Published online May 21, 2009. doi: 10.3748/wjg.15.2381
Revised: April 11, 2009
Accepted: April 18, 2009
Published online: May 21, 2009
AIM: To detect and evaluate the antibodies against Helicobacter pylori (H pylori) neutrophil-activating protein (HP-NAP) in patients with gastric cancer and other gastroduodenal diseases.
METHODS: Recombinant HP-NAP was prepared from a prokaryotic expression system in Escherichia coli. Serum positivity and level of HP-NAP-specific antibodies in sera from 43 patients with gastric cancer, 28 with chronic gastritis, 28 with peptic ulcer, and 89 healthy controls were measured by rHP-NAP-based ELISA. rHP-NAP-stimulated production of interleukin-8 (IL-8) and growth-related oncogene (GROα) cytokines in the culture supernatant of SGC7901 gastric epithelial cells was also detected.
RESULTS: The serum positivity and mean absorbance value of HP-NAP-specific antibodies in the gastric cancer group (97.7% and 1.01 ± 0.24) were significantly higher than those in the chronic gastritis group (85.7% and 0.89 ± 0.14, P < 0.005) and healthy control group (27.7% and 0.65 ± 0.18, P < 0.001). The sensitivity and specificity of ELISA for the detection of HP-NAP-specific antibodies were 95.5% and 91.5%, respectively. HP-NAP could slightly up-regulate IL-8 production in gastric epithelial cell lines but had no effect on GROα production.
CONCLUSION: Infection with virulent H pylori strains secreting HP-NAP is associated with severe gastroduodenal diseases, and HP-NAP may play a role in the development of gastric carcinoma. rHP-NAP-based ELISA can be used as a new method to detect H pylori infection. The direct effect of HP-NAP on gastric epithelial cells may be limited, but HP-NAP may contribute to inflammatory response or carcinogenesis by activating neutrophils.
-
Citation: Long M, Luo J, Li Y, Zeng FY, Li M. Detection and evaluation of antibodies against neutrophil-activating protein of
Helicobacter pylori in patients with gastric cancer. World J Gastroenterol 2009; 15(19): 2381-2388 - URL: https://www.wjgnet.com/1007-9327/full/v15/i19/2381.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.2381
Helicobacter pylori (H pylori), a microaerophilic Gram-negative bacterium, infects the stomach of more than 50% of human population worldwide and is a major cause of chronic gastritis and peptic ulcer. Furthermore, it is associated with gastric adenocarcinoma and gastric B cell lymphoma. In 1994, the World Health Organization classified H pylori infection as a definite (class 1) carcinogen[1]. H pylori colonization is followed by infiltration of neutrophils, macrophages and lymphocytes in gastric mucosa. The degree of mucosal damage is closely associated with the extent of neutrophil infiltration[2–4].
Multiple bacterial virulence factors, such as vacA, cagA and lipopolysaccharide (LPS), can modulate H pylori-induced inflammation. H pylori neutrophil-activating protein (HP-NAP), a 150-kDa iron-binding protein, is a ball-shaped dodecamer formed by four-helix bundled subunits with its sequence similar to that of bacterioferritins and DNA binding proteins[56]. It has been designated as a neutrophil-activating factor because it promotes the adherence of neutrophils to endothelial cells and stimulates production of reactive oxygen species (ROS) in neutrophils by activating nicotinamide adenine dinucleotide phosphate (NADPH) oxidase in plasma membrane[7–11]. Satin et al[12] demonstrated that the purified recombinant HP-NAP is chemotactic for human neutrophils and monocytes, induces surface expression of β2-integrin, which mediates endothelial transmigration, adhesion and accumulation of leucocytes at the site of H pylori infection. Recombinant HP-NAP induces the production of ROS by neutrophils via a cascade of intracellular activation events, including increased cytosolic calcium and phosphorylation of proteins, leading to the assembly of functional NADPH oxidase on neutrophil plasma membrane. In addition, HP-NAP increases the synthesis of tissue factor and secretion of type 2 inhibitor of plasminogen activator in monocytes[1314], contributing to the inflammation of gastric mucosa by fibrin deposition. These studies indicate that HP-NAP is a virulence factor relevant to the pathogenic effect of H pylori.
It was recently reported that HP-NAP promotes a Th1 immune response by inducing the expression of IL-12 and IL-23 in neutrophils and monocytes, and also elicits an antigen-specific Th1-polarized T cell response in gastric mucosa of H pylori-infected patients in vivo[15]. It has been shown that HP-NAP is able to shift antigen-activated human T cells from a Th2 to a Th1 cytotoxic phenotype characterized by production of IFN-γ and TNF-α[16]. Additionally, the majority of infected patients have antibodies against this antigen, and vaccination of mice with HP-NAP induces protection against a subsequent challenge with H pylori[12]. Therefore, HP-NAP is an immune modulator promoting Th1 immune responses and an important vaccine candidate antigen[1718].
Since HP-NAP is a powerful stimulant for the production of ROS, mediating damage to DNA and enhancing cell turnover[19], it may be a risk factor for H pylori-associated gastric cancer. Currently, it is uncertain whether HP-NAP is related to the occurrence of gastric cancer. Interleukin-8 (IL-8) and growth-related oncogene (GROα) are members of the CXC chemokine family that induce neutrophil chemotaxis and activation. It has been shown that IL-8 and GROα levels are elevated in H pylori-infected gastric mucosa[20]. In addition, H pylori water soluble surface proteins up-regulate the expression of IL-8 and GROα mRNA and protein by neutrophils[21]. Whether HP-NAP contributes to the inflammatory response or carcinogenesis by up-regulating IL-8 and GROα production in H pylori-infected gastric mucosa remains unknown. An understanding of the relation between HP-NAP and gastric cancer and the molecular mechanism(s) underlying HP-NAP-induced diseases should lead to improved approaches to the effective control of H pylori-associated gastric cancer.
