Published online Sep 15, 2004. doi: 10.3748/wjg.v10.i18.2759
Revised: December 3, 2003
Accepted: December 22, 2003
Published online: September 15, 2004
AIM: To investigate the expression of Survivin in pancreatic cancer and its correlation to the expression of Bcl-2.
METHODS: Survivin and Bcl-2 expressions were examined by immunohistochemistry in 42 tissue samples from pancreatic cancer and 10 from normal pancrease.
RESULTS: No survivin expression was detected in the tissue samples from normal pancrease, while it was detected in 34 of 42 tissue samples from pancreatic cancer (81.95%). There was a correlation between survivin expression and differentiation and stages of pancreatic cancer. Survivin positive cases were strongly correlated to Bcl-2 expression (28/30 vs 6/12, P < 0.05).
CONCLUSION: Overexpression of survivin plays an important role in the development and progression of pancreatic cancer, and correlates to the expression of Bcl-2. Survivin expression can be used as a prognostic factor.
- Citation: Qiao JG, Zhang YQ, Yin YC, Tan Z. Expression of Survivin in pancreatic cancer and its correlation to expression of Bcl-2. World J Gastroenterol 2004; 10(18): 2759-2761
- URL: https://www.wjgnet.com/1007-9327/full/v10/i18/2759.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i18.2759
The inhibitor of apoptosis protein (IAP) is a member of the widely-expressed gene family of apoptosis inhibitors. It can inhibit apoptosis induced by a variety of stimuli and plays a critical role in the physiologic activities of cells. Survivin is a recently-characterized gene, a member of the IAP family, and has a close relation between anti-apoptosis and tumors. Overexpression of survivin has been reported to play an important role in the development and progression of pancreatic cancer[1-5]. In this study, we investigated the relationship between the expression of survivin in patients with pancreatic cancer and the expression of Bcl-2 in order to provide a theoretical basis for the prevention, diagnosis, and treatment of pancreatic cancer.
Ten samples from normal pancreatic tissue and 42 samples from pancreatic cancer were collected. Normal pancreatic tissues were obtained from autopsy specimens in Department of Anatomy, Medical College, Wuhan University, and their mean age was 49.3 years (range, 33-78). Pancreatic cancer samples were obtained from surgically-resected specimens in Zhongnan Hospital, Wuhan University. All these cancers were diagnosed pathologically.
Primary antibody for survivin was purchased from Novus Co., Ltd (USA) and Bcl-2 was purchased from Boster Biological Technology Ltd (Wuhan). SP kit was purchased from Zhongshan Biotechnology Co., Ltd (Beijing).
Fixed in routinely-processed formalin and then embedded in paraffin, 4-μm thick sections were prepared from the cut surface of blocks at the maximum cross-section. For morphological analysis, the sections were routinely stained with hematoxylin and eosin. Immunohistochemical staining for survivin and Bcl-2 antigen was made by the standard streptavidin/peroxidase (SP) technique. The positive section was used as a positive control. As a negative control, PBS was used instead of the primary antibody for survivin and Bcl-2.
Cytoplasm staining with light yellow or brown was defined as a marker of positive cells. All sections were analyzed by an image analysis system. The mean percentage of positive cells for the expression of survivin and Bcl-2 was determined in at least 10 areas at 400-fold magnification, and the cases with less than 5% positively-stained cells were defined as being negative. The cases with 5% to 10% positively-stained cells were defined as having a positivity rate of “+”, 11% to 60% as “++”, and more than 60% as “+++” (Table 1).
| Survivin expression | P | r | |||
| (-,+) | (++,+++) | ||||
| Sex | Male | 11 | 7 | ||
| Female | 13 | 11 | > 0.05 | ||
| Location | Head | 16 | 14 | ||
| Body, Tail | 8 | 4 | > 0.05 | ||
| Differentiation | High | 14 | 4 | ||
| Low | 10 | 14 | < 0.05 | 0.361 | |
| Distant metastasis | Present | 8 | 8 | ||
| Absent | 16 | 10 | > 0.05 | ||
| Stage | I-II | 18 | 8 | ||
| III-IV | 6 | 10 | < 0.05 | 0.311 | |
All statistical analyses were performed with SPSS 11.0 software. Difference and correlation were analyzed by χ2 test. P < 0.05 was considered statistically significant.
