Published online May 21, 2009. doi: 10.3748/wjg.15.2329
Revised: March 28, 2009
Accepted: April 4, 2009
Published online: May 21, 2009
AIM: To evaluate the possible role of Tribble 3 (TRB3) in a rat model of non-alcoholic fatty liver disease (NAFLD) and its signal transduction mechanism.
METHODS: Thirty Sprague-Dawley rats were randomized into three groups: normal control group, non-alcoholic fatty liver group A (fed on a high-fat diet for 8 wk) and group B (fed on a high-fat diet for 16 wk). To determine the degree of hepatic steatosis in rats of each group, livers were stained with hematoxylin and eosin, and evaluated; real-time fluorescent quantitative reverse transcriptase-polymerase chain reaction was performed to measure the expression levels of TRB3 mRNA; and Western blotting analysis was done to determine the expression levels of protein kinase B (Akt) and phosphorylated protein kinase B (p-Akt-Thr308, p-Akt-Ser473).
RESULTS: Hepatic steatosis was evident in both NAFLD groups: mild to moderate hepatic steatosis occurred in group A, mainly as mild steatosis. Moderate to severe hepatic steatosis occurred in group B, mainly as severe steatosis. The expression level of TRB3 mRNA in group B was significantly higher than in the control group (122.28 ± 95.37 vs 3.06 ± 2.33, P = 0.001) and group A (122.28 ± 95.37 vs 5.77 ± 4.20, P = 0.001). There was no significant difference in the expression levels of Akt (1.03 ± 0.53 vs 1.12 ± 0.77, P = 0.729) and p-Akt-Thr308 (0.82 ± 0.45 vs 0.92 ± 0.38, P = 0.592) between group A and the control group. The expression level of Akt and p-Akt-Thr308 in group B was significantly lower than in group A (Akt 0.41 ± 0.16 vs 1.12 ± 0.77, P = 0.008; p-Akt-Thr308 0.47 ± 0.19 vs 0.82 ± 0.45, P = 0.036) and the control group (Akt 0.41 ± 0.16 vs 1.03 ± 0.53, P = 0.018; p-Akt-Thr308 0.47 ± 0.19 vs 0.92 ± 0.38, P = 0.010). The expression level of p-Akt-Ser473 in group A was significantly higher than in group B (1.48 ± 0.50 vs 0.81 ± 0.39, P = 0.041) as well as the control group (1.48 ± 0.50 vs 0.45 ± 0.26, P = 0.003).
CONCLUSION: TRB3 blocks insulin signaling by inhibiting Akt activation, which contributes to insulin resistance. It may be an important factor in the occurrence and development of NAFLD.
- Citation: Wang YG, Shi M, Wang T, Shi T, Wei J, Wang N, Chen XM. Signal transduction mechanism of TRB3 in rats with non-alcoholic fatty liver disease. World J Gastroenterol 2009; 15(19): 2329-2335
- URL: https://www.wjgnet.com/1007-9327/full/v15/i19/2329.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.2329
Three pseudo kinases of the Tribble family[12] have been recognized recently, which include Tribble 1 (TRB1), TRB2 and TRB3. Different from the typical kinase domain structure, tribbles lack a conventional ATP binding site and activity domain of protein kinases. Thus, no kinase activity by tribbles has been detected. They are classified as members of the family of pseudo-kinases[3]. TRB3 is a mammalian homolog[4] of Drosphila tribbles and is also called a neuronal cell death-inducible putative protein kinase gene in rodents. TRB3 is located on the 20p13 region of the human chromosome[5]. Its full-length translated region in mRNA is 1074 bp, its protein product is made of 358 amino acids and it is a kind of nucleoprotein.
Studies indicate that TRB3 is involved in many biological processes, including insulin resistance (IR), blocking of insulin signaling pathway[6], endoplasmic reticulum stress responses[7] and the regulation of cell growth and differentiation[89]. Du et al[6] have found that the expression of hepatic TRB3 increased in a rat model of diabetes. It inhibits the activation of the Akt/PKB signaling pathway by insulin, resulting in IR. TRB3 inhibits the phosphorylation of Thr-308 and Ser-473 by binding with them, thus inhibiting the activity of Akt. Then, the insulin signaling pathway is blocked.