In the present study, recombinant HP-NAP was prepared from a prokaryotic expression system in Escherichia coli, and the napA gene in 20 H pylori clinical isolates from South China was detected by PCR. The seropositivity and level of HP-NAP-specific antibodies in sera from 43 patients with gastric cancer, 28 with chronic gastritis, 28 with peptic ulcer, and 89 healthy controls were measured by rHP-NAP-based ELISA. The production of IL-8 and GROα cytokines in culture supernatant from SGC7901 gastric epithelial cells stimulated by rHP-NAP was also detected.
H pylori NCTC11639 strain was stored at -70°C in our department. Bacteria were routinely cultured on Columbia agar plates supplemented with 10% defibrinated sheep blood, 0.004% triphenyltetrazolium chloride, and Dent selective supplement (Oxoid, Basingstoke, UK) at 37°C for 3 d under a microaerophilic atmosphere containing 50 mL/L O2, 150 mL/L CO2 and 800 mL/L N2. Several colonies were then picked up and inoculated into 20 mL of Brucella broth (Becton Dickinson, Cockeysville, MS) containing 0.1% β-cyclodextrin supplemented with 5% (v/v) fetal calf serum. After 24 h, 2 mL of culture was transferred to 40 mL of fresh medium, and the same process was repeated twice. Finally, 1 mL of the incubated medium containing the bacterial cells, most of which were spiral rather than coccoid, was plated on Brucella agar (Becton Dickinson) containing 10% (v/v) defibrinated sheep blood and cultured at 37°C for an additional 3 d in a microaerophilic atmosphere containing 50 mL/L O2, 150 mL/L CO2 and 800 mL/L N2. Bacterial cells were harvested, washed twice with cold phosphate-buffered saline (PBS, 25 mmol/L sodium phosphate, pH 7.2, 0.9% NaCl), and then sedimented by centrifugation at 5000 ×g for 10 min at 4°C. The cell pellet was stored at -80°C.
Human gastric epithelial cells (SGC7901) were cultured at 37°C in RPMI-1640 (Gibco, USA) containing 10% FBS (Gibco) in a humidified atmosphere containing 50 mL/L CO2, and plated at 106 cells/well in 24-well plates. The medium was changed every 3 d and replaced with RPMI-1640 without serum before experiment.
H pylori infection was diagnosed by histological examination of endoscopic biopsy specimens and CLO testing. Forty-three serum samples were collected from patients with gastric cancer at Southern Hospital, Guangzhou, China. The age of patients ranged 27-83 years. Twenty-eight serum samples were also collected from patients with peptic ulcer or chronic gastritis at Southern Hospital. The age of patients ranged 28-67 years. Finally, 89 serum samples were collected from healthy blood donors at the age of 18-70 years.
Genomic DNA of H pylori was prepared using a Takara kit (Takara, Japan) according to its manufacturer’s instructions. The extracted genomic DNA was then used as a template for amplification of the NAP coding region using a Taq DNA polymerase PCR kit (Takara, Japan)[2223]. Two primer sequences corresponding to the 5′ and 3′ ends of the coding gene, including EcoRI and XhoI restriction sites, were used: P1: 5'CCGGAATTCATGAAAACATTTGAA-3′, P2: 5'CCGCTCGAGTTAAGCCAAATGGGC-3′.
The PCR product was cloned into the expression vector pGEX-4T-1 (Amersham Biosciences). The plasmid was then transformed into E. coli strain TOP10 (Invitrogen BV, Leek, The Netherlands), and NAP expression was induced with 1 mmol/L isopropyl-β-d-1-thiogalactopyranoside when the cells were grown to the log phase at room temperature. After 4 h, the cells were harvested by centrifugation and washed with ice-cold PBS containing 5 mmol/L EDTA and 2 mmol/L PMSF. All subsequent procedures were performed at 4°C. The NAP-GST fusion protein was purified by glutathione-sepharose 4B column chromatography.
Anti-H pylori IgG antibodies in serum samples were assayed by indirect ELISA using a diagnostic kit (BIOCUP, Shenzhen, China). According to the instructions, serum (l00 &mgr;L, diluted 1/100 in PBS) was added to ELISA plates pre-coated with purified H pylori antigens in duplicate and incubated for 1 h. Controls consisted of wells with PBS alone, H pylori negative and positive serum, which were considered blank, negative and positive controls, respectively. Peroxidase-conjugated anti-human IgG (1/5000) was added and incubated for 30 min, after which the plates were washed with PBS, and color reaction was initiated by the addition of TMB (100 &mgr;L). After 10 min, the reaction was terminated by the addition of 1 mol/L H2SO4 (100 &mgr;L). The plate reader was calibrated to the blank well and the absorbance at 450 nm was read. Cut-off value (C.O.) = 2.1 × Nc (Nc = the mean absorbance value for three negative controls). Samples with absorbance > 0.21 were considered positive. The anti-H pylori IgG seropositive individuals were considered to be H pylori-infected healthy individuals.
Recombinant HP-NAP was prepared and purified, and ELISA was carried out as previously described[24–27]. Briefly, immunoplates (Nunc, Denmark) were coated with rNAP (5&mgr;g/well) and incubated overnight at 4°C. After washed three times with a washing buffer containing PBS (pH 7.2) and 0.1% Tween 20, the plates were blocked with 200 &mgr;L of 10% bovine serum in PBS and incubated in a moist chamber for 1 h at 37°C, then washed three times with a washing buffer containing PBS (pH 7.2) and 0.1% Tween 20. One hundred microlitre of serum samples (1:100) from patients or healthy individuals was then added to the microtiter wells, and the plates were incubated at 37°C for 60 min. After washed with PBS, 100 &mgr;L of secondary antibody (goat anti-human IgG-HRP, 1:10 000) was added to each well and incubated for 60 min at 37°C. After washed with PBS, 100 &mgr;L of TMB/H2O2 substrate was added to the wells and incubated at room temperature for about 10 min. The reaction was terminated by adding 100 &mgr;L of 2 mol/L H2SO4. The absorbance of each well was read at 450 nm. Samples with absorbance > 0.78 were considered positive. Each sample was tested in duplicate.
