Published online Jul 28, 2009. doi: 10.3748/wjg.15.3480
Revised: May 29, 2009
Accepted: June 5, 2009
Published online: July 28, 2009
AIM: To explore the pathological findings in the entire esophagus in rats with reflux esophagitis, and the effects of ecabet sodium (ES).
METHODS: A rat model of chronic acid reflux esophagitis was used. In the treatment group, ES was administered after surgery (n = 16). No drug was administered postoperatively to the esophagitis group (n = 9). Sham-operated rats were used as a control group (n = 5). Rats were sacrificed on day 7 after the operation. The epithelial thickness and leukocyte infiltration were examined in the upper, middle and lower areas of the esophagus. The survival rate, incidence of esophageal ulcer, and mean surface area and number of esophageal ulcers were determined in the esophagitis and ES groups. Esophageal histology was assessed in all three groups.
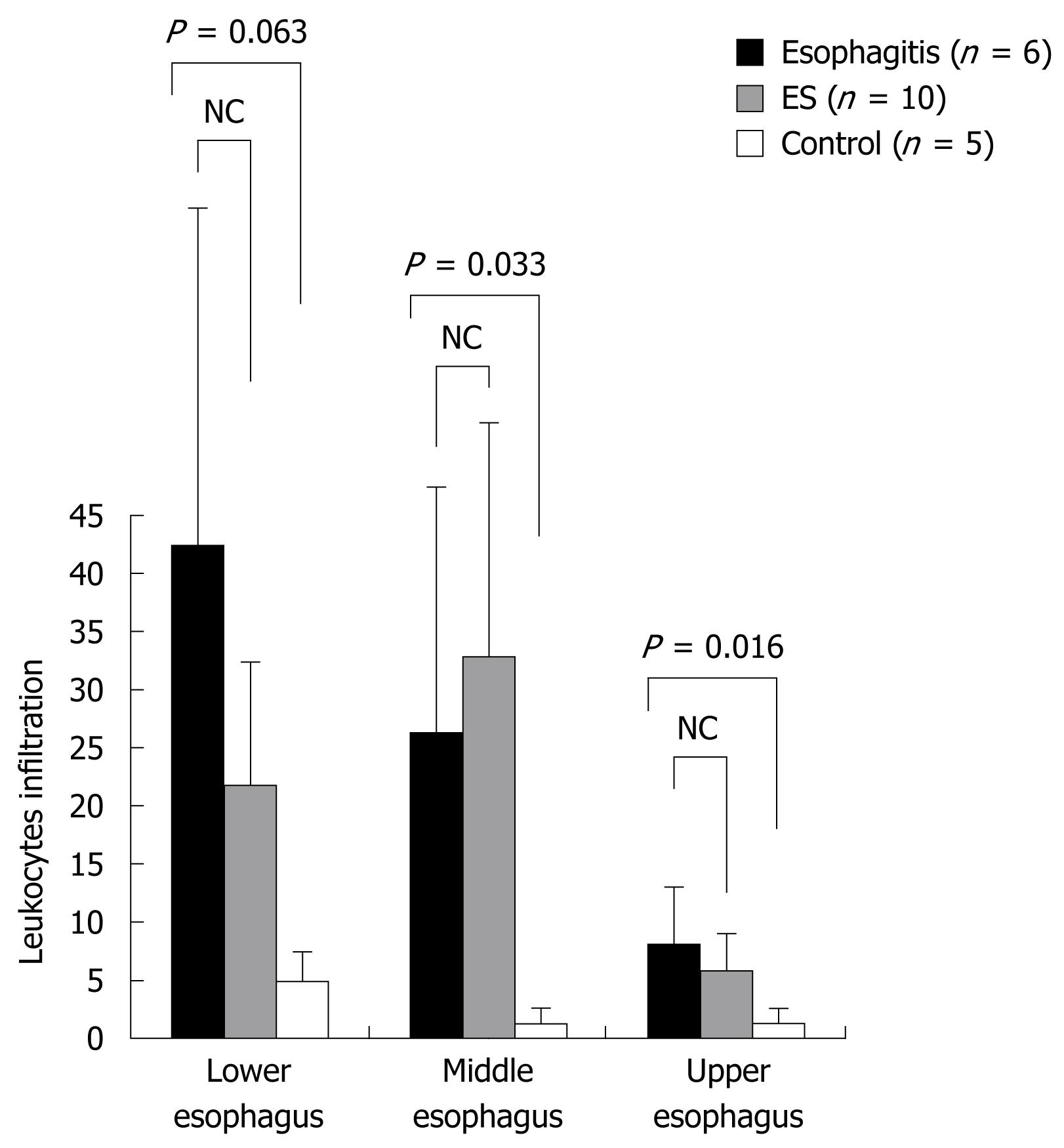
RESULTS: Leukocyte infiltration in the esophagitis group was 26.3 ± 22.0 in the middle esophagus and 8.2 ± 4.9 in the upper esophagus, which was significantly greater than that in the controls (1.3 ± 1.1 and 1.4 ± 1.0, respectively) (P < 0.05). The thickness of the epithelium in the esophagitis group was 210.8 ± 47.7 &mgr;m in the lower esophagus and 204.2 ± 60.1 &mgr;m in the middle esophagus, which was significantly greater than that in the controls (26.0 ± 5.5 and 21.0 ± 6.5 &mgr;m, respectively) (P < 0.05). The mean number of ulcers per animal in the ES group in the entire esophagus was 5.4 ± 2.5, which was significantly less than that in the esophagitis group (9.0 ± 3.5) (P < 0.05). The epithelial thickness in the ES group was 97.5 ± 32.2 &mgr;m in the lower esophagus, which was decreased compared with that in the esophagitis group (210.8 ± 47.7 &mgr;m) (P < 0.05).