Research by Chitturi et al[10] indicates that IR exits in about 98% of patients with non-alcoholic fatty liver disease (NAFLD). IR is possibly of key importance in inducing NAFLD. Therefore, any factor related to IR may play an important role in the development of NAFLD. Therefore, TRB3 may not only be a cause of IR, but also an important factor in the occurrence and development of NAFLD. Rat models of NAFLD have been developed by feeding them a high-fat diet. The objective was to study the expression of TRB3 mRNA using reverse transcriptase-polymerase chain reaction (RT-PCR) in rat models of NAFLD, and to evaluate the role of TRB3, using Western blotting analysis, in the occurrence and development of NAFLD.
Thirty healthy Sprague-Dawley rats weighing 210-260 g (15 male and 15 female) were purchased from Shanghai Slac Laboratory Animal Co. Ltd., Chinese Academy of Sciences. The rats were fed normal food for 1 wk.
The materials for the high-fat-diet rat models and the reagents for pathological tests were all purchased from Shanghai Lanji Technology Development Co., Ltd.; Trizol and SYBR Green I were purchased from Invitrogen. Akt (A444) antibody, p-Akt (S473) antibody and p-Akt (T308) antibody were obtained from Bioworld. Actin and horseradish peroxidase (HRP)-labeled goat anti-rabbit IgG (H + L) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). PVDF membranes were supplied by Millipore. Diaminobenzidine was purchased from Sigma. DEPC-treated Water, TBE and loading buffer were obtained from Shanghai Gene and Biotech Co., Ltd. Taq enzyme and random primers were supplied by Takara Biotechnology (Dalian) Co., Ltd. RNase inhibitor, dNTP, Moloney murine leukemia virus and TEMED were procured from Promega. DTT, SDS, Tris, Glycin, N, N'-Methylene bisacrylamide and acrylamide were obtained from Amresco. BSA was acquired from the Huamei Biotech Company (packaged separately after being imported). Protein markers were purchased by the Shanghai Institute of Biochemistry, Chinese Academy of Sciences. Methyl alcohol was purchased from the Sinopharm Reagent Group (Shanghai, China).
Applied Biosystems 7500 Real-Time PCR System, Vertical Electrophoresis Tank, Electrophoresis Apparatus PAC3000 and Semi-Dry Transfer Unit were purchased from Bio-Rad; high-speed freezing centrifuge, Centrifuge 5417R, was purchased from Eppendorf; Gel Imaging System (GIS)-2008 was purchased from Shanghai Tanon Science & Technology Co., Ltd.; electronic balance BP310P was from Sartorius; pipettes were from Gilson; glass homogenizer was from Ningbo Scientz Scientific Instruments Research Institute; UV-VIS Spectro Photometer Unico UV-2000 was from Unico (Shanghai) Instruments Co., Ltd.; ultrapure water system from Millipore; and ultrasonic cell disruption system Soniprep150 from SANYO.
The rats were divided into three groups according to random number tables. The control group (n = 10) was fed on a normal diet; NAFLD groups (n = 20) were fed on high-fat diet, which was prepared by adding 10% lard and 2% cholesterol to the normal diet. Group A (n = 10) was fed on high-fat diet for 8 wk and group B (n = 10) was fed on high-fat diet for 16 wk. All the rats lived in an air-conditioned room at room temperature at 18-23°C and 60% humidity. All the rats were fed ad libitum and had free access to water.
According to the schedule of the experiment, the rats were anesthetized with 2.5% pentobarbital sodium solution (1.5 mL/kg), injected into the abdominal cavity after an overnight fasting. They were sacrificed after blood samples were taken from the inferior vena cava, and the livers were removed immediately. Serum and liver paraffin embedded tissue sections were prepared according to routine methods. The liver specimens were fixed in a neutral formalin solution. The tissue sections were hematoxylin and eosin (HE), and the HE-stained sections were examined under a light microscope for the evaluation of hepatic steatosis.
Fluorescent quantitative RT-PCR for measurement of TRB3 mRNA expression levels: RNA extraction and cDNA synthesis: The Trizol method was used to extract total RNA from tissues and an UV-VIS Spectrophotometer was used to determine the purity and concentration. Two micrograms of total RNA was reverse transcribed into cDNA.