A receiver operating curve (ROC) was plotted to calculate the cut-off values for antibodies against HP-NAP at a 95% accuracy level[26]. In addition, the cut-off values were determined by mean plus 2 × SD and mean plus 3 × SD, derived from P values in healthy individuals.
Genomic DNA of 20 H pylori clinical isolates was prepared and used as a template for amplification of the NAP coding region with two primer sequences as preciously described[28]. PCR was carried out in a final volume of 60 &mgr;L. A preliminary denaturation step at 95°C for 5 min was followed by 30 amplification cycles, each consisting of denaturation at 94°C for 30 s, annealing at 50°C for 30 s, extension at 72°C for 45 s, and a final extension at 72°C for 7 min. Then, 10 &mgr;L of amplicon was transferred onto a 1.5% (w/v) agarose gel in 1 × TBE buffer, and electrophoresis was performed at 80 V for 30 min in a DNA submarine plate. The gel was then stained with ethidium bromide.
Before use, human gastric epithelial cells (SGC7901) growing in 24-well plates were washed and replaced with RPMI-1640 without serum, and then stimulated with 10 &mgr;g rHP-NAP for 12 h and 36 h, respectively. The supernatant was centrifuged to remove particulate debris and stored in aliquots at -70°C. IL-8 and GROα concentrations in the culture supernatant were measured by ELISA using a Quantikine immunoassay kit (R&D, USA) according to its manufacturer’s instructions. Before detection, the culture supernatant was filtered through a sterile membrane filter (0.22 &mgr;m. pore size).
Statistical analyses were conducted using the SPSS software package. One-way ANOVA was performed to assess differences among groups. Post hoc multiple comparisons between different infectious groups were done by LSD analysis. Comparisons between the seropositivity of anti-HP-NAP antibodies in patients with gastric cancer and other gastroduodenal diseases were done by chi-square test.
The gene encoding for HP-NAP was obtained by PCR amplification using genomic DNA extracted from H pylori as a template. Agarose gel electrophoresis analysis of the PCR product is shown in Figure 1.
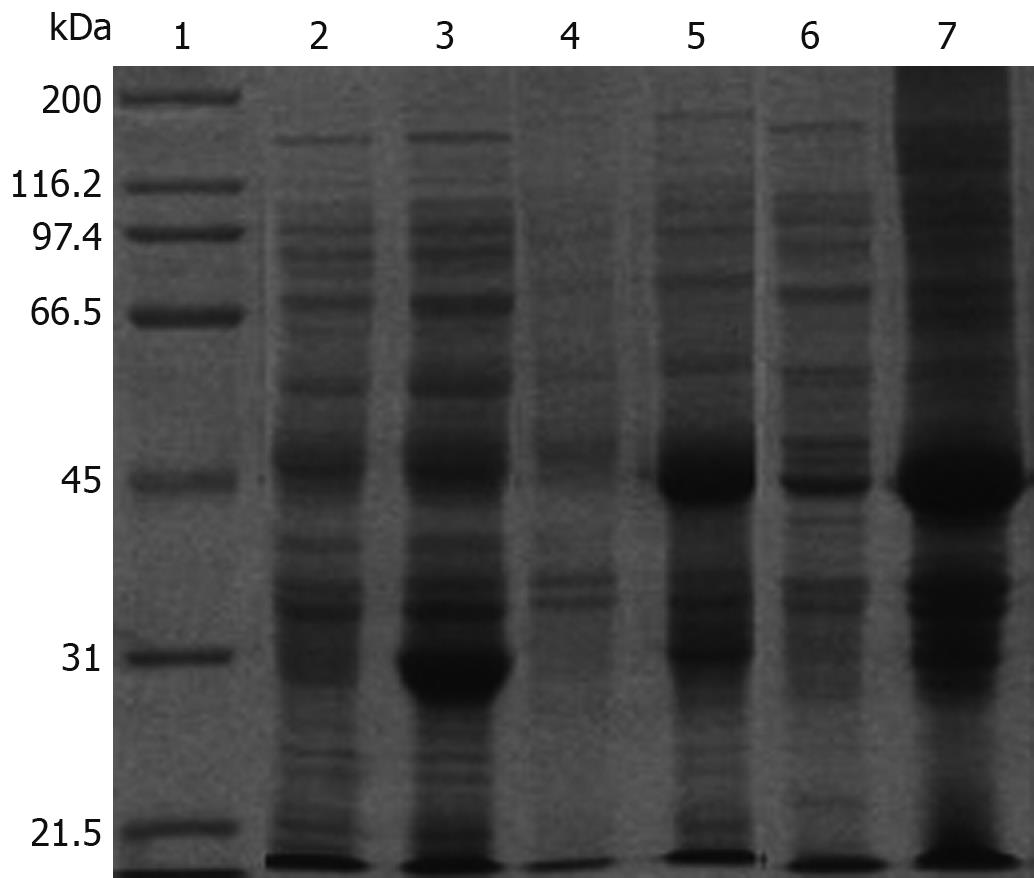
A 435 bp DNA fragment was detected, which corresponded to the size of the HP-NAP gene. The DNA sequence of the hypothetical HP-NAP gene of H pylori was determined (data not shown) and submitted to GenBank (accession No. DQ341279). To facilitate purification, HP-NAP was expressed as a GST fusion protein in E. coli Top10 cells. Expression of the recombinant NAP-GST was examined by SDS-PAGE. A pronounced band with an approximate molecular weight of 44 kDa appeared in the supernatant of cell lysate after induction but not in control cells, suggesting that the fusion protein can be successfully expressed in bacterial cells (Figure 2).
