Published online Jan 1, 2004. doi: 10.3748/wjg.v10.i1.17
Revised: August 20, 2003
Accepted: October 25, 2003
Published online: January 1, 2004
AIM: Disruption of cell cycle regulation is a critical event in carcinogenesis, and alteration of the retinoblastoma (pRb) tumour suppressor pathway is frequent. The aim of this study was to compare alterations in this pathway in proximal and distal gastric carcinogenesis in an effort to explain the observed striking epidemiological differences.
METHODS: Immunohistochemistry was performed to investigate expression of p16 and pRb in the following groups of both proximal (cardia) and distal (antral) tissue samples: (a) biopsies showing normal mucosa, (b) biopsies showing intestinal metaplasia and, (c) gastric cancer resection specimens including uninvolved mucosa and tumour.
RESULTS: In the antrum there were highly significant trends for increased p16 expression with concomitant (and in the group of carcinomas inversely proportional) decreased pRb expression from normal mucosa to intestinal metaplasia to uninvolved mucosa (from cancer resections) to carcinoma. In the cardia, there were no differences in p16 expression between the various types of tissue samples whereas pRb expression was higher in normal mucosa compared with intestinal metaplasia and tissue from cancer resections.
CONCLUSION: Alterations in the pRb pathway appear to play a more significant role in distal gastric carcinogenesis. It may be an early event in the former location since the trend towards p16 overexpression with concomitant pRb underexpression was seen as early as between normal mucosa and intestinal metaplasia. Importantly, the marked differences in expression of pRb and p16 between the cardia and antrum strongly support the hypothesis that tumours of the two locations are genetically different which may account for some of the observed epidemiological differences.
- Citation: Gulmann C, Hegarty H, Grace A, Leader M, Patchett S, Kay E. Differences in proximal (cardia) versus distal (antral) gastric carcinogenesis via retinoblastoma pathway. World J Gastroenterol 2004; 10(1): 17-21
- URL: https://www.wjgnet.com/1007-9327/full/v10/i1/17.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i1.17
In the last decades the pattern of incidence of gastric cancer in the Western world has changed. Distal (corpus and antrum) cancers have decreased slightly whereas proximal (cardia/ gastro-oesophageal) cancers have increased more than any other cancer[1,2]. The tumours are morphologically indistinguishable and intestinal metaplasia (IM) appears to be an important step in carcinogenesis in both sites[3,4]. The observed epidemiological differences are likely to be due to differences in the genetic pathways of carcinogenesis. However, so far they are poorly evaluated[4-6].
Abnormal regulation of the cell cycle is a feature of many neoplasms[7]. Regulation of the G1/S checkpoint is critical and is controlled by the retinoblastoma protein (pRb). Phosphorylated pRb releases E2F transcription factors which activate genes involved in DNA synthesis and cause G1/S transition. Phosphorylation of pRb is stimulated by the cyclin dependent kinase (CDK) 4-cyclin D complex. p16 specifically binds CDK4 which displaces it from cyclin D and thus acts to maintain pRb in an underphosphorylated state which causes G1 arrest[8]. Disruption of this so-called ‘Rb pathway’ is a critical event in many tumours. It is resulted from primary inactivation of Rb function, by overexpression of CDKs, or through loss of p16[7] which has a similar effect of G1/S progression. Several studies have shown a reciprocal expression of p16 and pRb[9,10]. The role of the pRb pathway in gastric carcinogenesis is the subject of many papers. In distal gastric cancers it may be that disruption of p16 is an early event[11] and a recent immunohistochemical study[12] has shown a progressive decrease in expression from gastritis to atrophy and dysplasia. Less information is available on the role of pRb expression.