CONCLUSION: Mucosal inflammation extended to the upper esophagus close to the hypopharynx. Our study suggested that ES may have a useful defensive role in reflux esophagitis.
- Citation: Asaoka D, Nagahara A, Oguro M, Izumi Y, Kurosawa A, Osada T, Kawabe M, Hojo M, Otaka M, Watanabe S. Characteristic pathological findings and effects of ecabet sodium in rat reflux esophagitis. World J Gastroenterol 2009; 15(28): 3480-3485
- URL: https://www.wjgnet.com/1007-9327/full/v15/i28/3480.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.3480
Against the background of an aging society and changing dietary habits that now include western-style food, and despite a low rate of infection with Helicobacter pylori (H pylori), the number of patients with gastroesophageal reflux disease (GERD) has increased recently in Japan[12]. The symptoms of GERD decrease quality of life[3], and long-term reflux of gastric acid is known to increase the risk of Barrett’s esophagus and Barrett’s adenocarcinoma. As a result of the global ground swell of GERD, a worldwide consensus definition of GERD has been developed recently[4]. Laryngopharyngeal reflux disease (LPRD) is a common condition in the primary care setting and is one of the extraesophageal syndromes regarded as secondary to GERD[56]. Exactly how reflux of gastric juice influences the esophagus and/or extraesophageal structures is unknown, and the causal association between gastric-juice reflux and pathogenesis of reflux esophagitis is a controversial subject.
There are limitations associated with the investigation of the pathophysiology of GERD in humans, thus, experiments using animal models are fundamental to this investigation. Proton pump inhibitors (PPIs) and histamine-2 receptor antagonists are likely to be used for reflux esophagitis, but there have been few reports about using mucosal-protective drugs. We previously induced chronic acid-reflux esophagitis in a rat model and investigated the underlying mechanism of reflux esophagitis, with a focus on the mechanism of esophageal mucosal resistance[78].
In the current study, we used a rat model of chronic acid-reflux esophagitis to explore the esophageal mucosal damage macroscopically and microscopically throughout the entire esophagus, including the upper esophagus close to the hypopharynx, and to investigate the protective effects of ecabet sodium (ES) on the esophageal mucosa.
Specific-pathogen-free male Wistar rats aged 9 wk were purchased from SLC (Tokyo, Japan). They were used after acclimatization for 1 wk in an animal room with a controlled temperature (23 ± 2°C). The rats were fed a standard diet but fasted for 12 h prior to the surgical induction of chronic acid-reflux esophagitis, which was induced by modifying the method of Omura et al[9]. Anesthesia was induced by inhalation of isoflurane. After laparotomy, duodenal stenosis was accomplished by wrapping the duodenum near the pylorus with a piece of 18 Fr Nelaton catheter (length: 3.0 mm, diameter: 4.0 mm; Terumo Inc., Tokyo, Japan). To prevent dislodgement, we sutured the edge of the catheter to the serosa of the pylorus using 4-0 nylon thread. The transitional zone between the fore-stomach and the glandular portion (i.e. the limiting ridge) was ligated with 2-0 silk thread. The animals were fasted for 48 h after the operation but were allowed free access to drinking water. In those allocated to the ES treatment group (n = 16), ES (65 mg/kg) was intragastrically administered only once immediately after surgery, and drinking water including ES (21.39 ± 2.74 mg/kg per day) was given from the day after surgery until day 7. ES was obtained from Tanabe Seiyaku Co. Ltd. (Osaka, Japan). No drug was administered after surgery to the animals in the esophagitis group (n = 9). Sham-operated rats were used as a control group (n = 5). The animals were sacrificed on day 7 after the operation. All the procedures performed on laboratory animals were approved by the Institutional Animal Care and Use Committee of Juntendo University School of Medicine (Tokyo, Japan), and all the animal experiments were carried out in compliance with the guidelines for animal experimentation of Juntendo University School of Medicine.