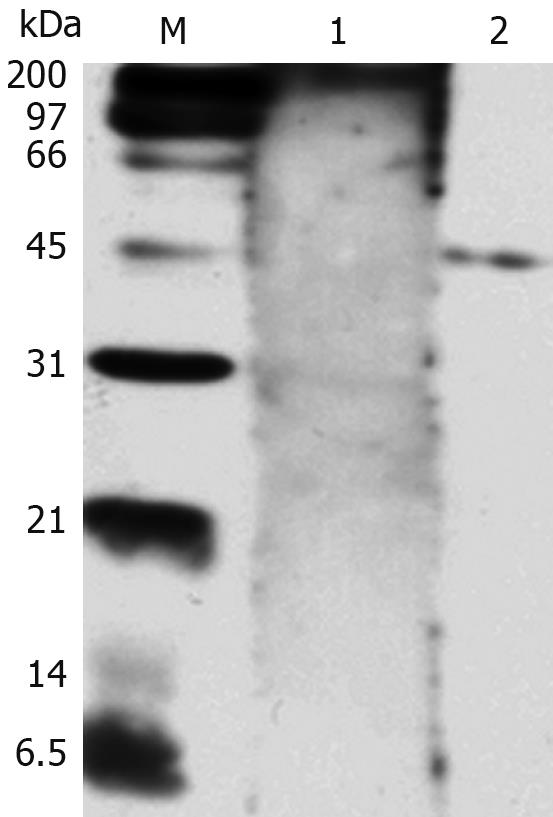
The purified NAP-GST fusion protein was further confirmed by Western blotting. Serum from H pylori-infected patients specifically recognized the recombinant NAP-GST fusion protein, while negative serum did not (Figure 3).
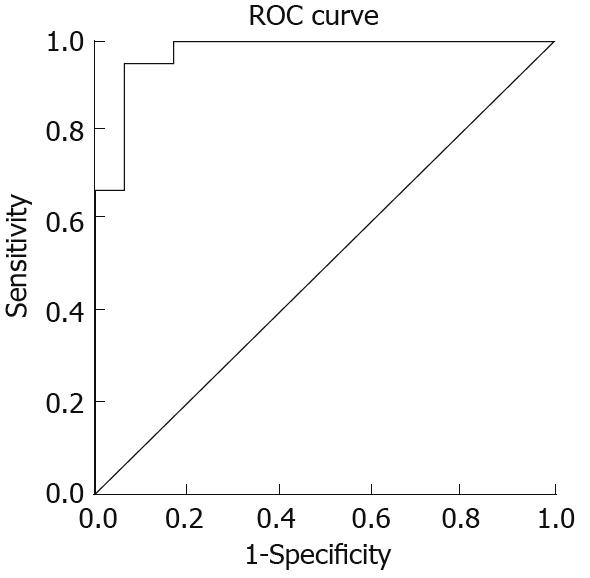
A cut-off value of 0.78 was determined for ELISA of HP-NAP antibodies using ROC analysis (Figure 4). The area under the ROC curve was 0.97. Overall, ELISA yielded a sensitivity of 95.5% (95% confidence interval) and a specificity of 91.5% (95% confidence interval) for the detection of antibodies against HP-NAP in serum.
Anti-H pylori IgG seropositivity was detected in 47 of the 89 healthy subjects who were considered H pylori-infected healthy individuals. As shown in Table 1, the anti-H pylori IgG and anti-HP-NAP seroposivity in healthy individuals was 52.8% (47/89) and 14.6% (13/89), respectively. The anti-H pylori IgG seropositivity was much higher than the anti-HP-NAP antibody seropositivity (P < 0.005). The anti-HP-NAP antibody seropositivity, however, was 27.7% (13/47) in H pylori-infected healthy individuals.
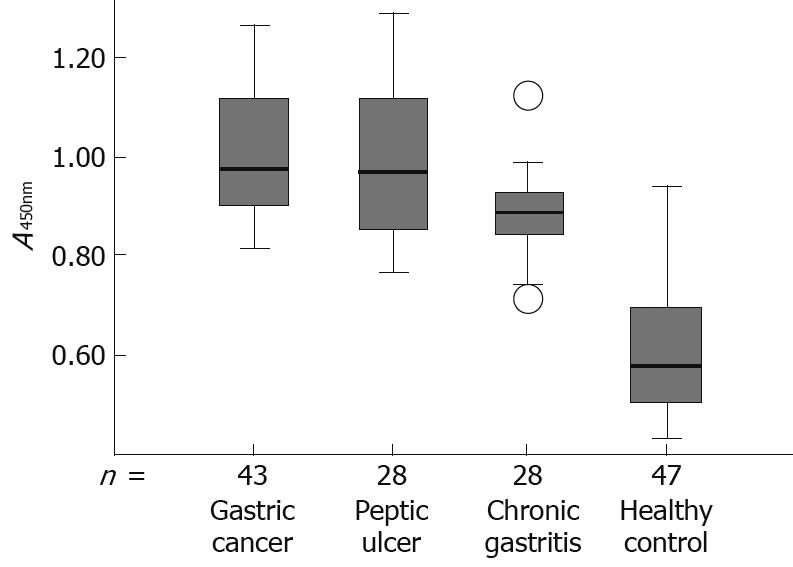
The seropositivity for antibodies against HP-NAP in gastric cancer and peptic ulcer patients was 97.7% (42/43) and 92.8% (26/28), respectively, while the seropositivity for antibodies against HP-NAP in chronic gastritis patients was 85.7% (24/28). The seropositivity for antibodies against HP-NAP in infected healthy controls was 27.7%. The seropositivity for HP-NAP-specific antibodies in gastric cancer patients was higher than that in chronic gastritis patients (P < 0.05) and in infected healthy controls (P < 0.01). The difference in the mean absorbance value for antibodies against HP-NAP between groups was also confirmed by one-way ANOVA analysis (F = 4.014, P = 0.023). The mean absorbance value for anti-HP-NAP antibodies in gastric cancer patients was 1.01 ± 0.24 (range 0.748-1.269), significantly higher than that in chronic gastritis patients (0.89 ± 0.14, range 0.711-1.122, P < 0.005) and in infected healthy controls (0.65 ± 0.18, range 0.451-0.948, P < 0.001). There was no significant difference, however, in the mean absorbance value for anti-HP-NAP antibodies between patients with gastric cancer (1.01 ± 0.24, range 0.748-1.269) and peptic ulcer (0.98 ± 0.32, range 0.771-1.265) (Table 2).