Real-time fluorescent quantitative PCR: The SYBR Green I dye method was adopted. GADPH and TRB3 were amplified by reverse transcription. After gel electrophoresis of amplified products, a fully-automatic Gel Imaging System was used to analyze mRNA expression to compare the intensity between the groups. All the results were normalized to GADPH. The GADPH primers were used as follows: upstream primer: 5'-ACCACAGTCCATGCCATCAC-3', downstream primer: 5'-TCCACCACCCTGTTGCTGTA-3'. The length of the amplified product was 440 bp. The TRB3 primers were used as follows: upstream primer: 5'-TCATCTTGCGCGACCTCAA-3', downstream primer: 5'-TCCACCACCCTGTTGCTGTA-3'. The length of the amplified product was 296 bp. Thirty-six cycles of pre-degeneration at 95°C for 2 min, degeneration at 95°C for 10 s, annealing at 50°C for 10 s, and extension at 72°C for 45 s were used for all experiments.
Western blotting analysis for the expression levels of total Akt and phosphorylated Akt (p-Akt-Thr308, p-Akt-Ser473): Equal samples of tissue were prepared and put into protein extracts to be ground as plasm form. Then the plasm was high-speed centrifuged under freezing conditions for protein extraction. The protein concentration was determined according to the fixed steps. The protein samples (30 &mgr;L) were subjected to SDS-PAGE electrophoresis, transferred to PVDF membranes, and shaken on a rotary shaker at room temperature for 2 h. After that, a TBST buffer solution was used to wash the membrane three times. Then, Akt-related antibodies (Akt1, p-Akt-Thr308, p-Akt-Ser473) were incubated at 4°C overnight under constant shaking on a rotary shaker. After three washes with TBST buffer solution, HRP-labeled goat anti-rabbit IgG (H + L) was incubated at room temperature while shaking on a rotary shaker for 2 h. NBT/BCIP reagent was applied for color development. The membrane was rinsed with deionized water. All the procedures were repeated three times. β-actin was chosen as an internal control. The GIS was used for data analysis, and statistical analysis was used to detect differences between samples and the internal control.