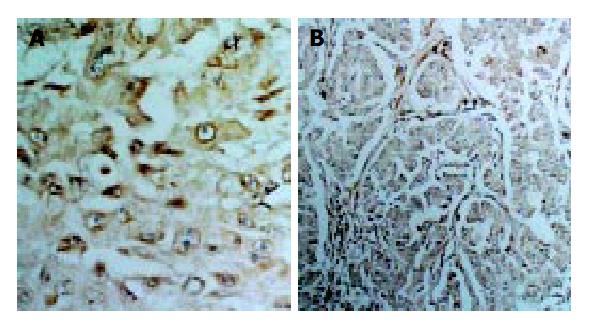
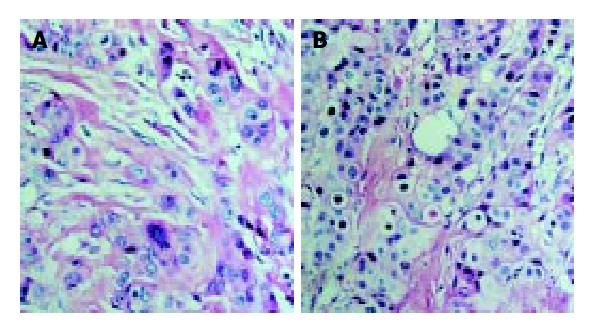
With immunohistochemical staining, we examined the expression of survivin in pancreatic cancer. The results are shown in Figure 1. The expression of survivin was localized in cytoplasm of tumor cells, which are shown as brown granules in Figure 1 (SP), and in Figure 2 (HE). Survivin was expressed in 34 of 42 pancreatic cancer samples, but not expressed in the 10 samples from normal pancreatic tissue. The expression rate was 81.95%.
To study the relationship between the expressions of survivin and Bcl-2, 42 cases were analyzed. The results are shown in Table 2.
| Survivin expression | P | r | |||
| (+) | (-) | ||||
| Bcl-2 (+) | 28 | 2 | |||
| Bcl-2 (-) | 6 | 6 | < 0.01 | 0.499 | |
Survivin, a recently-characterized member of IAP family, was isolated from the human gene bank by Altieri et al[6] in 1997 using effector cell protease receptor-1 (EPR-1) cDNA. Recently, great progress has been made in the structure and function of survivin and its correlation to malignant tumors. Many findings have suggested that survivin may express selectively in different tissues. Survivin was max expressed or poorly expressed in normal terminally-differentiated tissues, whereas it was extensively expressed in many kinds of human tumor tissues[7-14].
Studies have shown that Caspase is responsible for apoptosis, which can activate in cascade and lyze protein, thus determining the pattern of apoptosis. Survivin could directly inhibit the activities of Caspase-3 and 7 and block the process of apoptosis and indirectly inhibit Caspase through P21.Therefore survivin would bind to cell cycle apoptosis factor CDK4 to form survivin-CDK4 complex, and then release of P21 from CDK4 complex.When P21 was bound to mitochondrial Caspase-3, it could inhibit its activity, thus preventing apoptosis[15-17].
The results of our study showed that survivin was highly expressed in pancreatic tumor tissues, but not in normal pancreatic tissues, suggesting that it might play a critical role in the development and progression of pancreatic cancer. Furthermore, there was a positive correlation between survivin expression and tumor TNM staging and differentiation grade. It can be concluded that survivin expression is indicative of higher invasiveness or poor prognosis in pancreatic cancer.
Bcl-2 was the first characterized anti-apoptotic gene with an inhibiting apoptotic pathway different from that of survivin. Bcl-2 could regulate apoptosis by preventing cytochrome C release from mitochondrion to cytoplasm. Whereas, survivin acted by direct inhibition of the terminal effector proteases of apoptosis, i.e., Caspase-3 and Caspase-7.
Our study demonstrated that the expression of survivin was positively correlated with that of Bcl-2. It might be caused by the same transcription and activation mechanism of survivin and Bcl-2, or both might be regulated by GC-rich promoters, and were then transcripted and activated to enhance cell proliferation, acting synergistically to inhibit apoptosis[18-29].
Asanuma et al[2,5] investigated whether survivin expression could directly regulate cancer sensitivity to radiotherapy using gene-transducted pancreatic cancer cell strain (MIAPaCa-2). Their results showed that survivin expression could directly down-regulate pancreatic cancer sensitivity to radiotherapy.
In addition, Rohayem et al[12,18,31] observed that specific anti-survivin antibody could be detected in serum of patients with cancers of the lung and colon, suggesting that this antibody could be used as a new diagnostic marker for cancers of the lung and colon. Furthermore, Smith et al[31], Moore et al[32], and Swana et al[33] found that survivin levels in urine could be used to diagnose primary and recurrent bladder carcinomas, thus providing a new idea for the diagnosis of pancreatic cancer[34-38].
In conclusion, Survivin can be used as a cancer therapeutic target because of its selective expression in the tissue concerned. Moreover, natural antisense nucleic acid for Survivin-endogenous EPR-1 has become a new hot issue for the tumor gene therapy.