| Patient group | Positivity for HP-NAP antibodies | Negativity for HP-NAP antibodies | Absorbance (mean ± 2SD) (cut-off value 0.78) | Range |
| Gastric cancer group (n = 43) | 42 (97.7) | 1 (2.3) | 1.01 ± 0.24 | 0.748-1.269 |
| Peptic ulcer group (n = 28) | 26 (92.8) | 2 (7.1) | 0.98 ± 0.32 | 0.771-1.265 |
| Chronic gastritis group (n = 28) | 24 (85.7) | 4 (14.3) | 0.89 ± 0.14 | 0.711-1.122 |
| Healthy persons (HP-IgG positive n = 47) | 13 (27.7) | 34 (72.3) | 0.65 ± 0.18 | 0.451-0.948 |
Box plots of the antibodies against HP-NAP in sera from patients with gastric cancer, peptic ulcer, chronic gastritis, and healthy controls are shown in Figure 5, with the 90th percentile range and 75th and 25th percentiles.
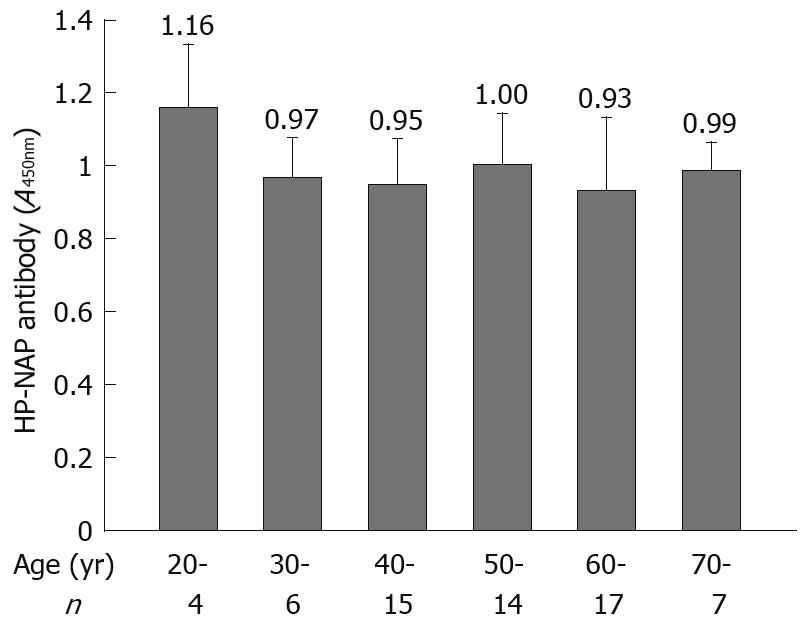
Most H pylori-associated diseases occurred in subjects at the ages of 40-70 years (53/63). The mean absorbance value for anti-HP-NAP antibodies was not significantly different in patients at different ages (Figure 6).
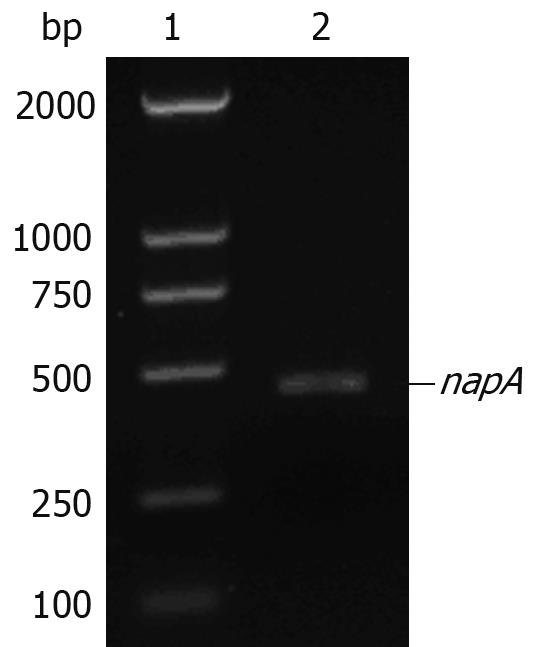
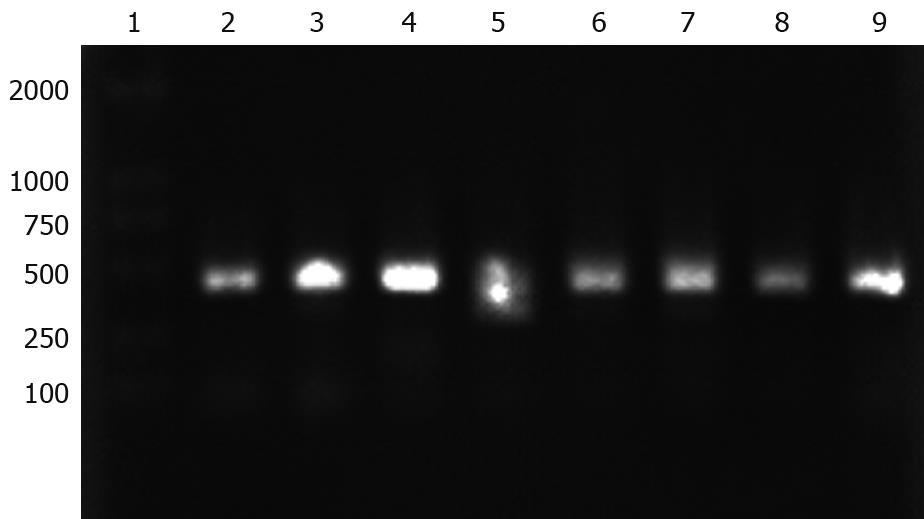
The napA gene was detected by PCR in 20 H pylori clinical isolates. All examined H pylori strains carried the napA gene. The electrophoresis results of PCR products of the napA gene from 8 representative H pylori strains are shown in Figure 7.
