19]. However, in this study it was demonstrated that the HBV infection was transmitted through an HBV infected surgeon during surgery.
For the other qualitative variables: dialyses, endovenous illicit drug use, tattoos, acupuncture, “piercings” and sexually promiscuous lifestyle, the associations were not analyzed because we had low frequencies of observed cases.
In quantitative-variable analysis (age, disease diagnosis time and number of colonoscopies), the P value results have statistical significance (P = 0.001). The average age of patients with positive anti-HBc was higher (47.7 years) than patients with negative anti-HBc (39.0 years). In the literature, the positive anti-HBc prevalence was associated with ages older than 50 years in CD and in UC[11]. These data were found, we believe, because older patients probably have a longer disease duration time and therefore have had more time to develop complica-tions requiring surgical and endoscopic interventions. However, we were not able to prove the veracity of these assumptions.
Biancone et al have shown (P = 0.37) that disease duration time (number of months since IBD diagnosis) is associated with incidence of positive anti-HBc in UC patients[1].
Steroids, immunosuppressant drugs and the anti-TNF antibodies (anti-necrosis tumor antibodies-Infliximab®) in IBD patients[2324], as some studies have demonstrated, can influence the course of hepatic disease when used in HBV infected patients, mainly patients with positive HBsAg and anti-HBc and negative HBV-DNA (called inactive bearers)[12–14]. It is also important to note that in patients with positive anti-HBc and negative HBsAg, the HBV can replicate because the virus stays inside the hepatocytes although there is an apparent serologic cure[23]. These studies show that immunological suppression caused by these drugs could cause viral replication and spread infection inside hepatocytes. When these drugs were suspended and the immunological reaction was restored, the infected hepatocytes were be destroyed quickly and there was an increase in the transaminases levels ("flare") and an accentuated viremia reduction[1525]. Two cases of fulminant hepatitis were identified after use of Infliximab® in rheumatoid arthritis patients infected by HBV[26] and one case of hepatic insufficiency and death in a CD patient treated with Infliximab®[27,28]. The reactivation of HBV can happen also to inactive bearers submitted to transplants or in cancer patients who are submitted to chemotherapy. Such patients need higher immunosuppressant drug doses than do IBD patients[12].
Patients with positive HBsAg and anti-HBc and negative HBV-PCR DNA have increased risk of reactivating their HBV infections. Therefore, the use of lamivudine is recommended before immunological suppression therapies[29]. Lau and collaborators demon-strated that patients with lymphoma infected by HBV who were submitted to chemotherapy did not have HBV infection reactivated when they used lamivudina one week before chemotherapy was begun[30].
In conclusion, our study demonstrated that there were high incidences of positive anti-HBc (17%) and positive HBsAg (2.3%) in IBD patients in Clementino Fraga Filho University Hospital when compared with the overall population (7.9%).
These data show that it is important to have an early diagnosis of HBV infection in diagnosed IBD patients before any IBD treatment is initiated using steroids, immunosuppressant drugs, or anti-TNF antibodies, as that IBD treatment may worsen quiescent HBV hepatic disease. We also recommend HBV vaccination in this group of patients.
Hepatitis B virus (HBV) infection is considered a worldwide public health problem. Inflammatory bowel disease (IBD) patients have a high risk of acquiring HBV infection because they sometimes need blood transfusions, invasive surgical and endoscopic procedures. The objective of this study is to verify the seroprevalence of HBV infection and to identify the infection transmission risk factors in IBD patients at Clementino Fraga Filho University Hospital.
The statistical analysis cannot identify one possible risk factor for HBV transmission but the study found among the IBD patients 4 persons with positive HBsAg who were called inactive bearers. Studies show that immunological suppression caused by steroids, immunosupressants drugs and the anti-TNF antibodies (anti necrosis antibodies-Infliximab) in IBD patients can influence the course of hepatic disease once used in HBsAg positive patients. Theses drugs would take a viral replication and infection spread inside hepatocytes. It has already been related to 1 case of hepatic insufficiency and death in a Crohn’s disease (CD) patient and 1 case of fulminant hepatitis in rheumatoid arthritis patient, both with positive HBsAg and treated with these drugs. In patients with positive HBsAg lamivudine use would be recommended before immunological suppression.
