Published online Oct 21, 2009. doi: 10.3748/wjg.15.4952
Revised: August 31, 2009
Accepted: September 7, 2009
Published online: October 21, 2009
AIM: To investigate the inhibitory effects of genistein on metastasis of MHCC97-H hepatocellular carcinoma cells and to explore the underlying mechanism.
METHODS: MHCC97-H hepatocellular carcinoma cells were exposed to genistein. A cell attachment assay was carried out in a microculture well pre-coated with fibronectin. The invasive activity of tumor cells was assayed in a transwell cell culture chamber, and cell cycle and apoptosis were evaluated by a functional assay. In addition, the expression and phosphorylation of FAK were detected by Western blotting. In situ xenograft transplantation of hepatocellular carcinoma was performed in 12 nude mice and lung metastasis of hepatocellular carcinoma was observed.
RESULTS: Genistein significantly inhibited the growth of MHCC97-H cells in vitro. Adhesion and invasiveness of MHCC97-H cells were inhibited in a concentration-dependent fashion, and the inhibitory effect of genistein was more potent in the 10 μg/mL and 20 μg/mL genistein-treated groups. Genistein caused G0/G1 cell cycle arrest, an S phase decrease, and increased apoptosis. The expression and phosphorylation of FAK in MHCC-97H cells were significantly decreased. In situ xenograft transplantation of hepatocellular carcinoma was also significantly suppressed by genistein. The number of pulmonary micrometastatic foci in the genistein group was significantly lower compared with the control group (12.3 ± 1.8 vs 16.6 ± 2.6, P < 0.05).
CONCLUSION: Genistein appears to be a promising agent in the inhibition of metastasis of hepatocellular carcinoma.
- Citation: Gu Y, Zhu CF, Dai YL, Zhong Q, Sun B. Inhibitory effects of genistein on metastasis of human hepatocellular carcinoma. World J Gastroenterol 2009; 15(39): 4952-4957
- URL: https://www.wjgnet.com/1007-9327/full/v15/i39/4952.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4952
As a common malignancy, hepatocellular carcinoma (HCC) is chemoresistant to most currently available chemotherapeutic agents, and is the leading cause of cancer related deaths in the world, with increasing incidence in many countries[1]. In China, primary liver cancer, of which more than 90% is HCC, remains the second leading cancer killer. HCC mainly affects middle-aged people, those in the prime of their most productive years[2]. The high incidence of metastasis accounts for the poor overall survival in HCC patients. Research into interventions for liver cancer metastasis has special priority in the anti-cancer campaign. The MHCC97-H hepatocellular carcinoma cell line has high metastatic potential and was established from a subcutaneous tumor in a high-metastatic-potential model of human HCC cells in BALB/c nu/nu mice (LCI-D20). Lung is the preferential metastasis target of MHCC97-H cells[3].
In this study, we investigated the inhibitory effects of genistein on metastasis of human HCC cells and explored the underlying mechanism.
The human HCC cell line, MHCC97-H, was obtained from the Liver Cancer Institute of Fudan University in Shanghai. The cells were cultured at 37°C in 50 mL/L CO2 air in high glucose Dulbecco’s Modified Essential Medium (DMEM; Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS; Gibco BRL, Grand Island, NY, USA). Genistein (5,7,4’-trihydroxyisoflavone) purchased from Sigma Chemical Co. (St. Louis, MO, USA) was suspended in dimethyl sulfoxide (DMSO) for the experiments.
A 96-well plate was incubated with exponentially growing cells at a density of 1 × 104 per well. Following incubation of MHCC97-H cells with or without genistein in different columns of 96-well microtiter plates, on day 1-6 methyl thiazol terazolium (MTT) was added to each well and incubated at 37°C for another 4 h before spectrophotometric detection (A595). Each assay was performed in quadruplicate.
The inhibitory rate of tumor cell growth was calculated as: (average A595 value of control group-average A595 value of genistein group)/average A595 value of control group[4].
Pre-coating of 96-well microtiter plates was performed by incubating wells with 20 mg/L fibronectin at 4°C overnight. The wells were then blocked with 2% bovine serum albumin (BSA) for 45 min at 37°C. Cells were cultured in medium (DMEM with 2% FBS) for 24 h, then harvested at about 70% confluence, resuspended in serum-free DMEM medium supplemented with 0.1% BSA and distributed in the wells (8 × 104 cells/well). The cells were incubated at 37°C in a 50 mL/L CO2 atmosphere for 20, 40, 60 and 90 min with or without genistein. The wells were washed 3 times with phosphate buffered saline (PBS) to remove unattached cells, and the attached cells were then incubated with MTT and the A595 was measured.