SPSS11 software was used for the statistical analysis. All the statistical data were expressed as mean ± SD for single factor analysis of variance and paired comparisons were performed by the least-square deconvolution method. Two-tailed tests (α = 0.05) were used for statistical treatment. P < 0.05 was considered a significant difference.
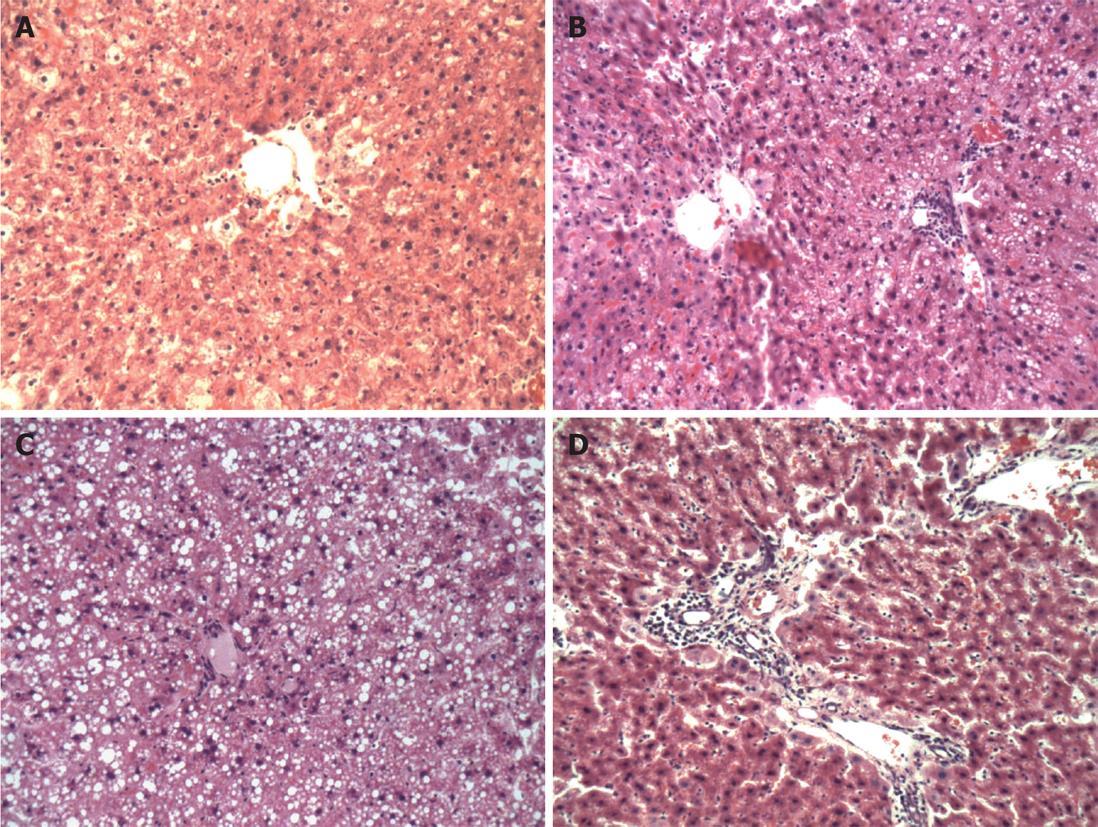
A high-fat diet causes obvious hepatic steatosis in rats, which was evident for both model groups. Mild to moderate hepatic steatosis occurred in group A, mainly as mild steatosis. Moderate to severe hepatic steatosis occurred in model group B, mainly as severe steatosis. Mild hepatic steatosis tissue was defined as hepatic steatosis that accounted for 30%-50% of the total liver cells in the microscopic field; for moderate hepatic steatosis, liver cells with hepatic steatosis accounted for 50%-75% of the total liver cells; and for severe hepatic steatosis tissues, liver cells with hepatic steatosis accounted for over 75% of the total liver cells. In portal areas, severe inflammation featured infiltration of large numbers of diffuse lymphocytes and neutrophils, destroyed limiting plates and hepatic lobules were infiltrated by inflammatory cells that surrounded liver cells (Figure 1).
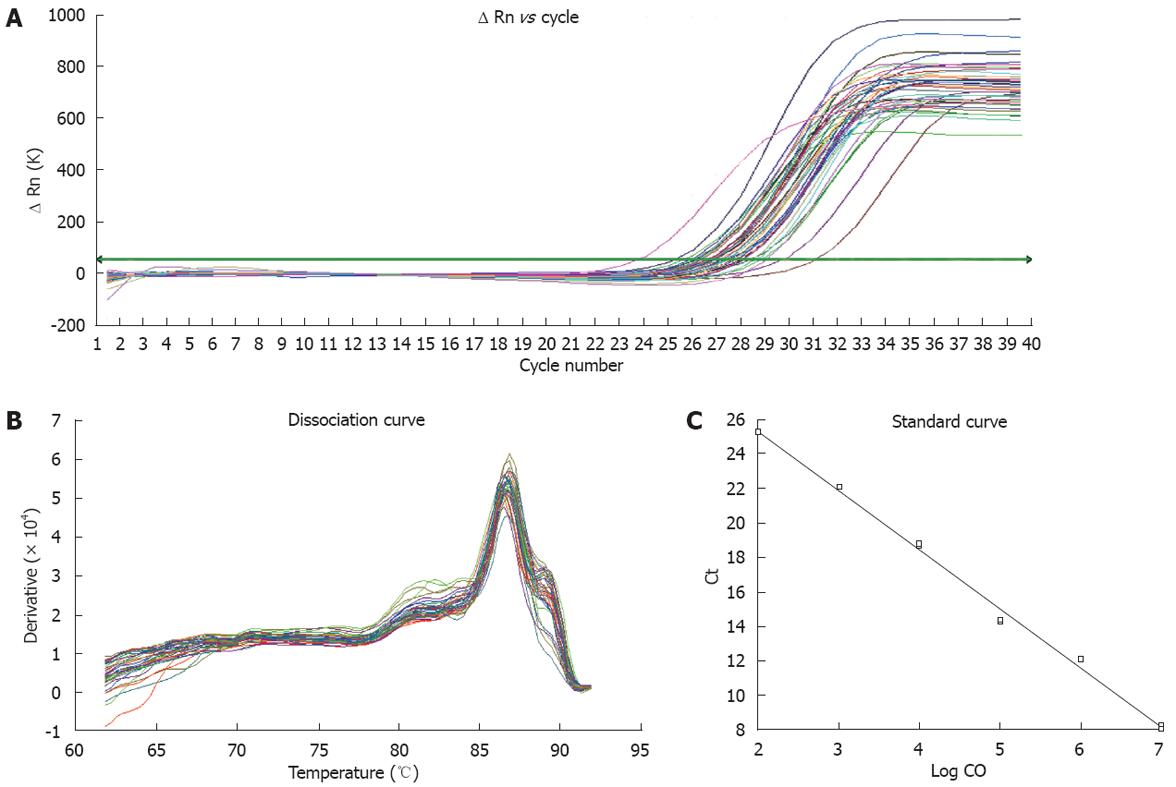
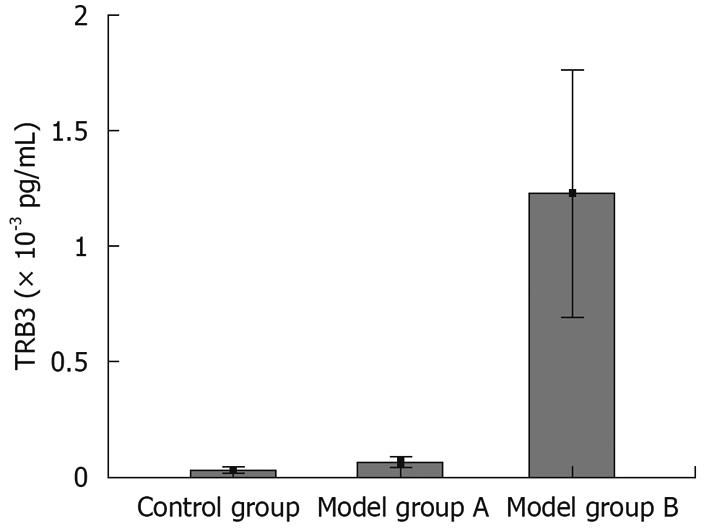
We adopted real-time fluorescent quantitative RT-PCR methods to measure the expression levels of TRB3 mRNA in rats (Figure 2A-C). The relation (r = 0.99) between amplified results of PCR and Ct value of standard samples is shown in Figure 2C. From the melting curve, no primer dimer formation was detected during the PCR reaction (Figure 2B). We found that the expression level of TRB3 mRNA in group B was significantly higher than in the control group (122.28 ± 95.37 vs 