The IL-8 and GROα levels in culture supernatant of human gastric epithelial cell line SGC7901 were measured by ELISA, showing that HP-NAP slightly up-regulated the IL-8 production (Table 3) but had no effect on GROα production by gastric epithelial cells (data not shown).
HP-NAP, an important virulence factor for H pylori, induces adherence of neutrophils to gastric epithelial cells and causes an inflammatory reaction by activating neutrophils. In the present study, we cloned and expressed HP-NAP, which was then used as an antigen to detect HP-NAP-specific antibodies in serum samples. The seropositivity for anti-HP-NAP antibodies was detected in 89 healthy subjects and compared with that for anti-H pylori IgG antibodies in the same subjects. Meanwhile, the seropositivity and levels of HP-NAP-specific antibodies in 43 patients with gastric cancer, 28 with chronic gastritis, 28 with peptic ulcer, were measured by rHP-NAP-based ELISA. Moreover, the napA gene in 20 H pylori clinical isolates from South China was detected by PCR. The production of IL-8 and GROα cytokines in the culture supernatant of SGC7901 gastric epithelial cells stimulated with rHP-NAP was also detected.
The seropositivity for anti-H pylori IgG was 52.8% (47/89) in healthy subjects, which is in agreement with the average prevalence (about 50%-60%) of H pylori in the adult Chinese population. The 47 subjects who were serum positive for anti-H pylori IgG were considered to be H pylori-infected healthy individuals. The seropositivity for anti-HP-NAP antibodies was 27.7% (13/47) in H pylori-infected healthy individuals, due to variations in HP-NAP expression in different H pylori strains. It has been shown that the neutrophil adhesion-promoting activity in different H pylori strains varies considerably[2930], suggesting that HP-NAP is differently expressed in different H pylori strains[31]. Our results also show that the mean absorbance value for anti-HP-NAP antibodies was not significantly different in different age groups. Our work and other studies, however, have found that the napA gene is present in all H pylori clinical isolates.
H pylori is a clear (class 1) carcinogen. HP-NAP stimulates neutrophils to infiltrate gastric mucosa and subsequently causes ROS production by activating NADPH oxidase in plasma membrane. It was reported that ROS can cause a variety of DNA lesions and produce mutations in mammalian cells[32]. ROS production is significantly decreased in gastric mucosa of patients with H pylori successfully eradicated[33]. It has also been shown that HP-NAP can be involved in extravasation of leukocytes, and ROS can play a role in the carcinogenic process in gastric mucosa during chronic H pylori infection[3435], indicating that HP-NAP may be a risk factor for H pylori-associated gastric cancer.
In the present study, the seropositivity and mean absorbance value for HP-NAP-specific antibodies in gastric cancer patients (97.7% and 1.01 ± 0.24) were significantly higher than those in chronic gastritis patients (85.7% and 0.89 ± 0.14, P < 0.005) and in infected healthy controls (0.65 ± 0.18, range 0.451-0.948, P < 0.001). There was no difference, however, in the seropositivity or mean absorbance value for HP-NAP antibodies between the patients with gastric cancer and peptic ulcer, indicating that HP-NAP specific antibodies are correlated with severe gastroduodenal diseases and HP-NAP may contribute to the pathogenesis of H pylori-associated gastric cancer. Our results also show that HP-NAP had a strong antigenicity, and the majority of infected patients produced antibodies against HP-NAP.
ELISA for the detection of antibodies against HP-NAP in this study had a sensitivity of 95.5% and a specificity of 91.5%. Since the level of HP-NAP-specific antibodies may be correlated with the severity of H pylori infection, ELISA can be used in immunodiagnostic assay for H pylori-infection and in screening for a high-risk population with H pylori-associated gastric cancer.
In this study, HP-NAP slightly up-regulated IL-8 production by gastric epithelial cell lines, but had no effect on GROα production, suggesting that the direct effect of HP-NAP on gastric epithelial cells may be limited, but HP-NAP may contribute to the inflammatory response or carcinogenesis by activating neutrophils.
In conclusion, infection with virulent H pylori strains secreting HP-NAP is associated with severe gastroduodenal diseases and HP-NAP may play a role in the development of gastric carcinoma. rHP-NAP-based ELISA can be used as a new method to detect H pylori infection. We have recently developed a monoclonal antibody against HP-NAP, which might be used to detect HP-NAP expression in gastric mucosa. It would be desirable to carry out a comparative analysis to define the characteristic differences in HP-NAP expression between patients with gastric cancer and other gastroduodenal diseases. Further characterization of the interactions between HP-NAP and gastric cancer should aid in the development of novel strategies against H pylori-associated gastric cancer.
Helicobacter pylori (H pylori) infection increases the risk of developing peptic ulcer, gastric adenocarcinoma, and gastric B cell lymphoma. H pylori neutrophil-activating protein (HP-NAP), a virulence factor, promotes the adherence of neutrophils to gastric mucosa endothelial cells and stimulates high production of reactive oxygen species (ROS) in neutrophils. Since ROS can mediate DNA damage and enhance cell turnover, HP-NAP may be a risk factor for H pylori-associated gastric cancer. To prevent H pylori-related cancer, mass screening for and treatment of H pylori infection are cost-effective. Whether HP-NAP is related to the occurrence of gastric cancer is still uncertain.
HP-NAP is a virulence factor for H pylori infection and a vaccine candidate antigen. The majority of infected patients have antibodies against this antigen, and vaccination of HP-NAP in mice can protect against a subsequent challenge with H pylori. H pylori colonization is followed by infiltration of neutrophils, macrophages and lymphocytes in gastric mucosa. The degree of mucosal damage is closely correlated with the extent of neutrophil infiltration. It has been shown that HP-NAP can be involved in the extravasation of leukocytes, and ROS can play a role in the carcinogenic process of gastric mucosa during chronic H pylori infection.