The invasive activity of MHCC97-H cells was assayed in Transwell cell chambers (Corning Inc., Corning, NY, USA), according to the method reported by Kido et al[5]. Polyvinylpyrrolidone-free polycarbonate filters with an 8.0 μm pore size were pre-coated with 5 μg of fibronectin in a volume of 50 μL on the lower surface. The Matrigel was diluted to 100 μg/mL with cold PBS, applied to the upper surface of the filters (5 μg/filter), and dried overnight under a hood at room temperature. Log-phase cell cultures of MHCC97-H cells were harvested and washed 3 times with serum-free DMEM, then resuspended at a final concentration of 2 × 106 cells/mL in DMEM with 0.1% BSA. Cell suspensions (100 μL) with or without genistein were added to the upper compartment and incubated for 20 h at 37°C in a 50 mL/L CO2 atmosphere. The filters were fixed with methanol and stained with Giemsa. The cells on the upper surface of the filters were removed by wiping with cotton swabs. The cells invading the lower surface of the filter through the Matrigel and the filter were manually counted under a microscope at a magnification of × 400. Each assay was performed in triplicate. The inhibitory rate of adhesion and invasion were calculated[4].
MHCC97-H cells were seeded at a density of 5 × 105 cells/well in six-well dishes. After 24 h the cells were treated with or without genistein for 72 h and harvested by trypsinization. The cells were then centrifuged at 300 g for 10 min, washed in PBS, and resuspended in cold 70% ethanol. The cells were then subjected to flow cytometric analysis on a FACScan cytofluorimeter (Becton Dickinson, Franklin Lakes, NJ, USA) after propidium iodide labeling.
Control and genistein-treated cell extracts were prepared and the proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), followed by Western blot analysis using a FAK monoclonal antibody (1:1000; SC-713; Cell signaling, USA) and pFAK polyclonal antibody (1:500; SC-6243; Cell signaling, USA) as primary antibodies. Anti-rabbit/mouse immunoglobulin G (IgG)-HRP (Beyotime Biotech, China) was used as a secondary antibody, followed by the detection of chemiluminescence using chemiluminescence kits (Beyotime Biotech, China). The optical density was determined using a scanning densitometer and analyzed using Quantity One software (Bio-Rad). House-keeping gene β-actin was used as an internal standard.
Male athymic BALB/c nu/nu mice (46-wk-old), were obtained from the Shanghai Institute of Materia Medica, Chinese Academy of Science and maintained in specific pathogen-free (SPF) conditions and fed with sterilized MF pellets and distilled water. All studies on the mice were conducted in accordance with the National Institutes of Health “Guide for the Care and Use of Laboratory Animals”. The mouse study protocol was approved by the Shanghai Medical Experimental Animal Care Commission.
MHCC97-H cells (5 × 106) in 0.2 mL of serum-free culture medium were injected subcutaneously into the upper flank region of nude mice, and the mice were observed for tumor growth. When a subcutaneous tumor had reached approximately 1.5 cm in diameter, a small piece was removed and cut into pieces of approximately 1 mm × 1 mm × 1 mm which were subsequently implanted into the livers of 12 new recipient nude mice by a method described previously[6]. Of the 12 recipient nude mice, bearing an orthotopic tumor implant, 6 were randomly selected for treatment with genistein and the remaining 6, which did not receive genistein treatment, served as controls. Genistein (50 mg/kg) was administered intraperitoneally to each mouse in the genistein group daily for 20 d, while control animals were administered the same vehicle. The mice were observed for 35 d, and then killed by cervical dislocation. The liver and lungs were excised during autopsy, lungs were fixed and embedded in paraffin and coronal sections were cut. After hematoxylin and eosin staining, micrometastatic foci were counted microscopically.
All data are the mean ± SD. The statistical significance of differences between the treated and control groups were determined by applying the one-way ANOVA and χ2 test. The statistical analysis software package Stata 6.0 was used for the tests, and P < 0.05 was considered statistically significant.