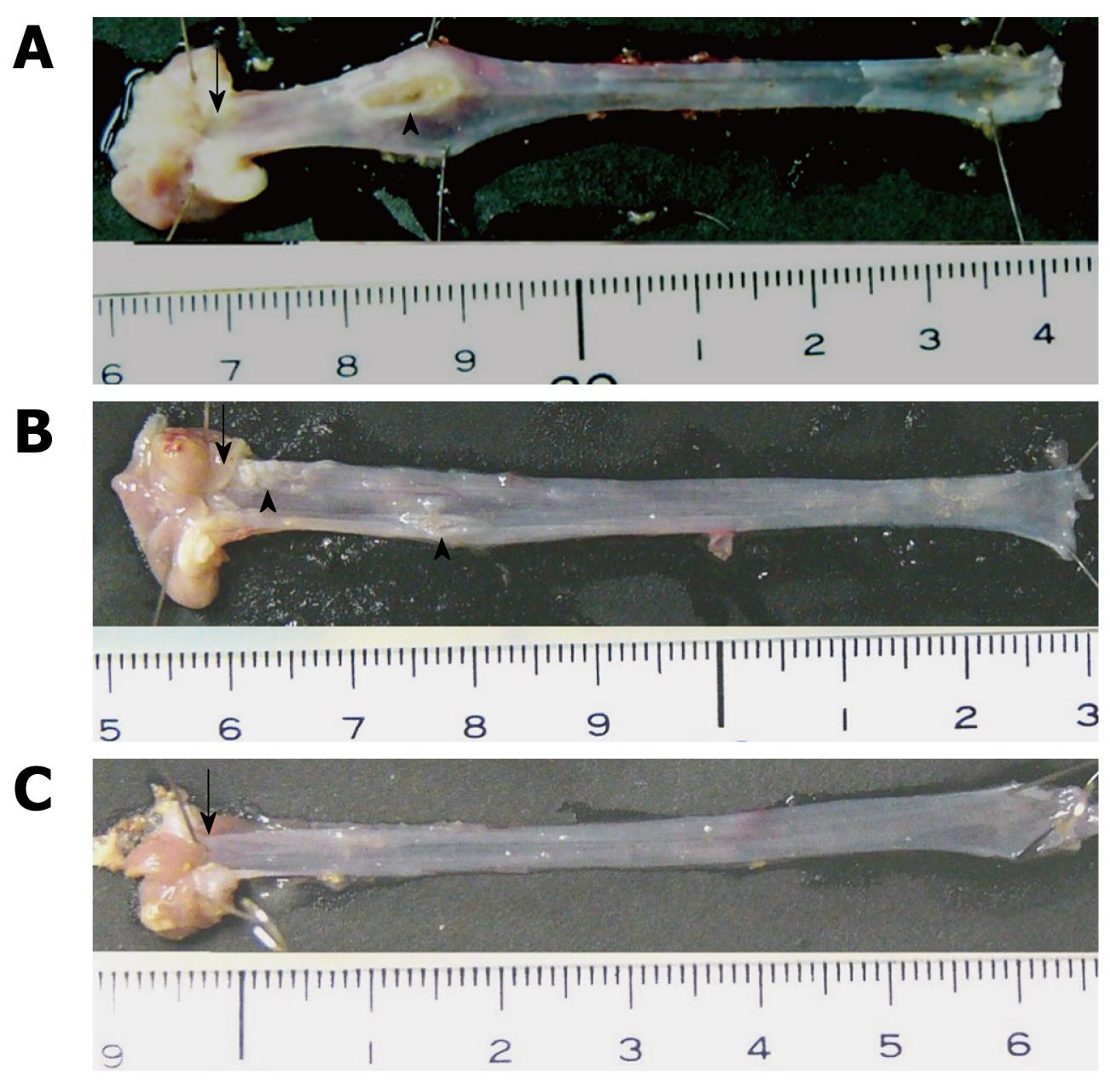
In the chronic acid-reflux esophagitis and ES groups, the survival rate and incidence of esophageal ulcers were determined. The esophagus was resected up to the upper segment close to the hypopharynx. After taking photographs of esophageal lesions, the numbers of ulcers were measured in the upper, middle and lower segments of the esophagus. The surface areas of ulcers in these three segments of the esophagus were measured using a high-resolution computerized image analyzer (KS 400; Carl Zeiss Imaging Solutions GmbH, Hallbergmoos, Germany).
After being fixed in 10% buffered formalin, the tissues were embedded in paraffin, and 3-&mgr;m sections were prepared and stained with HE. The epithelial thickness was assessed in the upper (approximately 5 mm below the cricopharyngeus), middle [midpoint between the cricopharyngeus and the esophagogastric (EG) junction] and lower (approximately 0.5 mm above the EG junction) segments of the esophagus, using light microscopy (high-power fields). The numbers of leukocytes that infiltrated each high-power field were counted in these three segments of the esophagus.
All data are presented as mean ± SD. Student’s t test and χ2 test were used, and P < 0.05 was regarded as statistically significant.
In the esophagitis group, the 7-d survival rate after the operation was 66.7% (6/9 rats), and the incidence of ulcers in the survivors was 100%. In the ES group, the 7-d survival rate after the operation was 62.5% (10/16), and the incidence of ulcers was 90.0% (9/10 rats). There were no statistically significant between-group differences in the survival rate and incidence of ulcers.