After this study, we recommend HBV vaccination for IBD patients that have never been infected by HBV and also recommend lamivudine for patients with positive anti-HBc and needs to use steroids and immunomodulators.
This article did not identify one risk factor for HBV infection transmission in IBD patients but it shows us that these patients have high risk of acquiring this infection because they need invasive procedures. IBD patients that have been infected already must receive lamivudine before immunological suppression. It is very interesting.
| 1. | World Health Organization. Hepatitis B. World Health Organization. 2000;204. [Cited in This Article: ] |
| 2. | Kim WR, Benson JT, Therneau TM, Torgerson HA, Yawn BP, Melton LJ 3rd. Changing epidemiology of hepatitis B in a U.S. community. Hepatology. 2004;39:811-816. [Cited in This Article: ] |
| 3. | D'Amelio R, Matricardi PM, Biselli R, Stroffolini T, Mele A, Spada E, Chionne P, Rapicetta M, Ferrigno L, Pasquini P. Changing epidemiology of hepatitis B in Italy: public health implications. Am J Epidemiol. 1992;135:1012-1018. [Cited in This Article: ] |
| 4. | Maddrey WC. Hepatitis B: an important public health issue. J Med Virol. 2000;61:362-366. [Cited in This Article: ] |
| 5. | Chan HL, Ghany MG, Lok ASF. Hepatitis B. Diseases of the liver. 8th ed. Lippincott Williams & Wilkins: Philadelphia 1999; 758-791. [Cited in This Article: ] |
| 6. | Lok ASF, Chan HL. Viral Hepatitis B and D. Comprehensive Clinical Hepatology. 1st ed. Mosby: London 2000; 12.1-12.10. [Cited in This Article: ] |
| 7. | Nishioka Sde A, Gyorkos TW, MacLean JD. Tattoos and transfusion-transmitted disease risk: implications for the screening of blood donors in Brazil. Braz J Infect Dis. 2002;6:172-180. [Cited in This Article: ] |
| 8. | Broome U, Glaumann H, Hellers G, Nilsson B, Sorstad J, Hultcrantz R. Liver disease in ulcerative colitis: an epidemiological and follow up study in the county of Stockholm. Gut. 1994;35:84-89. [Cited in This Article: ] |
| 9. | Gaeta GB, Stroffolini T, Taliani G, Ippolito FM, Giusti G, De Bac C. Surgical procedures as a major risk factor for chronic hepatitis C virus infection in Italy: evidence from a case-control study. Int J Infect Dis. 1999;3:207-210. [Cited in This Article: ] |
| 10. | Pallone F, Boirivant M, Stazi MA, Cosintino R, Prantera C, Torsoli A. Analysis of clinical course of postoperative recurrence in Crohn's disease of distal ileum. Dig Dis Sci. 1992;37:215-219. [Cited in This Article: ] |
| 11. | Biancone L, Pavia M, Del Vecchio Blanco G, D'Inca R, Castiglione F, De Nigris F, Doldo P, Cosco F, Vavassori P, Bresci GP. Hepatitis B and C virus infection in Crohn's disease. Inflamm Bowel Dis. 2001;7:287-294. [Cited in This Article: ] |
| 12. | Biancone L, Del Vecchio Blanco G, Pallone F, Castiglione F, Bresci G, Sturniolo G. Immunomodulatory drugs in Crohn's disease patients with hepatitis B or C virus infection. Gastroenterology. 2002;122:593-594. [Cited in This Article: ] |
| 13. | Perrillo RP. Acute flares in chronic hepatitis B: the natural and unnatural history of an immunologically mediated liver disease. Gastroenterology. 2001;120:1009-1022. [Cited in This Article: ] |
| 14. | Marusawa H, Uemoto S, Hijikata M, Ueda Y, Tanaka K, Shimotohno K, Chiba T. Latent hepatitis B virus infection in healthy individuals with antibodies to hepatitis B core antigen. Hepatology. 2000;31:488-495. [Cited in This Article: ] |
| 15. | Perrillo RP, Schiff ER, Davis GL, Bodenheimer HC Jr, Lindsay K, Payne J, Dienstag JL, O’Brien C, Tamburro C, Jacobson IM. A randomized, controlled trial of interferon alfa-2b alone and after prednisone withdrawal for the treatment of chronic hepatitis B. The Hepatitis Interventional Therapy Group. N Engl J Med. 1990;323:295-301. [Cited in This Article: ] |