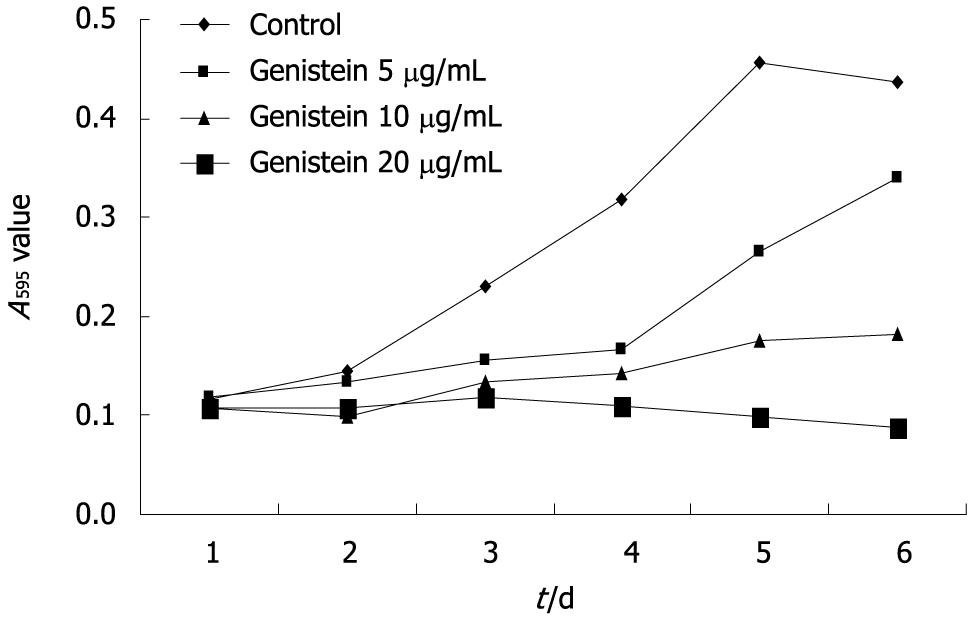
Genistein significantly inhibited MHCC97-H cell growth over the 6-d experimental period. The inhibitory rate of tumor cell growth in the 5, 10 and 20 μg/mL genistein groups was 22.3%, 58.2%, and 80.1%, respectively. The inhibitory rate of MHCC97-H cell growth in the 10 and 20 μg/mL genistein groups was significantly higher than the 5 μg/mL genistein group (P < 0.05). The concentration-dependent effects of genistein on MHCC97-H cell growth are shown in Figure 1.
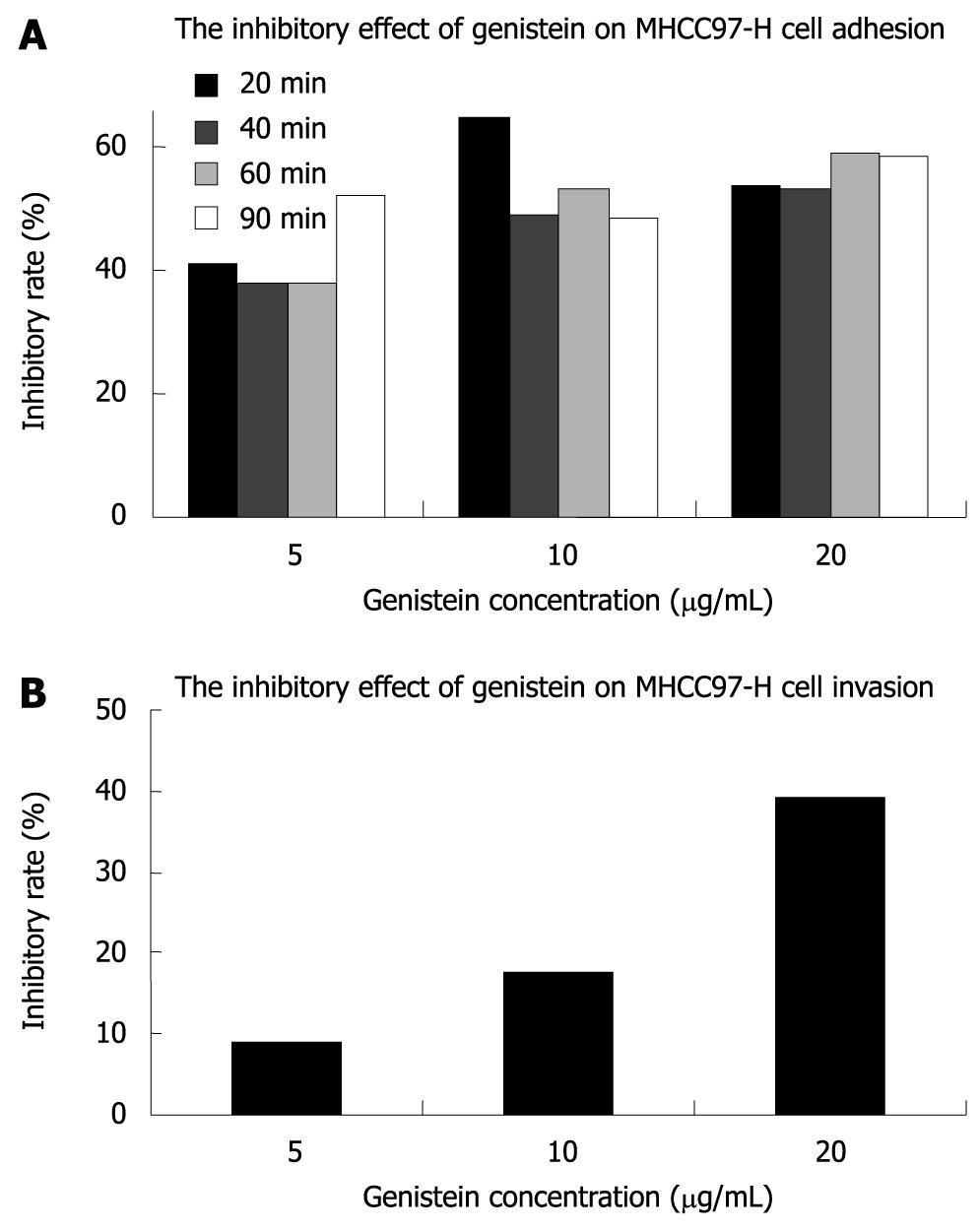
The A595 for MHCC97-H cells treated with or without genistein in fibronectin pre-coated 96-well microtiter plates at 20, 40, 60 and 90 min is shown in Table 1. Our results showed that genistein significantly inhibited tumor cell adhesion to fibronectin-coated substrates in a concentration-dependent fashion (P < 0.05), and the inhibitory effect of genistein on adhesion was more potent in the 10 and 20 μg/mL genistein groups. The inhibitory rate of genistein on MHCC97-H cell adhesion is shown in Figure 2.