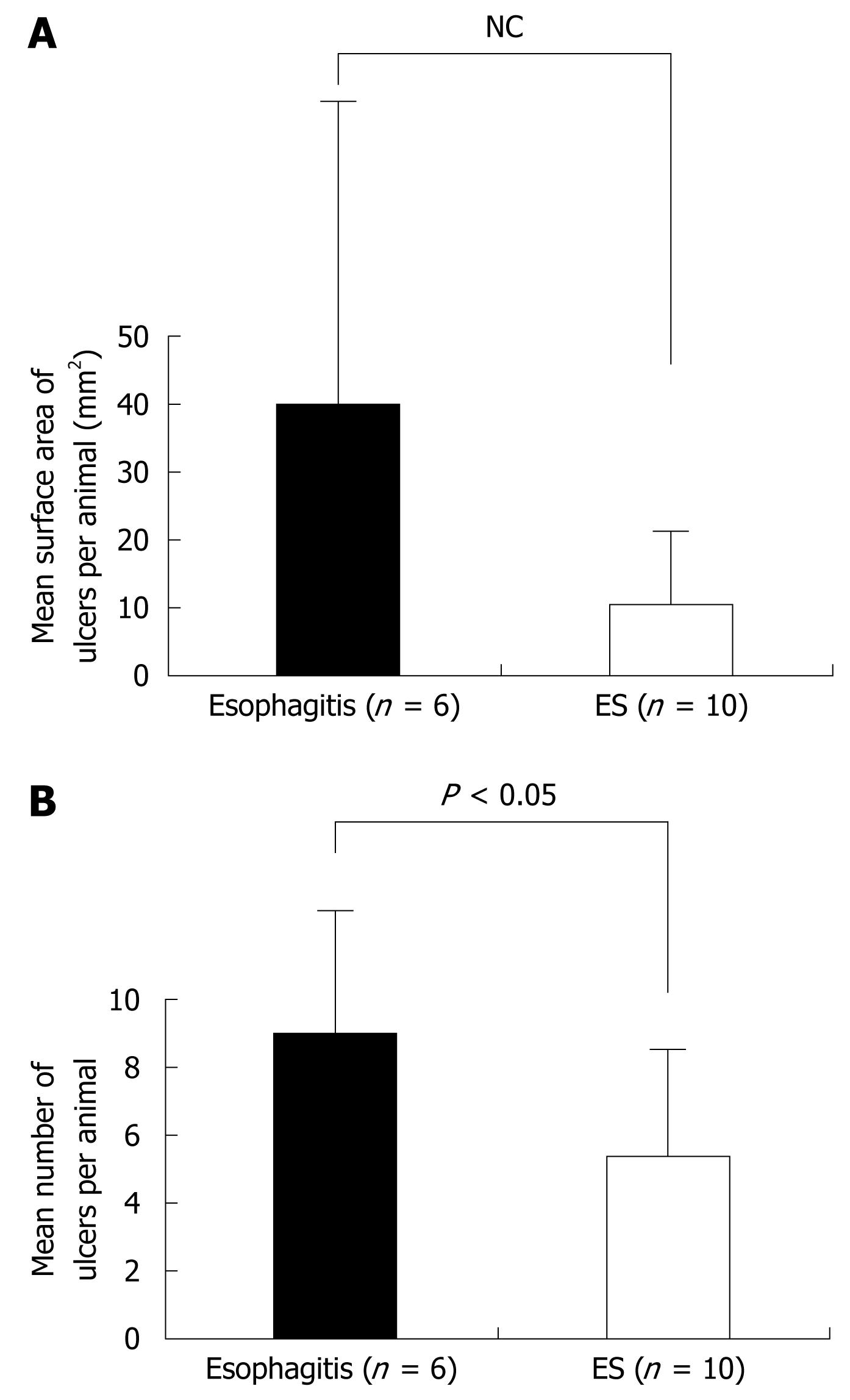
In both the esophagitis and ES groups, ulcers were noted in the mucosa, but none occurred in the control group. Also in the esophagitis and ES groups, macroscopic esophageal ulcers were found in the lower and middle parts of the esophagus in most cases (Figure 1). The mean number of ulcers per animal in the ES group in the entire esophagus was 5.4 ± 2.5, which was significantly less than the number in the esophagitis group (9.0 ± 3.5) (P < 0.05) (Figure 2). The mean surface area of ulcers per animal was 40.0 ± 56.8 and 10.5 ± 8.6 mm2 in the esophagitis and ES groups, respectively (P = 0.120) (Figure 2). No significant between-group differences were found in terms of the number and area of ulcers in each of the three individual segments of the esophagus (Figure 3).