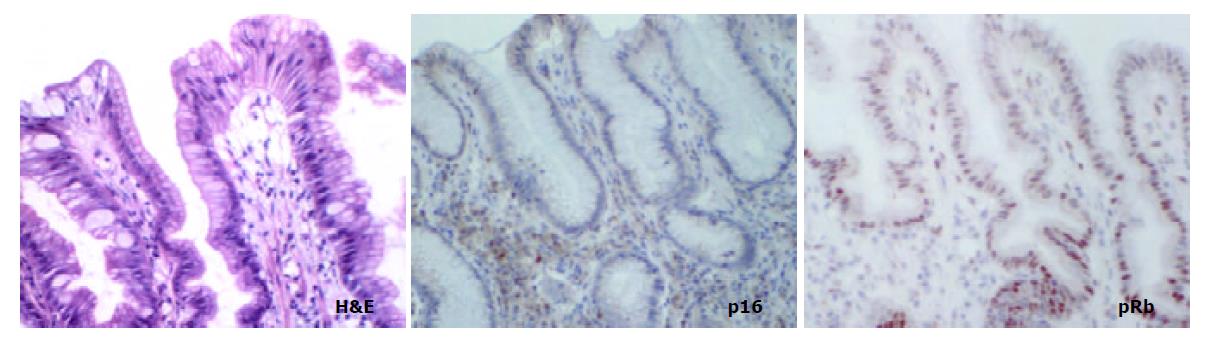
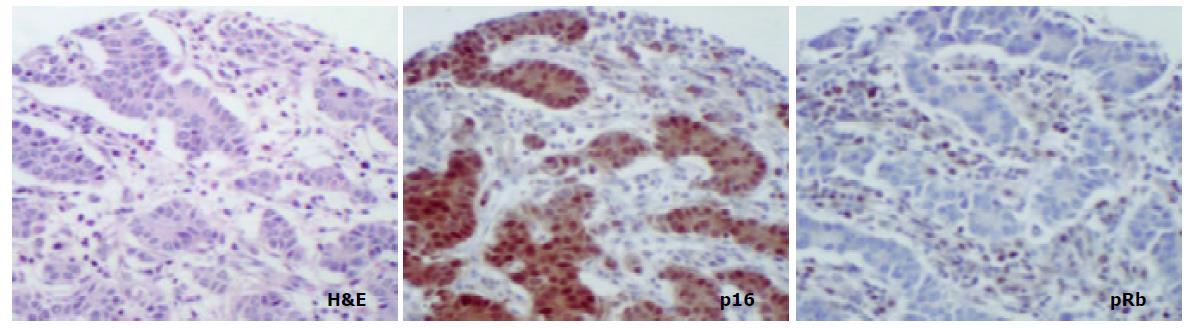
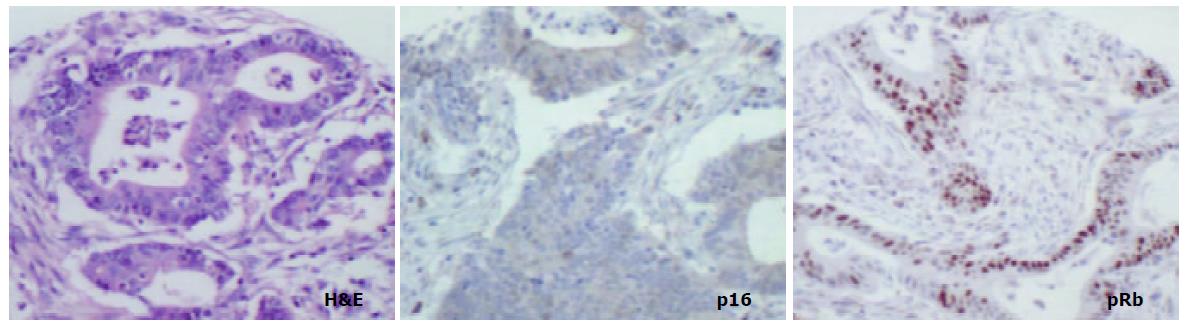
The aim of the present study was to compare the role of the pRb pathway in proximal and distal gastric cancers. p16 and pRb immunoexpression was investigated in the following groups of both proximal (cardia) and distal (antral) tissue samples: (a) biopsies showing normal mucosa, (b) biopsies showing intestinal metaplasia and, (c) gastric cancer resection specimens including uninvolved mucosa and tumour. (Figure 1).