We also investigated the capability of metastatic tumor cells to migrate through reconstituted basement membrane (Matrigel). The cells invading the lower surface of the filter through Matrigel in the control group, 5, 10, and 20 μg/mL genistein groups were 234.20 ± 12.36/field, 213.60 ± 14.98/field, 193.80 ± 19.92/field, and 142.80 ± 23.66/field, respectively. The inhibitory rate of invasion is shown in Figure 2. Our results showed that genistein inhibited the in vitro invasion of MHCC97-H cells. The inhibitory effect on invasion of MHCC97-H cells in the 20 μg/mL genistein group was more significant than that in the 5 and 10 μg/mL genistein groups (P < 0.05).
The effects of genistein on the cell cycle in MHCC97-H cells were determined by flow cytometry. After treatment with genistein, the number of MHCC97-H cells in the G0/G1 phase increased significantly compared with control cells (P < 0.05). The number of cells in the G2/M phase was decreased, but there was no statistical significance between the control group and the 10 and 20 μg/mL genistein-treated groups (P > 0.05). S phase fractions decreased significantly in cells treated with 10 and 20 μg/mL genistein (P < 0.01). The percentage of apoptotic cells in the genistein-treated groups increased significantly compared with the control group (P < 0.05). The results of the cell cycle analysis of MHCC97-H cells by flow cytometry are presented in Table 2.
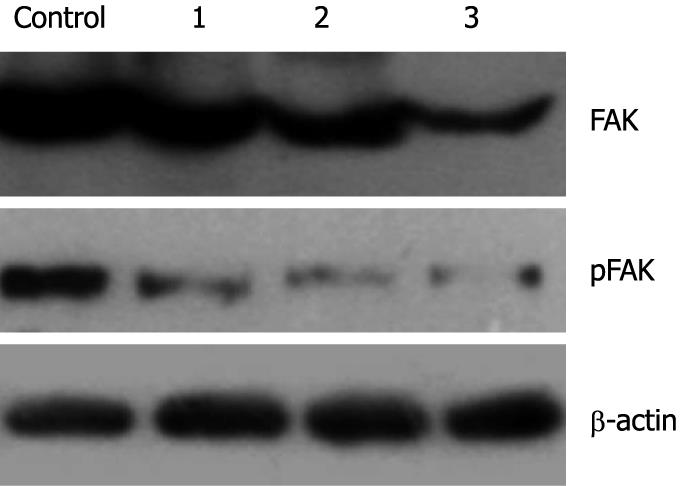
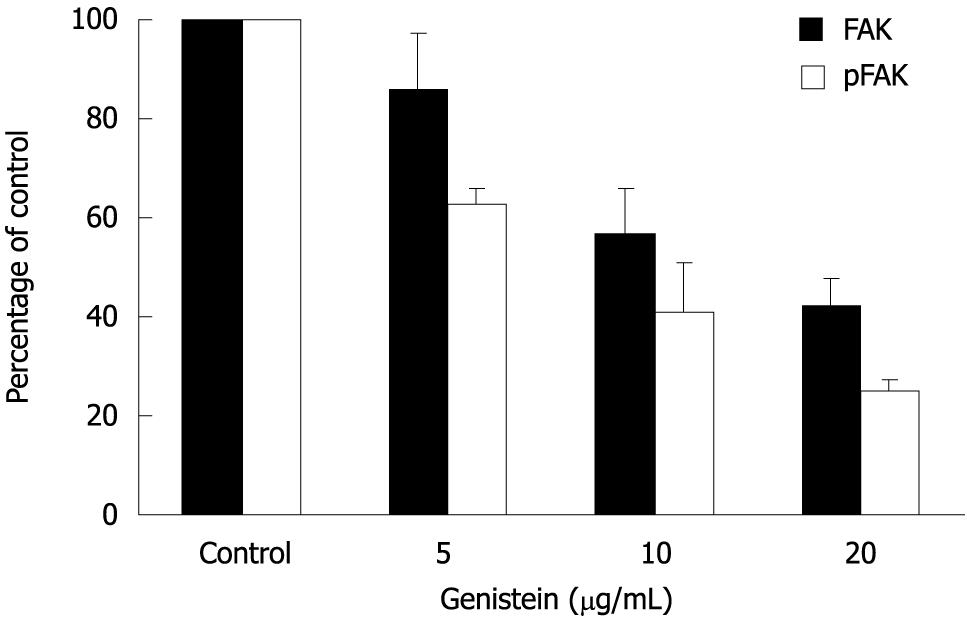
The effect of genistein on expression and phosphorylation of FAK in MHCC97-H cells was determined using Western blotting. After treatment with genistein, the expression level of total FAK was decreased when the genistein concentration increased (P < 0.05). The optical density in the 10 μg/mL and 20 μg/mL genistein groups were significantly lower than that in the control group (P < 0.05). More interestingly, at the same time phosphorylated FAK also decreased. The 5 μg/mL genistein treatment inhibited about 40% of FAK phosphorylation. These parameters were further significantly inhibited in the presence of genistein in a concentration-dependent fashion (P < 0.05). Data are shown in Figures 3 and 4.