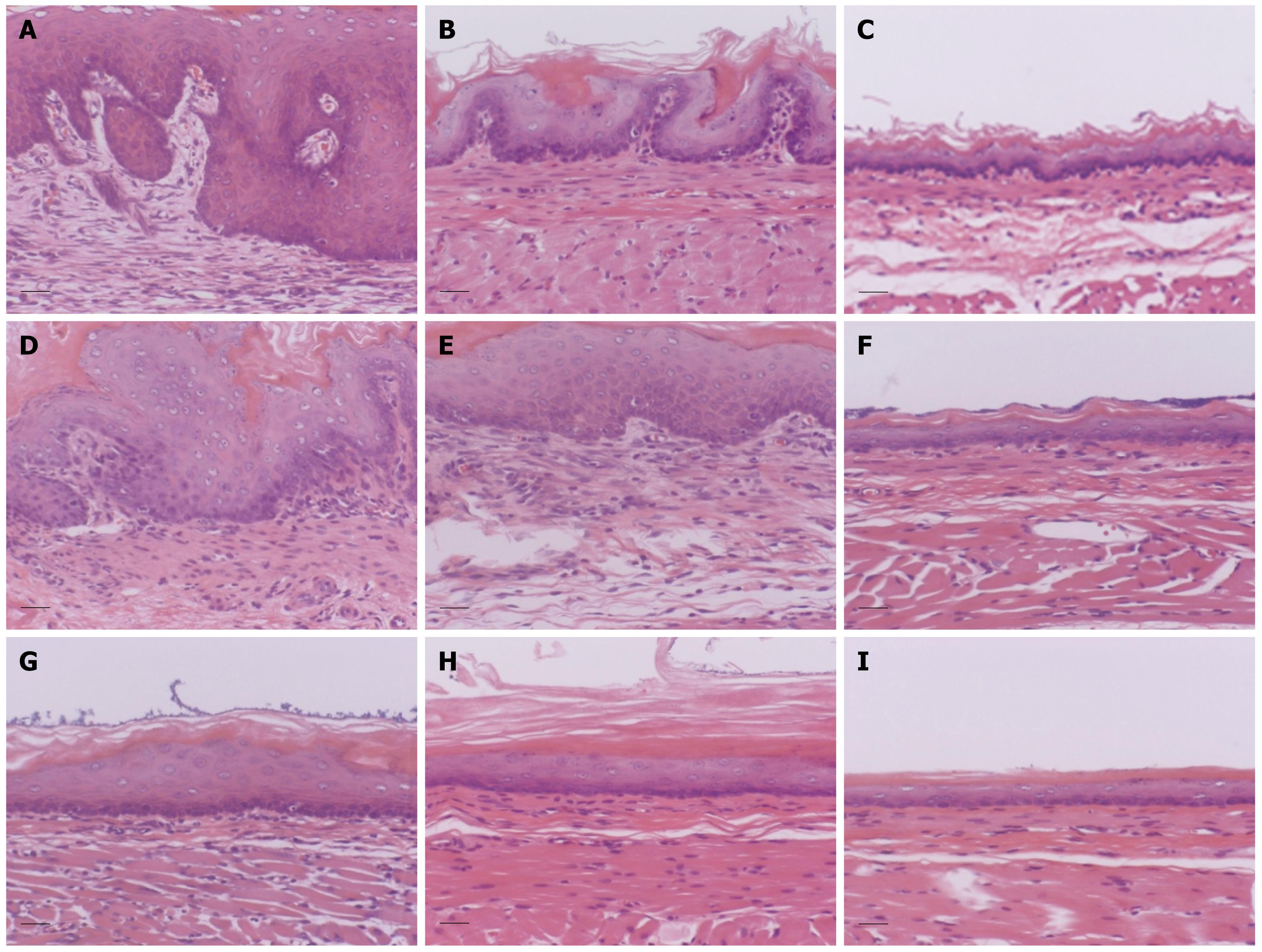
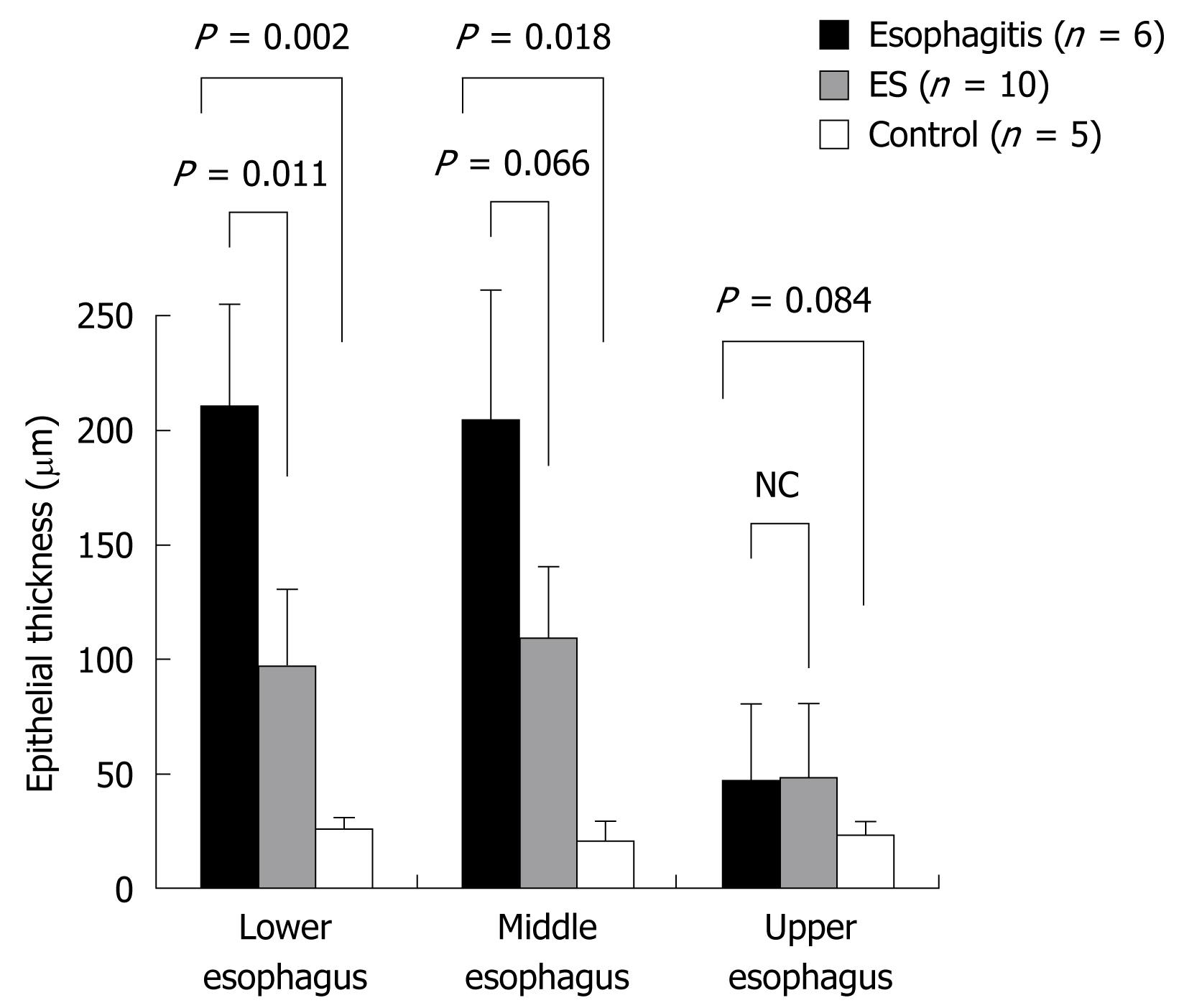
Histologically, the esophagus showed a thin epithelial layer with squamous cells in the control group. In the esophagitis group, the epithelium was thickened markedly, and elongation of the lamina propria papillae into the epithelium was noted. Basal cell hyperplasia and inflammatory cell infiltration were marked in the lamina propria (Figure 4). The thickness of the epithelium in the lower esophagus was the most severely affected of the three areas. The thickness of the epithelium in the esophagitis group was 210.8 ± 47.7 &mgr;m in the lower esophagus and 204.2 ± 60.1 &mgr;m in the middle esophagus, which was significantly greater than that in the controls (26.0 ± 5.5 and 21.0 ± 6.5 &mgr;m, respectively) (P < 0.05) (Figure 5). Also, the epithelial thickness in the ES group was 97.5 ± 32.2 &mgr;m in the lower esophagus, which was decreased compared with that in the esophagitis group (210.8 ± 47.7 &mgr;m) (P < 0.05), and the epithelial thickness in the ES group in the middle esophagus also tended to be less than that in the esophagitis group.
Leukocyte infiltration in the esophagitis group was 26.3 ± 22.0 in the middle esophagus and 8.2 ± 4.9 in the upper esophagus, which was significantly greater than that in the controls (1.3 ± 1.1 and 1.4 ± 1.0, respectively) (P < 0.05) (Figure 6).
We revealed that epithelial thickening occurs at the same time as inflammatory cell infiltration in the middle to lower esophagus in chronic acid-reflux esophagitis. An imbalance between offensive factors (e.g. gastric acid and pepsin) and defensive factors (e.g. mucin, saliva and the esophageal epithelial lining) results in the development of reflux esophagitis[71011]. However, there have been few reports about the pathological findings in the esophageal squamous epithelium, and there are differing opinions among pathologists about the findings considered characteristic of chronic reflux esophagitis[12–14]. Some authors have reported the relationship between chronic inflammation and epithelial changes in other parts of the gastrointestinal tract. Yasunaga et al[15] have suggested that increased interleukin 1β and hepatocyte growth factor production caused by H pylori infection may contribute to fold thickening of the stomach by stimulating epithelial cell proliferation and foveolar hyperplasia in patients with enlarged fold gastritis[15]. In patients with erosive reflux disease, the thickness of the basal cell layer and length of the papillae were associated with the severity of esophagitis. After esomeprazole treatment, the basal layer thickness and length of papillae were reduced in both non-erosive and erosive reflux disease[16]. However, these studies were performed in human subjects, so detailed pathological investigation in chronic reflux esophagitis was not performed.