An understanding of the relation between HP-NAP and gastric cancer should lead to improved approaches to the effective control of H pylori-associated gastric cancer. Seropositivity and mean absorbance value for HP-NAP-specific antibodies in gastric cancer patients were significantly higher than those in chronic gastritis patients and in infected healthy controls. There was no difference, however, in serum positivity or mean absorbance value for HP-NAP antibodies between patients with gastric cancer and peptic ulcer. These findings indicate that HP-NAP specific antibodies are correlated with severe gastroduodenal diseases and HP-NAP may contribute to the pathogenesis of H pylori-associated stomach cancer.
ELISA used for the detection of antibodies against HP-NAP in this study had a sensitivity of 95.5% and a specificity of 91.5%. Since the level of HP-NAP-specific antibodies may be correlated with the severity of H pylori infection, ELISA can be used to screen for a high-risk population with H pylori-associated gastric cancer.
H pylori, a gram-negative microaerophilic bacterium, causes a long-term mild inflammation of stomach lining and is strongly linked to the development of duodenal and gastric ulcers and stomach cancer. Over 50% of the world’s population harbor H pylori in their upper gastrointestinal tract, and infection is more prevalent in developing countries.
In this study, the authors detected and evaluated the level of antibodies against HP-NAP in patients with gastric cancer and other gastroduodenal diseases. The results suggest that HP-NAP-specific antibodies are correlated with severe gastroduodenal diseases and HP-NAP may contribute to the pathogenesis of H pylori-associated stomach cancer. ELISA can be used to screen for a high-risk population with H pylori-associated gastric cancer.
| 1. | Pounder RE, Ng D. The prevalence of Helicobacter pylori infection in different countries. Aliment Pharmacol Ther. 1995;9 Suppl 2:33-39. [Cited in This Article: ] |
| 2. | D'Elios MM, Montecucco C, de Bernard M. VacA and HP-NAP, Ying and Yang of Helicobacter pylori-associated gastric inflammation. Clin Chim Acta. 2007;381:32-38. [Cited in This Article: ] |
| 3. | Polenghi A, Bossi F, Fischetti F, Durigutto P, Cabrelle A, Tamassia N, Cassatella MA, Montecucco C, Tedesco F, de Bernard M. The neutrophil-activating protein of Helicobacter pylori crosses endothelia to promote neutrophil adhesion in vivo. J Immunol. 2007;178:1312-1320. [Cited in This Article: ] |
| 4. | Brisslert M, Enarsson K, Lundin S, Karlsson A, Kusters JG, Svennerholm AM, Backert S, Quiding-Järbrink M. Helicobacter pylori induce neutrophil transendothelial migration: role of the bacterial HP-NAP. FEMS Microbiol Lett. 2005;249:95-103. [Cited in This Article: ] |
| 5. | Zanotti G, Papinutto E, Dundon W, Battistutta R, Seveso M, Giudice G, Rappuoli R, Montecucco C. Structure of the neutrophil-activating protein from Helicobacter pylori. J Mol Biol. 2002;323:125-130. [Cited in This Article: ] |
| 6. | Cooksley C, Jenks PJ, Green A, Cockayne A, Logan RP, Hardie KR. NapA protects Helicobacter pylori from oxidative stress damage, and its production is influenced by the ferric uptake regulator. J Med Microbiol. 2003;52:461-469. [Cited in This Article: ] |
| 7. | Allen LA. The role of the neutrophil and phagocytosis in infection caused by Helicobacter pylori. Curr Opin Infect Dis. 2001;14:273-277. [Cited in This Article: ] |
| 8. | Dundon WG, Nishioka H, Polenghi A, Papinutto E, Zanotti G, Montemurro P, Del GG, Rappuoli R, Montecucco C. The neutrophil-activating protein of Helicobacter pylori. Int J Med Microbiol. 2002;291:545-550. [Cited in This Article: ] |
| 9. | Teneberg S, Miller-Podraza H, Lampert HC, Evans DJ Jr, Evans DG, Danielsson D, Karlsson KA. Carbohydrate binding specificity of the neutrophil-activating protein of Helicobacter pylori. J Biol Chem. 1997;272:19067-19071. [Cited in This Article: ] |
| 10. | Evans DJ Jr, Evans DG, Takemura T, Nakano H, Lampert HC, Graham DY, Granger DN, Kvietys PR. Characterization of a Helicobacter pylori neutrophil-activating protein. Infect Immun. 1995;63:2213-2220. [Cited in This Article: ] |
| 11. | Montecucco C, de Bernard M. Molecular and cellular mechanisms of action of the vacuolating cytotoxin (VacA) and neutrophil-activating protein (HP-NAP) virulence factors of Helicobacter pylori. Microbes Infect. 2003;5:715-721. [Cited in This Article: ] |
| 12. | Satin B, Del Giudice G, Della Bianca V, Dusi S, Laudanna C, Tonello F, Kelleher D, Rappuoli R, Montecucco C, Rossi F. The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a protective antigen and a major virulence factor. J Exp Med. 2000;191:1467-1476. [Cited in This Article: ] |
| 13. | Montemurro P, Barbuti G, Dundon WG, Del Giudice G, Rappuoli R, Colucci M, De Rinaldis P, Montecucco C, Semeraro N, Papini E. Helicobacter pylori neutrophil-activating protein stimulates tissue factor and plasminogen activator inhibitor-2 production by human blood mononuclear cells. J Infect Dis. 2001;183:1055-1062. [Cited in This Article: ] |
| 14. | Ljungh A. Helicobacter pylori interactions with plasminogen. Methods. 2000;21:151-157. [Cited in This Article: ] |
| 15. | Amedei A, Cappon A, Codolo G, Cabrelle A, Polenghi A, Benagiano M, Tasca E, Azzurri A, D'Elios MM, Del Prete G. The neutrophil-activating protein of Helicobacter pylori promotes Th1 immune responses. J Clin Invest. 2006;116:1092-1101. [Cited in This Article: ] |