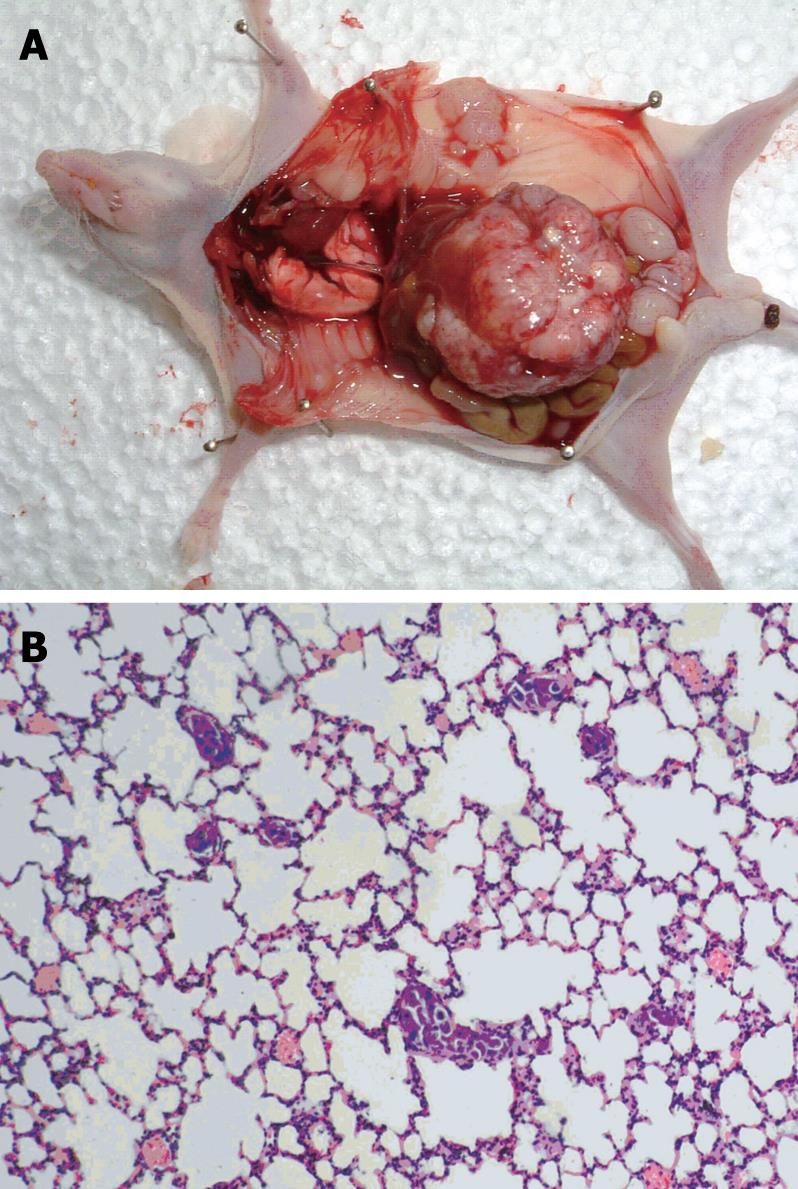
The anti-tumor activity of genistein was evaluated in nude mice bearing orthotopic tumor implants
(Figure 5A). Treatment with genistein significantly inhibited local tumor growth, compared with the control group. At the end of treatment, the tumor weight in the genistein group was significantly less than that in the control group (1.99 ± 2.07 g vs 2.63 ± 1.31 g, P < 0.05). Tumors in mice treated with genistein were reduced in weight by 24.3% compared with the control group.