Recently, the mechanism by which acid may induce inflammation has been proposed by Tobey et al[17]. They clarified that the permeability of the esophageal epithelium to acid is increased by dilatation of the intracellular space, and PPIs may decrease inflammation of the esophageal epithelium[1718]. These results suggest that acid may diffuse through the esophageal epithelium and induce infiltration of inflammatory cells. This increases the release of proinflammatory cytokines, which may induce thickening of the esophageal epithelium. Furthermore, we demonstrated that inflammatory cells infiltrated the epithelium of the upper esophagus close to the hypopharynx, where there was no evidence of ulcers. It has been reported previously that inflammation mainly occurs in the lower esophagus near the EG junction in cases of reflux esophagitis[8], but how the reflux of gastric juice influences esophageal and/or extraesophageal symptoms is unknown. Recently, GERD-related extraesophageal syndromes have attracted attention and it has been suggested that gastric-juice reflux can extend to the upper esophagus close to the hypopharynx. In a study of the pathogenesis of LPRD, which is one of the extraesophageal syndromes, Tokashiki et al[19] have found that patients with LPRD show a significantly longer acid reflux time in the upper esophagus than healthy volunteers do.
In our chronic acid-reflux esophagitis model, gastric juice passed through the EG junction and diffused directly into the esophagus. This model resembles the reflux esophagitis that is seen in the clinical setting. The direct acid injury to the hypopharynx appeared to be reflected in the microscopic pathological changes in the upper esophagus.
Secondarily, we demonstrated that ES inhibited the epithelial thickening of the lower esophagus, which was the most severely inflamed segment of the esophagus, and ES also tended to inhibit the epithelial thickening of the middle esophagus. ES, a dehydroabietic acid derivative from pine resin, has been used clinically in the treatment of gastritis and gastric ulcer, and is believed to exert its effects through various mechanisms[20–24]. ES binds directly to the gastric mucosa, thereby protecting the mucosa against ethanol binding, and it has been shown to inhibit pepsin activity in rat and human gastric juices.
In the present study, ES may decrease the number of ulcers in the entire esophagus by binding directly to the esophageal mucosa and inhibiting pepsin activity. ES was shown to be useful in preventing inflammation from the lower to the middle esophagus. ES may inhibit the increase in cytokines which are released as part of the inflammatory process and induce epithelial thickening. However, the relationship between the occurrence of ulcers and epithelial thickening is unknown, and further study about this relationship is necessary.
In conclusion, this study revealed that mucosal inflammation extended to the upper esophagus close to the hypopharynx, even where there was no evidence of ulcers. This finding of inflammation suggested that direct injury to the hypopharynx may occur as a result of the reflux of gastric juice. Our study also suggested that ES may play a useful defensive role in the prevention of reflux esophagitis. Further studies are necessary to explore the factors that are responsible for the protective effects of ES in reflux esophagitis.
Recently, the number of patients with gastroesophageal reflux disease (GERD) has increased in Japan. Although GERD-related extraesophageal syndromes have attracted attention, how gastric-juice reflux influences the esophagus and/or extraesophageal structures is unknown. In the present study, the authors explored the pathological findings in the entire esophagus and the effects of ecabet sodium (ES).
The authors revealed that epithelial thickening occurred at the same time as inflammatory cell infiltration in the middle to lower esophagus in chronic acid-reflux esophagitis. Furthermore, they demonstrated that inflammatory cells infiltrated the epithelium of the upper esophagus close to the hypopharynx, where there was no evidence of ulcers. These findings suggested that the reflux of gastric juice can extend to the upper esophagus close to the hypopharynx.
Laryngopharyngeal reflux disease is a common condition in the primary care setting and is one of the extraesophageal syndromes regarded as secondary to GERD.
The authors evaluated the pathological findings in rat chronic acid-reflux esophagitis and the defensive effects of ES on esophageal mucosal injury. This an interesting, well-executed study.