Six groups were included in this cross sectional study on archival, paraffin embedded tissues. The material was retrieved from the files in Beaumont Hospital, Dublin, Ireland and the local ethics committee approved the study. Tissue samples from the gastric cardia included endoscopic biopsies showing histologically normal mucosa (n = 56) or IM (n = 49) and material from gastric cancer resection specimens (n = 39). Non-involved mucosa as well as tumour were investigated in the latter specimens. Tissue samples from the gastric antrum included the same groups: Normal (n = 52), IM (n = 50) as well as non-involved mucosa and tumour from cancer resections (n = 78). All patients were Caucasians. Clinical details are shown in Table 1. (Figure 2).
| Proximal | Distal | |||||
| Biopsies normal | Biopsies IM | Cancer resections | Biopsies normal | Biopsies IM | Cancer resections | |
| Number of subjects | 56 | 49 | 39 | 52 | 50 | 78 |
| Gender{F/M} | 28/28 | 22/27 | 13/26 | 30/22 | 29/21 | 34/44 |
| Age, years | 50 (16) | 58 (16)A | 67 (11)B | 52 (18) | 59 (14)C | 69 (10)D |
| Mean and (SD) | ||||||
| Tumour type | 30 Intestinal | 42 Intestinal | ||||
| 9 Diffuse | 36 Diffuse | |||||
| TNM-stage, | T 3.1 (0.6) | T 3.0 (1.1) | ||||
| mean and (SD) | N 1.0 (0.8) | N 1.0 (0.8) | ||||
Endoscopic biopsy material was obtained from patients who had presented to the endoscopy service with a variety of upper gastrointestinal symptoms. Biopsies of the cardia were only included when clearly labelled as such on the original request form without any endoscopic suspicion of Barrett’s oesophagus or any prior or subsequent oesophageal biopsies showing Barrett’s metaplasia. The distinction between proximal and distal gastric cancers was made on the basis of the clinical data as well as macroscopic description of the resection specimens on the pathology report. A case was labelled as proximal gastric cancer if it straddled the gastro-oesophageal junction with approximately equal amounts in the oesophagus and stomach and no histological evidence of Barrett’s mucosa in the oesophagus. Distal gastric cancers were labelled as such when the tumour was clinically, macroscopically and histologically (i.e. at no point adjacent to squamous mucosa) confined to the more distal stomach. Tumours were classified according to the Lauren classification[13] as diffuse or intestinal. (Figure 3).
Initially, 4 µm sections were cut from all blocks and stained with haematoxylin and eosin. Tissue microarrays (TMAs) were constructed[14]. The technique involves taking cylindrical core biopsies from ‘donor’ blocks with subsequent precise arraying into a new ‘recipient’ paraffin block using a precision instrument (Beecher Instruments, Silver Spring, MD, USA)[14,15]. Two different types of TMAs were constructed. Endoscopic biopsy fragments from wax blocks were arrayed using 2 mm punches. This size punch covers most endoscopic fragments in toto and all individual fragments from a donor wax block were sampled in separate cores (in this study 1-8 cores per donor block). Therefore, all the tissue from an original donor block containing endoscopic biopsy material was arrayed into the TMA block. Each ‘biopsy-TMA’ could hold up to 40 2 mm cores (i.e. up to 40 donor blocks depending on numbers of tissue fragments per original donor block). In the cancer resection specimens a different type of TMA was constructed due to the large size of tissue pieces in each block. The whole-section glass-slides were evaluated and areas of tumour as well as uninvolved mucosa were marked on the glass slides and identified in the corresponding wax blocks. Four 0.6 mm core biopsies were taken from each area. In 31 cases tumour and uninvolved mucosa were not present in the same wax block and two separate blocks were used. In this fashion, a total of 8 cores were taken per case (4 from tumour and 4 from uninvolved mucosa) with 35-40 cases (a total of 280-320 cores) fitted on to each TMA-block. Using TMAs the total number of TMA-blocks constructed was twenty-one. (Figure 4).