| 16. | D'Elios MM, Amedei A, Cappon A, Del Prete G, de Bernard M. The neutrophil-activating protein of Helicobacter pylori (HP-NAP) as an immune modulating agent. FEMS Immunol Med Microbiol. 2007;50:157-164. [Cited in This Article: ] |
| 17. | Nishioka H, Baesso I, Semenzato G, Trentin L, Rappuoli R, Del Giudice G, Montecucco C. The neutrophil-activating protein of Helicobacter pylori (HP-NAP) activates the MAPK pathway in human neutrophils. Eur J Immunol. 2003;33:840-849. [Cited in This Article: ] |
| 18. | Montemurro P, Nishioka H, Dundon WG, de Bernard M, Del Giudice G, Rappuoli R, Montecucco C. The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a potent stimulant of mast cells. Eur J Immunol. 2002;32:671-676. [Cited in This Article: ] |
| 19. | Shimoyama T, Fukuda S, Liu Q, Nakaji S, Fukuda Y, Sugawara K. Helicobacter pylori water soluble surface proteins prime human neutrophils for enhanced production of reactive oxygen species and stimulate chemokine production. J Clin Pathol. 2003;56:348-351. [Cited in This Article: ] |
| 20. | Sieveking D, Mitchell HM, Day AS. Gastric epithelial cell CXC chemokine secretion following Helicobacter pylori infection in vitro. J Gastroenterol Hepatol. 2004;19:982-987. [Cited in This Article: ] |
| 21. | Kim JS, Jung HC, Kim JM, Song IS, Kim CY. Helicobacter pylori water-soluble surface proteins activate human neutrophils and up-regulate expression of CXC chemokines. Dig Dis Sci. 2000;45:83-92. [Cited in This Article: ] |
| 22. | Niccolai A, Fontani S, Kapat A, Olivieri R. Maximization of recombinant Helicobacter pylori neutrophil activating protein production in Escherichia coli: improvement of a chemically defined medium using response surface methodology. FEMS Microbiol Lett. 2003;221:257-262. [Cited in This Article: ] |
| 23. | Kang QZ, Duan GC, Fan QT, Xi YL. Fusion expression of Helicobacter pylori neutrophil-activating protein in E.coli. World J Gastroenterol. 2005;11:454-456. [Cited in This Article: ] |
| 24. | Xiang Z, Bugnoli M, Ponzetto A, Morgando A, Figura N, Covacci A, Petracca R, Pennatini C, Censini S, Armellini D. Detection in an enzyme immunoassay of an immune response to a recombinant fragment of the 128 kilodalton protein (CagA) of Helicobacter pylori. Eur J Clin Microbiol Infect Dis. 1993;12:739-745. [Cited in This Article: ] |
| 25. | Kashyap RS, Rajan AN, Ramteke SS, Agrawal VS, Kelkar SS, Purohit HJ, Taori GM, Daginawala HF. Diagnosis of tuberculosis in an Indian population by an indirect ELISA protocol based on detection of Antigen 85 complex: a prospective cohort study. BMC Infect Dis. 2007;7:74. [Cited in This Article: ] |
| 26. | Tang JW, Rohwäder E, Chu IM, Tsang RK, Steinhagen K, Yeung AC, To KF, Chan PK. Evaluation of Epstein-Barr virus antigen-based immunoassays for serological diagnosis of nasopharyngeal carcinoma. J Clin Virol. 2007;40:284-288. [Cited in This Article: ] |
| 27. | Ohkusu K. Cost-effective and rapid presumptive identification of gram-negative bacilli in routine urine, pus, and stool cultures: evaluation of the use of CHROMagar orientation medium in conjunction with simple biochemical tests. J Clin Microbiol. 2000;38:4586-4592. [Cited in This Article: ] |
| 28. | Greco G, Corrente M, Martella V, Pratelli A, Buonavoglia D. A multiplex-PCR for the diagnosis of contagious agalactia of sheep and goats. Mol Cell Probes. 2001;15:21-25. [Cited in This Article: ] |
| 29. | Backert S, Kwok T, Schmid M, Selbach M, Moese S, Peek RM Jr, König W, Meyer TF, Jungblut PR. Subproteomes of soluble and structure-bound Helicobacter pylori proteins analyzed by two-dimensional gel electrophoresis and mass spectrometry. Proteomics. 2005;5:1331-1345. [Cited in This Article: ] |
| 30. | Sabarth N, Hurwitz R, Meyer TF, Bumann D. Multiparameter selection of Helicobacter pylori antigens identifies two novel antigens with high protective efficacy. Infect Immun. 2002;70:6499-6503. [Cited in This Article: ] |
| 31. | Thoreson AC, Hamlet A, Celik J, Byström M, Nyström S, Olbe L, Svennerholm AM. Differences in surface-exposed antigen expression between Helicobacter pylori strains isolated from duodenal ulcer patients and from asymptomatic subjects. J Clin Microbiol. 2000;38:3436-3441. [Cited in This Article: ] |
| 32. | Drake IM, Mapstone NP, Schorah CJ, White KL, Chalmers DM, Dixon MF, Axon AT. Reactive oxygen species activity and lipid peroxidation in Helicobacter pylori associated gastritis: relation to gastric mucosal ascorbic acid concentrations and effect of H pylori eradication. Gut. 1998;42:768-771. [Cited in This Article: ] |
| 33. | Tari A, Kitadai Y, Sumii M, Sasaki A, Tani H, Tanaka S, Chayama K. Basis of decreased risk of gastric cancer in severe atrophic gastritis with eradication of Helicobacter pylori. Dig Dis Sci. 2007;52:232-239. [Cited in This Article: ] |
| 34. | Momynaliev KT, Rogov SI, Selezneva OV, Chelysheva VV, Akopian TA, Govorun VM. Comparative analysis of transcription profiles of Helicobacter pylori clinical isolates. Biochemistry (Mosc). 2005;70:383-390. [Cited in This Article: ] |
| 35. | Wang G, Hong Y, Olczak A, Maier SE, Maier RJ. Dual Roles of Helicobacter pylori NapA in inducing and combating oxidative stress. Infect Immun. 2006;74:6839-6846. [Cited in This Article: ] |