| 1. | Fujimoto K. Review article: prevalence and epidemiology of gastro-oesophageal reflux disease in Japan. Aliment Pharmacol Ther. 2004;20 Suppl 8:5-8. [Cited in This Article: ] |
| 2. | Watanabe S, Hojo M, Nagahara A. Metabolic syndrome and gastrointestinal diseases. J Gastroenterol. 2007;42:267-274. [Cited in This Article: ] |
| 3. | Dimenäs E. Methodological aspects of evaluation of Quality of Life in upper gastrointestinal diseases. Scand J Gastroenterol Suppl. 1993;199:18-21. [Cited in This Article: ] |
| 4. | Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900-1920; quiz 1943. [Cited in This Article: ] |
| 5. | Cherry J, Margulies SI. Contact ulcer of the larynx. Laryngoscope. 1968;78:1937-1940. [Cited in This Article: ] |
| 6. | Richter JE. Review article: extraoesophageal manifestations of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2005;22 Suppl 1:70-80. [Cited in This Article: ] |
| 7. | Orlando RC. Review article: oesophageal mucosal resistance. Aliment Pharmacol Ther. 1998;12:191-197. [Cited in This Article: ] |
| 8. | Asaoka D, Miwa H, Hirai S, Ohkawa A, Kurosawa A, Kawabe M, Hojo M, Nagahara A, Minoo T, Ohkura R. Altered localization and expression of tight-junction proteins in a rat model with chronic acid reflux esophagitis. J Gastroenterol. 2005;40:781-790. [Cited in This Article: ] |
| 9. | Omura N, Kashiwagi H, Chen G, Suzuki Y, Yano F, Aoki T. Establishment of surgically induced chronic acid reflux esophagitis in rats. Scand J Gastroenterol. 1999;34:948-953. [Cited in This Article: ] |
| 10. | Nagahama K, Yamato M, Nishio H, Takeuchi K. Essential role of pepsin in pathogenesis of acid reflux esophagitis in rats. Dig Dis Sci. 2006;51:303-309. [Cited in This Article: ] |
| 11. | Okuyama K, Saito N, Kume E, Noto T, Nagasaki M. Ecabet sodium prevents esophageal lesions induced by the reflux of gastric juice in rats. Inflammopharmacology. 2007;15:90-94. [Cited in This Article: ] |
| 12. | Takubo K, Honma N, Aryal G, Sawabe M, Arai T, Tanaka Y, Mafune K, Iwakiri K. Is there a set of histologic changes that are invariably reflux associated? Arch Pathol Lab Med. 2005;129:159-163. [Cited in This Article: ] |
| 13. | Hongo M. Minimal changes in reflux esophagitis: red ones and white ones. J Gastroenterol. 2006;41:95-99. [Cited in This Article: ] |
| 14. | Collins BJ, Elliott H, Sloan JM, McFarland RJ, Love AH. Oesophageal histology in reflux oesophagitis. J Clin Pathol. 1985;38:1265-1272. [Cited in This Article: ] |
| 15. | Yasunaga Y, Shinomura Y, Kanayama S, Higashimoto Y, Yabu M, Miyazaki Y, Kondo S, Murayama Y, Nishibayashi H, Kitamura S. Increased production of interleukin 1 beta and hepatocyte growth factor may contribute to foveolar hyperplasia in enlarged fold gastritis. Gut. 1996;39:787-794. [Cited in This Article: ] |
| 16. | Vieth M, Kulig M, Leodolter A, Nauclér E, Jaspersen D, Labenz J, Meyer-Sabellek W, Lind T, Willich S, Malfertheiner P. Histological effects of esomeprazole therapy on the squamous epithelium of the distal oesophagus. Aliment Pharmacol Ther. 2006;23:313-319. [Cited in This Article: ] |
| 17. | Tobey NA, Hosseini SS, Argote CM, Dobrucali AM, Awayda MS, Orlando RC. Dilated intercellular spaces and shunt permeability in nonerosive acid-damaged esophageal epithelium. Am J Gastroenterol. 2004;99:13-22. [Cited in This Article: ] |
| 18. | van Malenstein H, Farré R, Sifrim D. Esophageal dilated intercellular spaces (DIS) and nonerosive reflux disease. Am J Gastroenterol. 2008;103:1021-1028. [Cited in This Article: ] |
| 19. | Tokashiki R, Nakamura K, Watanabe Y, Yamaguchi H, Suzuki M. The relationship between esophagoscopic findings and total acid reflux time below pH 4 and pH 5 in the upper esophagus in patients with laryngopharyngeal reflux disease (LPRD). Auris Nasus Larynx. 2005;32:265-268. [Cited in This Article: ] |
| 20. | Kinoshita M, Endo M, Yasoshima A, Saito N, Yamasaki K, Chishima S, Narita H. Ecabet sodium, a novel locally-acting anti-ulcer agent, protects the integrity of the gastric mucosal gel layer from pepsin-induced disruption in the rat. Aliment Pharmacol Ther. 1999;13:687-694. [Cited in This Article: ] |
| 21. | Ito Y, Nakamura S, Onoda Y, Sugawara Y, Takaiti O. Effects of the new anti-ulcer drug ecabet sodium (TA-2711) on pepsin activity. I. Inactivation of enzyme protein. Jpn J Pharmacol. 1993;62:169-174. [Cited in This Article: ] |
| 22. | Pearson JP, Roberts NB. Mucosal protective effects of ecabet sodium: pepsin inhibition and interaction with mucus. Clin Sci (Lond). 2001;100:411-417. [Cited in This Article: ] |
| 23. | Furukawa O, Kume E, Sugamoto S, Kawauchi S, Takeuchi K. Effect of ecabet disodium, a novel locally-acting antiulcer drug, on epithelial restitution following injury by hypertonic NaCl in bullfrog stomach in vitro. Digestion. 2000;62:116-125. [Cited in This Article: ] |
| 24. | Kinoshita M, Yamasaki K, Kokusenya Y, Tamaki H. Relationship between gastroprotective effect of locally acting antiulcer agent ecabet sodium and its binding to gastric mucosa in rats. Comparison with sucralfate. Dig Dis Sci. 1995;40:661-667. [Cited in This Article: ] |