| 16. | Froio N, Nicastri E, Comandini UV, Cherubini C, Felicioni R, Solmone M, Di Giulio S, Petrosillo N. Contamination by hepatitis B and C viruses in the dialysis setting. Am J Kidney Dis. 2003;42:546-550. [Cited in This Article: ] |
| 17. | Freeman R. Barriers to accessing and accepting dental care. Br Dent J. 1999;187:81-84. [Cited in This Article: ] |
| 18. | Secretaria do Estado de Saúde do Rio de Janeiro-Assessoria de Doenças Transmissíveis por Sangue e Hemoderivados. Hepatites Virais-9° Boletim Informativo, 2005. [Cited in This Article: ] |
| 19. | Spijkerman IJ, van Doorn LJ, Janssen MH, Wijkmans CJ, Bilkert-Mooiman MA, Coutinho RA, Weers-Pothoff G. Transmission of hepatitis B virus from a surgeon to his patients during high-risk and low-risk surgical procedures during 4 years. Infect Control Hosp Epidemiol. 2002;23:306-312. [Cited in This Article: ] |
| 20. | Ishino Y, Ido K, Sugano K. Contamination with hepatitis B virus DNA in gastrointestinal endoscope channels: risk of infection on reuse after on-site cleaning. Endoscopy. 2005;37:548-551. [Cited in This Article: ] |
| 21. | Schreiber GB, Busch MP, Kleinman SH, Korelitz JJ. The risk of transfusion-transmitted viral infections. The Retrovirus Epidemiology Donor Study. N Engl J Med. 1996;334:1685-1690. [Cited in This Article: ] |
| 22. | Longo F, Hebuterne X, Tran A, Staccini P, Hastier P, Schneider S, Benzaken S, Tirtaine C, Rampal P. [Prevalence of hepatitis C in patients with chronic inflammatory bowel disease in the region of Nice and evaluation of risk factors]. Gastroenterol Clin Biol. 2000;24:77-81. [Cited in This Article: ] |
| 23. | Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417-429. [Cited in This Article: ] |
| 24. | Cottone M, Magliocco A, Trallori G, Brignola C, Vandelli C, Ardizzone S, Meucci G, Zannoni F, Di Maio G, Astegiano M. Clinical course of inflammatory bowel disease during treatment with interferon for associated chronic active hepatitis. Ital J Gastroenterol. 1995;27:3-4. [Cited in This Article: ] |
| 25. | Esteve M, Saro C, Gonzalez-Huix F, Suarez F, Forne M, Viver JM. Chronic hepatitis B reactivation following infliximab therapy in Crohn's disease patients: need for primary prophylaxis. Gut. 2004;53:1363-1365. [Cited in This Article: ] |
| 26. | Michel M, Duvoux C, Hezode C, Cherqui D. Fulminant hepatitis after infliximab in a patient with hepatitis B virus treated for an adult onset still's disease. J Rheumatol. 2003;30:1624-1625. [Cited in This Article: ] |
| 27. | Oniankitan O, Duvoux C, Challine D, Mallat A, Chevalier X, Pawlotsky JM, Claudepierre P. Infliximab therapy for rheumatic diseases in patients with chronic hepatitis B or C. J Rheumatol. 2004;31:107-109. [Cited in This Article: ] |
| 28. | Millonig G, Kern M, Ludwiczek O, Nachbaur K, Vogel W. Subfulminant hepatitis B after infliximab in Crohn's disease: need for HBV-screening? World J Gastroenterol. 2006;12:974-976. [Cited in This Article: ] |
| 29. | Tillmann HL, Wedemeyer H, Manns MP. Treatment of hepatitis B in special patient groups: hemodialysis, heart and renal transplant, fulminant hepatitis, hepatitis B virus reactivation. J Hepatol. 2003;39 Suppl 1:S206-S211. [Cited in This Article: ] |
| 30. | Lau GK, Yiu HH, Fong DY, Cheng HC, Au WY, Lai LS, Cheung M, Zhang HY, Lie A, Ngan R. Early is superior to deferred preemptive lamivudine therapy for hepatitis B patients undergoing chemotherapy. Gastroenterology. 2003;125:1742-1749. [Cited in This Article: ] |