Monoclonal mouse antibodies directed against p16 (1:100. Clone G175-405, PharMingen, USA) and pRb (1:300. Clone M7131, DAKO, Denmark) were used. Immunostaining was performed using standard procedures. Heat mediated antigen retrieval using a pressure cooker was required to unmask the antigen sites. Antibody binding was detected using the Vectastain universal elite ABC-peroxidase kit (Vector Laboratories Inc., Burlingame, CA, USA). The positive p16 control was a case of severe uterine cervical dysplasia which strongly expressed p16 in dysplastic foci. The positive control for pRb was a multi-tissue block including tonsil. (Figure 3).
p16 Positive staining was defined as nuclear staining whereas cytoplasmic staining was considered non-specific and ignored. Extent was scored semi-quantitatively as negative (0) if < 5% of cells stained, 1 if 5%-25% of cells stained, 2 if 26%-50% of cells stained and 3 if > 50% cells stained. In cancer resections the average score was calculated from each of the four 0.6 mm TMA cores of either carcinoma or non-involved mucosa. The average score of each of the four decided the overall positivity/ negativity.
pRb Nuclear staining was considered positive. Extent was scored semi-quantitatively as negative (0) if < 5% of cells stained, 1 if 5%-25% of cells stained, 2 if 26%-50% of cells stained and 3 if > 50% cells stained. In cancer resections the average score was calculated from each of the four 0.6 mm TMA cores of either carcinoma or non-involved mucosa. The average score of each of the four decided the overall positivity/negativity.
Statistical significance was defined as P < 0.05. Chi-square test was used for comparison between groups. Logistic regression analysis on the actual numbers of positive cases was used to test trends between tissue types within the proximal and distal groups.
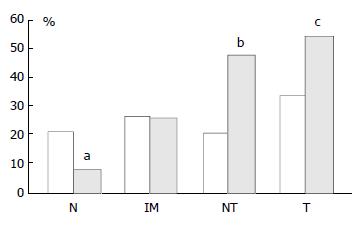
In mucosa from biopsies (i.e. not associated with carcinoma) with and without intestinal metaplasia there was a low level of expression in both proximal and distal locations, mainly within the neck regions of the glands and absent in superficial epithelium. In distal tissue samples there was a statistically significant stepwise increase from normal mucosa to intestinal metaplasia to non-involved mucosa from cancer resections to carcinoma (P < 0.0001, Table 2). In proximal tissue samples no such trend was noted and there were no differences between the different tissue samples (Figure 4). No differences in p16 expression were noted between tumour types in either of the two locations and there were no correlations between p16 expression and stage of tumour or any clinical parameters.
| Proximal | Distal | |||||||
| Biopsies | Biopsies | Resections | Resections | Biopsies | Biopsies | Resections | Resections | |
| normal | IM | UM | cancer | normal | IM | UM | cancer | |
| Number and (%) of cases | 12 (21%) | 13 (27%) | 8 (21%) | 13 (33%) | 4 (8%) | 13 (26%) | 37 (47%) | 42 (54%) |
| positive for p16 | ||||||||
| Mean (and SD) of extent | 0.21 (0.41) | 0.27 (0.45) | 0.23 (0.48) | 0.41 (0.68) | 0.077 (0.27) | 0.26 (0.44) | 0.49 (0.53) | 0.69 (0.79) |
| scores for p16 | ||||||||
| Number and (%) of cases | 52 (93%) | 37 (76%) | 31 (80%) | 30 (77%) | 46 (88%) | 35 (70%) | 51 (65%) | 38 (49%) |
| positive for pRb | ||||||||
| Mean (and SD) of extent | 0.41 (0.5) | 0.34 (0.48) | 0.33 (0.48) | 0.5 (0.51) | 0.42 (0.5) | 0.24 (0.43) | 0.17 (0.38) | 0.28 (0.45) |
| scores for pRb | ||||||||
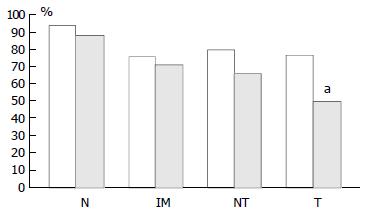
pRb was highly expressed in normal epithelium and intestinal metaplasia, mainly in the more proliferative areas, i.e. the neck region within the glandular epithelium. In both proximal and distal stomach there was a statistically significant trend for decreased pRb expression from normal mucosa to intestinal metaplasia to non-involved mucosa from cancer resections to carcinoma. It was more pronounced in the distal tissue samples (P < 0.0001) than in the proximal tissue samples (P = 0.035) and in the latter location it was mainly a function of the high expression in normal mucosa compared with the other histological subsets which showed very similar expression rates (Figure 5). No differences in pRb expression were noted between tumour types in the two locations and there were no correlations between pRb expression and stage of tumour or any clinical parameters.