3.06 ± 2.33, P = 0.001) and group A (122.28 ± 95.37 vs 5.77 ± 4.20, P = 0.001). There was no significant difference (5.77 ± 4.20 vs 3.06 ± 2.33, P = 0.914) in the expression levels of TRB3 between group A and the control group (Figure 3, Table 1). All these data indicate that a simple hepatic steatosis pathomorphism showed no significant difference between group A and the control group for the expression of TRB3 mRNA. As the time to set up the model increased, the degree of hepatic steatosis was raised. When the model deteriorated to show evidence of a fatty hepatitis pathomorphism, the expression of TRB3 mRNA was significantly higher.
The protein bands of Akt p-Akt-Thr308 and p-Akt-Ser473 are shown for each group. There was no significant difference in the expression levels of Akt (1.03 ± 0.53 vs 1.12 ± 0.77, P = 0.729) and p-Akt-Thr308 (0.82 ± 0.45 vs 0.92 ± 0.38, P = 0.592) between group A and the control group. However, the expression levels of Akt and p-Akt-Thr308 in group B was significantly lower than in group A (Akt 0.41 ± 0.16 vs 1.12 ± 0.77, P = 0.008; p-Akt-Thr308 0.47 ± 0.19 vs 0.82 ± 0.45, P = 0.036) and the control group (Akt 0.41 ± 0.16 vs 1.03 ± 0.53, P = 0.018; p-Akt-Thr308 0.47 ± 0.19 vs 0.92 ± 0.38, P = 0.010). The expression level of p-Akt-Ser473 in group A was significantly higher than in group B (1.48 ± 0.50 vs 0.81 ± 0.39, P = 0.041) as well as the control group (1.48 ± 0.50 vs 0.45 ± 0.26, P = 0.003) (Table 1). All these data indicate that a simple hepatic steatosis pathomorphism does not produce a significant difference between the model group and the control group in the expression of total Akt. As the rats were exposed to longer periods of a high-fat diet, the degree of hepatic steatosis was increased. When the rats deteriorated to a fatty hepatitis pathomorphism, the expression of total Akt, p-Akt-Thr308 and p-Akt-Ser473 was significantly lower than the simple fatty liver disease modeled by group A and the control group.