After orthotopic implantation of tumor tissues, pulmonary metastases occurred in the recipient mice by day 35 (Figure 5B). Microscopy showed that the number of micrometastatic foci in the genistein group (12.3 ± 1.8) was significantly lower than that in the control group (12.3 ± 1.8 vs 16.6 ± 2.6, P < 0.05).
Metastasis is a fundamental characteristic of cancer and the ultimate cause of most cancer mortality. Advances in surgical techniques and adjuvant therapies have proven useful in the treatment of primary tumors[7]. However, metastasis remains a major cause of poor prognosis and death in cancer patients even after curative resection. The process of metastasis consists of sequential steps that include detachment, motility, invasion of the extracellular matrix, intravasation, circulation, adhesion, extravasation into the organ parenchyma and growth[8]. The ability of cancer cells to form metastases depends on a set of unique biological properties that enable the malignant cells to complete all these steps of the metastatic process. Currently, only a few chemotherapeutic drugs are effective for the treatment of patients with malignant tumor metastasis, and there is a clear need to identify new anti-metastatic drugs[9].
Genistein, an isoflavonoid abundant in soy beans, is a planar molecule with an aromatic A ring, has a second oxygen atom from that in the A ring, and has a molecular mass similar to those of the steroidal estrogens[9-11]. Reports from epidemiological and experimental studies show that it plays an important role in the inhibition of tumors including breast cancer, prostate cancer, colon cancer, leukemia and melanoma[12,13]. Genistein has a wide range of biological actions that suggest it may be of use in cancer treatment. Its molecular actions include: an inhibitory effect on protein tyrosine kinases, DNA topoisomerase I and II, and ribosomal S6 kinase; anti-estrogenicity; antioxidant activity; anti-angiogenesis activity; suppression of cell proliferation; induction of differentiation and modulation of apoptosis[13-15]. However, few data are available on the effects of genistein on metastasis of HCC. The purpose of this study was to investigate the metastatic potential of genistein in vitro and in vivo in HCC and to gain primary insight into the underlying mechanism mediating the effects of genistein.
Several human and animal HCC cell clones have been established, but few of these have been suitable for the study of human HCC metastasis[3,16,17]. However, most showed tumorigenicity when inoculated into experimental animals, and rarely did they demonstrate the full potential for distant metastases, as seen so frequently in patients. The metastatic HCC cell line MHCC97-H is characterized by high pulmonary metastasis potential and is a suitable cell line for the study of liver cancer metastasis[3,18]. In this study, we found that genistein significantly inhibited MHCC97-H cell growth both in vitro and in vivo. The effect of genistein on MHCC97-H cells was concentration dependent; the in vitro inhibitory rates of tumor cell growth in the 10 μg/mL and 20 μg/mL genistein groups were about 58% and 80%, respectively. In vivo, tumor weight was significantly reduced by 24% in the genistein-treated group compared with the control group. We also found that genistein induced cell cycle progression arrest at the G0/G1 and G2/M phases. These data are in accordance with other reports which showed that genistein induced cell cycle progression arrest at the G2/M phase and that the number of S phase cells decreased in a progressive way as the genistein incubation time was increased[15,19,20]. Although the exact mechanisms of action of genistein have yet to be fully elucidated, induction of apoptosis may be partly responsible. In our studies, the percentage of MHCC97-H cells undergoing apoptosis was significantly higher in the genistein group than in the control group. This finding is consistent with apoptosis studies in breast, prostate and gastric cancer cell lines treated with genistein[21,22].
The adhesion and invasiveness of tumor cells represents one of the several important steps necessary for the formation of metastases. Cell migration depends on adhesion between cells which is partly adjusted by integrin[23]. We investigated the effect of genistein on the adhesive properties of MHCC97-H cells and found that genistein significantly inhibited MHCC97-H cell adhesion to fibronectin-coated substrates. The inhibition rate reached approximately 58% in the 20 μg/mL genistein group. This demonstrated that the reduction in cell adhesion caused by genistein may account for the ability of MHCC97-H cells to cross normal tissue boundaries and disperse to adjacent sites. Cell invasion was studied both in vitro and in vivo in our study. MHCC97-H cells which invaded the Matrigel to the lower surface of the Transwell filter were significantly inhibited in the genistein-treated groups compared with the control group. The inhibitory effect on invasion in the 20 μg/mL genistein group was 39%, which was significantly higher than that in the other groups. Our experiments with mice bearing orthotopic tumor implants further confirmed that treatment with genistein also significantly inhibited or halted lung metastasis of HCC, which correlated with the biological behavior in vitro.