In cancer resection specimens there was a significantly lower expression of p16 in proximal than distal location, both in uninvolved mucosa (P = 0.0048) and carcinoma (P = 0.0036) whereas the opposite was seen in normal mucosa (P = 0.0045) (Figure 4). pRb expression was significantly higher in proximal carcinomas (P = 0.0047) (Figure 5).
Disruption of cell cycle regulation is a critical event in carcinogenesis. Alteration of the retinoblastoma (pRb) tumour suppressor pathway, which controls the G1/S checkpoint, is a common event in many neoplasms and typically implicates abnormal expression of both pRb and p16 although other molecules may be involved.
The current study aimed to compare alterations in this pathway in proximal and distal gastric carcinogenesis in an effort to explain the observed epidemiological differences between the two sites. Immunohistochemistry was performed to investigate expression of p16 and pRb in various histological stages from normal mucosa to carcinoma in both proximal (cardia) and distal (antral) tissue samples.
The results of this study showed highly significant trends for increase in p16 expression with a concomitant decrease in pRb expression in distal tissue samples from normal mucosa to intestinal metaplasia to uninvolved mucosa from cancer resections to carcinoma. This suggests that the pRb pathway plays a definite role in distal gastric carcinogenesis. It may be an early event since the trend towards p16 overexpression and pRb underexpression was seen even between normal mucosa and intestinal metaplasia. In the proximal stomach no differences in p16 expression were seen between the various stages whereas pRb expression decreased slightly from normal mucosa to carcinoma. This seemed to be mainly an effect of a higher expression of pRb in normal mucosa compared with any of the other tissue samples. Furthermore, the trend for decreasing pRb was not matched by an increase in p16 expression and there was no inverse relationship between the two molecules in the group of carcinomas. Taken together this argues against the pRb pathway as important in proximal gastric carcinogenesis.
Other studies on (distal) gastric carcinogenesis have shown a similar p16 ‘overexpression’ in gastric carcinomas[16,17] with weak staining in non-involved mucosa. However, a recent immunohistochemical study found a progressive p16 decrease and a pRb increase from gastritis to dysplasia[12] in samples from the cardia. This is surprising in view of the opposite trend in distal gastric samples seen here and also by others[17]. The reason for this discrepancy is uncertain. In the above-mentioned study patients were from an area in China with a high incidence of gastric carcinoma and compared with the current study, different entities (chronic gastritis, dysplasia) were investigated. Also, racial differences may play a role since all the patiens in this study were Caucasians.
A study on p16 expression in normal tissues[18] showed some staining in gastric antral glands. The following scenario is therefore likely: p16 is expressed with a low frequency in the normal state, which may be related to a relatively low proliferative status. In support of this is the limited expression in glandular (proliferative area) rather than surface epithelium (quiescent area) seen here and also noted by others[17,18]. In tumours there may be loss of negative feedback through decrease in pRb expression which causes p16 overexpression and other studies have also shown an inverse relationship between expression of the two molecules[9,10]. The current study showed mainly cases of distal gastric cancer resections with gain of p16 expression in cancers compared with non-involved mucosa. However, loss of p16 expression in cancers compared with uninvolved mucosa was also seen in some cases and it is entirely possible that, whereas most cancers lose pRb function/ expression, some preferentially lose p16 expression.
This study did not entirely rule out a role for the pRb pathway in proximal gastric carcinogenesis since other molecules participate in this complex cell cycle control mechanism. It is unlikely, however, since altered expression of p16 and pRb would be expected if abnormal feedback from other molecules in this pathway existed.
In conclusion this study strongly suggests that alterations in the pRb pathway are significant in distal, but not proximal, gastric carcinogenesis. In the former location it may be an early event. Importantly, this study therefore supports the hypothesis that tumours of the two locations are genetically different which may account for some of the observed epidemiological differences.
Mr. Ronan Conroy is thanked for his help with the statistical analysis.