NAFLD has increased in recently years, and is one of the major causes for cryptogenic cirrhosis[1112]. The pathogenesis of NAFLD is complicated. There is an interaction between genetic susceptibility and multiple metabolic disorders involved in the disease. The pathophysiologic basis of the condition is mainly insulin resistance and oxidative stress. No perfect theory exists for all its clinical manifestations[1314]. At present, IR along with hepatocyte fatty degeneration is believed to be a key factor in the occurrence and development of fatty liver disease[1516]. Research indicates[10] that IR exits in about 98% of patients with NAFLD. IR is probably of key importance for the induction of NAFLD. Therefore, any factor related to IR may play an important role in NAFLD.
Studies indicate that TRB3 is involved in many biological processes, including IR and blocking of the insulin signaling pathway[617]. In this study, we generated a rat model of NAFLD using a high-fat diet. Group A modeled simple fatty liver, and we continued to feed the rats with the high-fat diet. Simple hepatic steatosis produced no significant difference between the model group and the control group in the expression of TRB3 mRNA. As the rats were exposed to longer periods with the high-fat diet, the extent of hepatic steatosis was raised. When the condition of the rats deteriorated to a fatty hepatitis pathomorphism (group B), the expression of TRB3 mRNA became significantly higher. This result indicates that TRB3 is involved in the occurrence and development of NAFLD.
On the basis of recent research, through this study, we made further efforts to discover the possible mechanism of TRB3 involved in the occurrence and development of NAFLD. One study has shown that IR is related to the insulin signaling pathway phosphoinositide 3-kinase/protein kinase B (PI3-K/PKB)[18]. Akt is a key protein[19] in the PI3-K insulin signaling pathway. Two of its sites need to be phosphorylated[2021] for its normal physiological function. One site is Thr-308, located in the kinase domain, and the other one is Ser-473, located in hydrophobic motif of the regulatory domain. After the binding of insulin and its receptors in the cell membrane, the upstream signaling proteins in this pathway are activated step by step. Thr-308 and Ser-473[2223] phosphorylation sites for Akt are activated, and then endocytose from membrane to cytoplasm, which starts a cascade of reactions of the downstream related substrate proteins. Through this process, insulin contributes to glycogen synthesis, glucose transport, glycolysis and the inhibition of gluconeogenesis[24]. The quantity of Akt decreases and its activity changes in rats with IR[25]. Ijuin et al[26] have found that TRB3 may inhibit the signal transduction of insulin-activated PI3-K in CHO cells, which suggests that TRB3 affects insulin signal transduction and inhibits uptake and utilization of glucose by cells. Du et al[6] and Matsushima et al[27] have found that in the hepatic cells of TRB3 transgenic rats, TRB3 inhibited the phosphorylation and activation of Thr-308 and Ser-473 of Akt, but did not affect the protein expression of Akt. Therefore, TRB3 may decrease glucose tolerance and cause blood glucose elevation. The phosphorylation of substrate proteins like glycogen synthase kinase-3β by Akt was inhibited, and glycogen synthesis and the function of insulin on glucose metabolism was also lowered. TRB3 plays an important role in IR. TRB3 gene knockouts may increase the sensitivity of hepatic cells to insulin stimulation. The activity of Akt may be enhanced, and blood glucose levels may be lower[28]. The above mentioned studies indicate that TRB3 could block the insulin signaling pathway through inhibiting Akt activation[6]. Since TRB3 inhibits the phosphorylation of Thr-308 and Ser-473 by binding with them, it inhibits the activity of Akt. As a result, the insulin signaling pathway is blocked. Our results also support this hypothesis. When the pathomorphism was simple hepatic steatosis, there was no significant difference between the model group and control group in the expression of TRB3 mRNA. The expression level of total Akt did not change much either. As the degree of hepatic steatosis was raised and deteriorated to fatty hepatitis, the expression of total Akt, p-Akt-Thr308 and p-Akt-Ser473 was significantly lower than that in the simple fatty liver model group.
The data for p-Akt-Ser473 in Table 1 show that in mild steatosis (group A), expression levels are much greater than control while in severe steatosis (group B), levels go back to the control value. We assume that the complex regulation of active molecules in vivo may lead to another pathway. Balendran et al[29] have shown that 3-phosphoinositide-dependent protein kinase-1, Akt and protein-kinase-C-related kinase-2 interact with each other after the phosphorylation of Thr308, which can be converted into 3-phosphoinositide-dependent protein kinase-2 (PDK2) and modify Ser473. Kroner et al[30] have found that the existence of PDK2 can be proven by the complex relationship between Thr308 and Ser473, which is phosphorylated independently. A study by Ferguson et al[31] has shown that Akt can be activated in a PI3-K-independent pathway. Therefore, this interesting phenomenon is worthy of further study.