FAK is a cytoplasmic tyrosine kinase which plays an important role in integrin-mediated signal transduction pathways closely related to cell adhesion, motility and growth. Upregulation of FAK expression is associated with oncogenesis, and a decrease in FAK is associated with loss of cell attachment, decreased migration and induction of apoptosis[24]. We have reported that FAK is overexpressed in HCC, where the expression of FAK in invasive or metastatic HCC is significantly higher than that in non-invasive or non-metastatic HCC[25]. Therefore, FAK seems to be an important pharmacologic target site. In this study, a significant downregulation of expression and phosphorylation of FAK after genistein treatment was observed, suggesting that genistein may serve as a potentially important anticancer agent for HCC progression by blocking the FAK signaling process, which plays a crucial role in the invasion and metastasis of HCC.
In conclusion, both in vitro and in vivo studies showed that genistein is a promising agent for inhibiting the metastatic potential of HCC. It may affect HCC progression as a result of its effects on cell cycle progression and apoptosis. However, because its precise mechanism of activity and its targets remain unclear, further in-depth studies of the molecular mechanism are needed to establish the scientific basis for the possible use of genistein in the treatment of HCC metastasis.
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer related deaths in the world. A high incidence of metastasis accounts for the poor survival of HCC patients, and research into interventions for liver cancer metastasis has special priority in the anti-cancer campaign.
Genistein, an isoflavonoid abundant in soy beans, has a wide range of biological actions in the inhibition of tumors including breast cancer, prostate cancer, colon cancer, leukemia and melanoma. However, few data are available on the effects of genistein on metastasis of HCC. In this study, the authors demonstrate that genistein inhibited the metastasis of HCC in vitro and in vivo. Genistein appears to be a promising agent for inhibiting the metastatic potential of HCC.
In this study, genistein was found to significantly inhibit the growth, attachment and invasion of MHCC97-H cells in vitro in a concentration-dependent fashion. The expression and phosphorylation of FAK in MHCC-97H cells were also decreased by genistein. In addition, our study on nude mice bearing orthotopic tumor implants showed that genistein significantly inhibited lung metastasis of MHCC97-H cells.
The findings from this study support the idea that genistein may serve as a potentially important anticancer agent for HCC progression by blocking the FAK signaling process.
Genistein is a planar molecule with an aromatic A ring, has a second oxygen atom from that in the A ring, and has a molecular mass similar to those of the steroidal estrogens. It binds to and inhibits protein tyrosine kinase, thereby disrupting signal transduction and inducing cell apoptosis and differentiation.
This paper reports the effect of genistein exposure on the growth, adhesion, migration and metastases of a highly metastatic HCC cell line MHCC97-H. They observed that genistein exposure significantly inhibited growth, adhesion to fibronectin, and invasion of the cell line. The paper is well written and has novel data.
Peer reviewer: Samuel B Ho, Professor, Gastroenterology, VA San Diego Healthcare System, San Diego 92161, United States
S- Editor Wang YR L- Editor Webster JR E- Editor Ma WH
| 1. | Yeh TC, Chiang PC, Li TK, Hsu JL, Lin CJ, Wang SW, Peng CY, Guh JH. Genistein induces apoptosis in human hepatocellular carcinomas via interaction of endoplasmic reticulum stress and mitochondrial insult. Biochem Pharmacol. 2007;73:782-792. [Cited in This Article: ] |
| 2. | Wang H, Dai J, Hou S, Qian W, Li B, Ma J, Fan X, Zhao J, Yang S, Sang H. Treatment of hepatocellular carcinoma with adenoviral vector-mediated Flt3 ligand gene therapy. Cancer Gene Ther. 2005;12:769-777. [Cited in This Article: ] |
| 3. | Li Y, Tang ZY, Ye SL, Liu YK, Chen J, Xue Q, Chen J, Gao DM, Bao WH. Establishment of cell clones with different metastatic potential from the metastatic hepatocellular carcinoma cell line MHCC97. World J Gastroenterol. 2001;7:630-636. [Cited in This Article: ] |
| 4. | Gu Y, Zhu CF, Iwamoto H, Chen JS. Genistein inhibits invasive potential of human hepatocellular carcinoma by altering cell cycle, apoptosis, and angiogenesis. World J Gastroenterol. 2005;11:6512-6517. [Cited in This Article: ] |