Edited by Wang XL
| 1. | Blot WJ, Devesa SS, Kneller RW, Fraumeni JF. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265:1287-1289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1287] [Cited by in F6Publishing: 1132] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 2. | Devesa SS, Blot WJ, Fraumeni JF. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049-2053. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 14] [Reference Citation Analysis (0)] |
| 3. | Jass JR. Role of intestinal metaplasia in the histogenesis of gastric carcinoma. J Clin Pathol. 1980;33:801-810. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 159] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Ruol A, Parenti A, Zaninotto G, Merigliano S, Costantini M, Cagol M, Alfieri R, Bonavina L, Peracchia A, Ancona E. Intestinal metaplasia is the probable common precursor of adenocarcinoma in barrett esophagus and adenocarcinoma of the gastric cardia. Cancer. 2000;88:2520-2528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 12] [Reference Citation Analysis (0)] |
| 5. | Morales TG, Camargo E, Bhattacharyya A, Sampliner RE. Long-term follow-up of intestinal metaplasia of the gastric cardia. Am J Gastroenterol. 2000;95:1677-1680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Sipponen P. Intestinal metaplasia and gastric carcinoma. Ann Clin Res. 1981;13:139-143. [PubMed] [Cited in This Article: ] |
| 7. | Sherr CJ. The Pezcoller lecture: cancer cell cycles revisited. Cancer Res. 2000;60:3689-3695. [PubMed] [Cited in This Article: ] |
| 8. | Liggett WH, Sidransky D. Role of the p16 tumor suppressor gene in cancer. J Clin Oncol. 1998;16:1197-1206. [PubMed] [Cited in This Article: ] |
| 9. | Yang G, Zhang Z, Liao J, Seril D, Wang L, Goldstein S, Yang CS. Immunohistochemical studies on Waf1p21, p16, pRb and p53 in human esophageal carcinomas and neighboring epithelia from a high-risk area in northern China. Int J Cancer. 1997;72:746-751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 10. | Lee WA, Woo DK, Kim YI, Kim WH. p53, p16 and RB expression in adenosquamous and squamous cell carcinomas of the stomach. Pathol Res Pract. 1999;195:747-752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Jang TJ, Kim DI, Shin YM, Chang HK, Yang CH. p16(INK4a) Promoter hypermethylation of non-tumorous tissue adjacent to gastric cancer is correlated with glandular atrophy and chronic inflammation. Int J Cancer. 2001;93:629-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Zhou Y, Gao SS, Li YX, Fan ZM, Zhao X, Qi YJ, Wei JP, Zou JX, Liu G, Jiao LH. Tumor suppressor gene p16 and Rb expression in gastric cardia precancerous lesions from subjects at a high incidence area in northern China. World J Gastroenterol. 2002;8:423-425. [PubMed] [Cited in This Article: ] |
| 13. | LAUREN P. THE TWO HISTOLOGICAL MAIN TYPES OF GASTRIC CARCINOMA: DIFFUSE AND SO-CALLED INTESTINAL-TYPE CARCINOMA. AN ATTEMPT AT A HISTO-CLINICAL CLASSIFICATION. Acta Pathol Microbiol Scand. 1965;64:31-49. [PubMed] [Cited in This Article: ] |
| 14. | Kononen J, Bubendorf L, Kallioniemi A, Bärlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844-847. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2991] [Cited by in F6Publishing: 2918] [Article Influence: 112.2] [Reference Citation Analysis (0)] |
| 15. | Moch H, Kononen T, Kallioniemi OP, Sauter G. Tissue microarrays: what will they bring to molecular and anatomic pathology. Adv Anat Pathol. 2001;8:14-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 100] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Rocco A, Schandl L, Nardone G, Tulassay Z, Staibano S, Malfertheiner P, Ebert MP. Loss of expression of tumor suppressor p16(INK4) protein in human primary gastric cancer is related to the grade of differentiation. Dig Dis. 2002;20:102-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Tsujie M, Yamamoto H, Tomita N, Sugita Y, Ohue M, Sakita I, Tamaki Y, Sekimoto M, Doki Y, Inoue M. Expression of tumor suppressor gene p16(INK4) products in primary gastric cancer. Oncology. 2000;58:126-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Nielsen GP, Stemmer-Rachamimov AO, Shaw J, Roy JE, Koh J, Louis DN. Immunohistochemical survey of p16INK4A expression in normal human adult and infant tissues. Lab Invest. 1999;79:1137-1143. [PubMed] [Cited in This Article: ] |