In conclusion, TRB3 can block the insulin signaling pathway by inhibiting the activation of Akt[3233], and contributing to IR. Therefore, TRB3 may be an important factor in the occurrence and development of NAFLD. This study provides an experimental basis for future studies about the role of TRB3 in NAFLD. The control of the expression level of TRB3 in liver may become a new target for NAFLD therapy.
Tribble 3 (TRB3) is involved in many biological processes, including insulin resistance (IR), blocking of the insulin signaling pathway, endoplasmic reticulum stress responses, and the regulation of cell growth and differentiation. Any factor related to IR will play an important role in the development of non-alcoholic fatty liver disease (NAFLD). Therefore, the authors of this study investigated the relationship between TRB3 and IR, and aimed to establish its importance in the occurrence and development of NAFLD.
The study is the first to evaluate the role of TRB3 with IR in NAFLD. The potential effect of TRB3 is likely to block the insulin signaling pathway through inhibiting Akt activation. TRB3 may play an important role in the occurrence and development of NAFLD.
This study explained one of the possible mechanisms of IR, which could produce a potentially facilitative effect on the occurrence and development of NAFLD.
This study provides an experimental basis for future studies on the role of TRB3 in NAFLD. The control of the expression level of TRB3 in liver may become a new target for therapy for NAFLD.
In the present study, the authors tested the effect of TRB3 in NAFLD in rats, and found a facilitative effect in the occurrence and development of NAFLD.
| 1. | Mata J, Curado S, Ephrussi A, Rørth P. Tribbles coordinates mitosis and morphogenesis in Drosophila by regulating string/CDC25 proteolysis. Cell. 2000;101:511-522. [Cited in This Article: ] |
| 2. | Seher TC, Leptin M. Tribbles, a cell-cycle brake that coordinates proliferation and morphogenesis during Drosophila gastrulation. Curr Biol. 2000;10:623-629. [Cited in This Article: ] |
| 3. | Boudeau J, Miranda-Saavedra D, Barton GJ, Alessi DR. Emerging roles of pseudokinases. Trends Cell Biol. 2006;16:443-452. [Cited in This Article: ] |
| 4. | Grosshans J, Wieschaus E. A genetic link between morphogenesis and cell division during formation of the ventral furrow in Drosophila. Cell. 2000;101:523-531. [Cited in This Article: ] |
| 5. | Bowers AJ, Scully S, Boylan JF. SKIP3, a novel Drosophila tribbles ortholog, is overexpressed in human tumors and is regulated by hypoxia. Oncogene. 2003;22:2823-2835. [Cited in This Article: ] |
| 6. | Du K, Herzig S, Kulkarni RN, Montminy M. TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science. 2003;300:1574-1577. [Cited in This Article: ] |
| 7. | Selim E, Frkanec JT, Cunard R. Fibrates upregulate TRB3 in lymphocytes independent of PPAR alpha by augmenting CCAAT/enhancer-binding protein beta (C/EBP beta) expression. Mol Immunol. 2007;44:1218-1229. [Cited in This Article: ] |
| 8. | Hegedus Z, Czibula A, Kiss-Toth E. Tribbles: novel regulators of cell function; evolutionary aspects. Cell Mol Life Sci. 2006;63:1632-1641. [Cited in This Article: ] |
| 9. | Sung HY, Francis SE, Crossman DC, Kiss-Toth E. Regulation of expression and signalling modulator function of mammalian tribbles is cell-type specific. Immunol Lett. 2006;104:171-177. [Cited in This Article: ] |
| 10. | Chitturi S, Farrell GC. Etiopathogenesis of nonalcoholic steatohepatitis. Semin Liver Dis. 2001;21:27-41. [Cited in This Article: ] |
| 11. | Jansen PL. Nonalcoholic steatohepatitis. Neth J Med. 2004;62:217-224. [Cited in This Article: ] |
| 12. | Kallwitz ER, McLachlan A, Cotler SJ. Role of peroxisome proliferators-activated receptors in the pathogenesis and treatment of nonalcoholic fatty liver disease. World J Gastroenterol. 2008;14:22-28. [Cited in This Article: ] |