| 5. | Kido A, Krueger S, Haeckel C, Roessner A. Inhibitory effect of antisense aminopeptidase N (APN/CD13) cDNA transfection on the invasive potential of osteosarcoma cells. Clin Exp Metastasis. 2003;20:585-592. [Cited in This Article: ] |
| 6. | Sun FX, Tang ZY, Lui KD, Ye SL, Xue Q, Gao DM, Ma ZC. Establishment of a metastatic model of human hepatocellular carcinoma in nude mice via orthotopic implantation of histologically intact tissues. Int J Cancer. 1996;66:239-243. [Cited in This Article: ] |
| 7. | Entschladen F, Drell TL 4th, Lang K, Joseph J, Zaenker KS. Tumour-cell migration, invasion, and metastasis: navigation by neurotransmitters. Lancet Oncol. 2004;5:254-258. [Cited in This Article: ] |
| 8. | Steeg PS, Theodorescu D. Metastasis: a therapeutic target for cancer. Nat Clin Pract Oncol. 2008;5:206-219. [Cited in This Article: ] |
| 9. | Zhou HB, Chen JM, Cai JT, Du Q, Wu CN. Anticancer activity of genistein on implanted tumor of human SG7901 cells in nude mice. World J Gastroenterol. 2008;14:627-631. [Cited in This Article: ] |
| 10. | Dixon RA, Ferreira D. Genistein. Phytochemistry. 2002;60:205-211. [Cited in This Article: ] |
| 11. | Sarkar FH, Li Y. Soy isoflavones and cancer prevention. Cancer Invest. 2003;21:744-757. [Cited in This Article: ] |
| 12. | Sarkar FH, Li Y. The role of isoflavones in cancer chemoprevention. Front Biosci. 2004;9:2714-2724. [Cited in This Article: ] |
| 13. | Polkowski K, Popiolkiewicz J, Krzeczynski P, Ramza J, Pucko W, Zegrocka-Stendel O, Boryski J, Skierski JS, Mazurek AP, Grynkiewicz G. Cytostatic and cytotoxic activity of synthetic genistein glycosides against human cancer cell lines. Cancer Lett. 2004;203:59-69. [Cited in This Article: ] |
| 14. | Brownson DM, Azios NG, Fuqua BK, Dharmawardhane SF, Mabry TJ. Flavonoid effects relevant to cancer. J Nutr. 2002;132:3482S-3489S. [Cited in This Article: ] |
| 15. | Chang KL, Kung ML, Chow NH, Su SJ. Genistein arrests hepatoma cells at G2/M phase: involvement of ATM activation and upregulation of p21waf1/cip1 and Wee1. Biochem Pharmacol. 2004;67:717-726. [Cited in This Article: ] |
| 16. | Chiu JH, Chang HM, Kao HL, Wu LH, Lui WY. Establishment and characterization of two cell lines derived from a single hepatocellular carcinoma containing multiploid DNA distribution. Cancer Detect Prev. 1996;20:43-51. [Cited in This Article: ] |
| 17. | Ogawa K, Nakanishi H, Takeshita F, Futakuchi M, Asamoto M, Imaida K, Tatematsu M, Shirai T. Establishment of rat hepatocellular carcinoma cell lines with differing metastatic potential in nude mice. Int J Cancer. 2001;91:797-802. [Cited in This Article: ] |
| 18. | Xu Y, Sun HC, Tian B, Li Y, Chen J, Chen J, Gao DM, Xue Q, Tang ZY. Establishment of green fluorescent protein-expressing hepatocellular carcinoma cell lines with different metastatic potential: relevant models for in vivo monitoring of metastasis and angiogenesis. J Cancer Res Clin Oncol. 2004;130:375-382. [Cited in This Article: ] |
| 19. | Ravindranath MH, Muthugounder S, Presser N, Viswanathan S. Anticancer therapeutic potential of soy isoflavone, genistein. Adv Exp Med Biol. 2004;546:121-165. [Cited in This Article: ] |
| 20. | Chodon D, Banu SM, Padmavathi R, Sakthisekaran D. Inhibition of cell proliferation and induction of apoptosis by genistein in experimental hepatocellular carcinoma. Mol Cell Biochem. 2007;297:73-80. [Cited in This Article: ] |
| 21. | Perabo FG, Von Low EC, Ellinger J, von Rucker A, Muller SC, Bastian PJ. Soy isoflavone genistein in prevention and treatment of prostate cancer. Prostate Cancer Prostatic Dis. 2008;11:6-12. [Cited in This Article: ] |
| 22. | Hilakivi-Clarke L. Nutritional modulation of terminal end buds: its relevance to breast cancer prevention. Curr Cancer Drug Targets. 2007;7:465-474. [Cited in This Article: ] |
| 23. | Mousa SA. Cell adhesion molecules: potential therapeutic & diagnostic implications. Mol Biotechnol. 2008;38:33-40. [Cited in This Article: ] |
| 24. | Agochiya M, Brunton VG, Owens DW, Parkinson EK, Paraskeva C, Keith WN, Frame MC. Increased dosage and amplification of the focal adhesion kinase gene in human cancer cells. Oncogene. 1999;18:5646-5653. [Cited in This Article: ] |
| 25. | Gu Y, Chen JS, Wang J, Zhou XD, Gao JS. Overexpression of focal adhesion kinase (FAK) and its relationship with the invasion and metastasis of human hepatocellular carcinoma. Zhonghua Shiyan Waike Zazhi. 2003;20:4-5. [Cited in This Article: ] |