| 13. | Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842-845. [Cited in This Article: ] |
| 14. | Adams LA, Angulo P, Lindor KD. Nonalcoholic fatty liver disease. CMAJ. 2005;172:899-905. [Cited in This Article: ] |
| 15. | Choudhury J, Sanyal AJ. Insulin resistance in NASH. Front Biosci. 2005;10:1520-1533. [Cited in This Article: ] |
| 16. | Duvnjak M, Lerotić I, Barsić N, Tomasić V, Virović Jukić L, Velagić V. Pathogenesis and management issues for non-alcoholic fatty liver disease. World J Gastroenterol. 2007;13:4539-4550. [Cited in This Article: ] |
| 17. | Ding J, Kato S, Du K. PI3K activates negative and positive signals to regulate TRB3 expression in hepatic cells. Exp Cell Res. 2008;314:1566-1574. [Cited in This Article: ] |
| 18. | Whiteman EL, Cho H, Birnbaum MJ. Role of Akt/protein kinase B in metabolism. Trends Endocrinol Metab. 2002;13:444-451. [Cited in This Article: ] |
| 19. | Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85-96. [Cited in This Article: ] |
| 20. | Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 2002;14:381-395. [Cited in This Article: ] |
| 21. | Neri LM, Borgatti P, Capitani S, Martelli AM. The nuclear phosphoinositide 3-kinase/AKT pathway: a new second messenger system. Biochim Biophys Acta. 2002;1584:73-80. [Cited in This Article: ] |
| 22. | Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655-1657. [Cited in This Article: ] |
| 23. | Hanada M, Feng J, Hemmings BA. Structure, regulation and function of PKB/AKT--a major therapeutic target. Biochim Biophys Acta. 2004;1697:3-16. [Cited in This Article: ] |
| 24. | Asano T, Fujishiro M, Kushiyama A, Nakatsu Y, Yoneda M, Kamata H, Sakoda H. Role of phosphatidylinositol 3-kinase activation on insulin action and its alteration in diabetic conditions. Biol Pharm Bull. 2007;30:1610-1616. [Cited in This Article: ] |
| 25. | Kim YB, Peroni OD, Franke TF, Kahn BB. Divergent regulation of Akt1 and Akt2 isoforms in insulin target tissues of obese Zucker rats. Diabetes. 2000;49:847-856. [Cited in This Article: ] |
| 26. | Ijuin T, Takenawa T. SKIP negatively regulates insulin-induced GLUT4 translocation and membrane ruffle formation. Mol Cell Biol. 2003;23:1209-1220. [Cited in This Article: ] |
| 27. | Matsushima R, Harada N, Webster NJ, Tsutsumi YM, Nakaya Y. Effect of TRB3 on insulin and nutrient-stimulated hepatic p70 S6 kinase activity. J Biol Chem. 2006;281:29719-29729. [Cited in This Article: ] |
| 28. | He L, Marecki JC, Serrero G, Simmen FA, Ronis MJ, Badger TM. Dose-dependent effects of alcohol on insulin signaling: partial explanation for biphasic alcohol impact on human health. Mol Endocrinol. 2007;21:2541-2550. [Cited in This Article: ] |
| 29. | Balendran A, Casamayor A, Deak M, Paterson A, Gaffney P, Currie R, Downes CP, Alessi DR. PDK1 acquires PDK2 activity in the presence of a synthetic peptide derived from the carboxyl terminus of PRK2. Curr Biol. 1999;9:393-404. [Cited in This Article: ] |
| 30. | Kroner C, Eybrechts K, Akkerman JW. Dual regulation of platelet protein kinase B. J Biol Chem. 2000;275:27790-27798. [Cited in This Article: ] |
| 31. | Ferguson KM, Kavran JM, Sankaran VG, Fournier E, Isakoff SJ, Skolnik EY, Lemmon MA. Structural basis for discrimination of 3-phosphoinositides by pleckstrin homology domains. Mol Cell. 2000;6:373-384. [Cited in This Article: ] |
| 32. | Bi XP, Tan HW, Xing SS, Wang ZH, Tang MX, Zhang Y, Zhang W. Overexpression of TRB3 gene in adipose tissue of rats with high fructose-induced metabolic syndrome. Endocr J. 2008;55:747-752. [Cited in This Article: ] |
| 33. | He L, Simmen FA, Mehendale HM, Ronis MJ, Badger TM. Chronic ethanol intake impairs insulin signaling in rats by disrupting Akt association with the cell membrane. Role of TRB3 in inhibition of Akt/protein kinase B activation. J Biol Chem. 2006;281:11126-11134. [Cited in This Article: ] |