Edited by Wang XL and Chen ZR Proofread by Xu FM
| 1. | Satoh K, Kaneko K, Hirota M, Masamune A, Satoh A, Shimosegawa T. Expression of survivin is correlated with cancer cell apoptosis and is involved in the development of human pancreatic duct cell tumors. Cancer. 2001;92:271-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 12] [Reference Citation Analysis (0)] |
| 2. | Asanuma K, Kobayashi D, Furuya D, Tsuji N, Yagihashi A, Watanabe N. A role for survivin in radioresistance of pancreatic cancer cells. Jpn J Cancer Res. 2002;93:1057-1062. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Sarela AI, Verbeke CS, Ramsdale J, Davies CL, Markham AF, Guillou PJ. Expression of survivin, a novel inhibitor of apoptosis and cell cycle regulatory protein, in pancreatic adenocarcinoma. Br J Cancer. 2002;86:886-892. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 142] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 4. | Jinfeng M, Kimura W, Sakurai F, Moriya T, Takeshita A, Hirai I. Histopathological study of intraductal papillary mucinous tumor of the pancreas: special reference to the roles of Survivin and p53 in tumorigenesis of IPMT. Int J Gastrointest Cancer. 2002;32:73-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Asanuma K, Moriai R, Yajima T, Yagihashi A, Yamada M, Kobayashi D, Watanabe N. Survivin as a radioresistance factor in pancreatic cancer. Jpn J Cancer Res. 2000;91:1204-1209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 147] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 6. | Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917-921. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2342] [Cited by in F6Publishing: 2349] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 7. | Hausladen DA, Wheeler MA, Altieri DC, Colberg JW, Weiss RM. Effect of intravesical treatment of transitional cell carcinoma with bacillus Calmette-Guerin and mitomycin C on urinary survivin levels and outcome. J Urol. 2003;170:230-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003;3:46-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 931] [Cited by in F6Publishing: 928] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 9. | Altieri DC. Survivin and apoptosis control. Adv Cancer Res. 2003;88:31-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 116] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Altieri DC. The molecular basis and potential role of survivin in cancer diagnosis and therapy. Trends Mol Med. 2001;7:542-547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 313] [Cited by in F6Publishing: 333] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 11. | Carter BZ, Milella M, Altieri DC, Andreeff M. Cytokine-regulated expression of survivin in myeloid leukemia. Blood. 2001;97:2784-2790. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 146] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 12. | Kawasaki H, Altieri DC, Lu CD, Toyoda M, Tenjo T, Tanigawa N. Inhibition of apoptosis by survivin predicts shorter survival rates in colorectal cancer. Cancer Res. 1998;58:5071-5074. [PubMed] [Cited in This Article: ] |
| 13. | Lu CD, Altieri DC, Tanigawa N. Expression of a novel antiapoptosis gene, survivin, correlated with tumor cell apoptosis and p53 accumulation in gastric carcinomas. Cancer Res. 1998;58:1808-1812. [PubMed] [Cited in This Article: ] |
| 14. | Li F, Ackermann EJ, Bennett CF, Rothermel AL, Plescia J, Tognin S, Villa A, Marchisio PC, Altieri DC. Pleiotropic cell-division defects and apoptosis induced by interference with survivin function. Nat Cell Biol. 1999;1:461-466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 450] [Cited by in F6Publishing: 473] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 15. | Suzuki A, Ito T, Kawano H, Hayashida M, Hayasaki Y, Tsutomi Y, Akahane K, Nakano T, Miura M, Shiraki K. Survivin initiates procaspase 3/p21 complex formation as a result of interaction with Cdk4 to resist Fas-mediated cell death. Oncogene. 2000;19:1346-1353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 186] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Ito T, Shiraki K, Sugimoto K, Yamanaka T, Fujikawa K, Ito M, Takase K, Moriyama M, Kawano H, Hayashida M. Survivin promotes cell proliferation in human hepatocellular carcinoma. Hepatology. 2000;31:1080-1085. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 267] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 17. | García JF, Camacho FI, Morente M, Fraga M, Montalbán C, Alvaro T, Bellas C, Castaño A, Díez A, Flores T. Hodgkin and Reed-Sternberg cells harbor alterations in the major tumor suppressor pathways and cell-cycle checkpoints: analyses using tissue microarrays. Blood. 2003;101:681-689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 193] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 18. | Rohayem J, Diestelkoetter P, Weigle B, Oehmichen A, Schmitz M, Mehlhorn J, Conrad K, Rieber EP. Antibody response to the tumor-associated inhibitor of apoptosis protein survivin in cancer patients. Cancer Res. 2000;60:1815-1817. [PubMed] [Cited in This Article: ] |
| 19. | Sharief MK, Semra YK. Down-regulation of survivin expression in T lymphocytes after interferon beta-1a treatment in patients with multiple sclerosis. Arch Neurol. 2002;59:1115-1121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Agui T, McConkey DJ, Tanigawa N. Comparative study of various biological parameters, including expression of survivin, between primary and metastatic human colonic adenocarcinomas. Anticancer Res. 2002;22:1769-1776. [PubMed] [Cited in This Article: ] |
| 21. | Gradilone A, Gazzaniga P, Ribuffo D, Scarpa S, Cigna E, Vasaturo F, Bottoni U, Innocenzi D, Calvieri S, Scuderi N. Survivin, bcl-2, bax, and bcl-X gene expression in sentinel lymph nodes from melanoma patients. J Clin Oncol. 2003;21:306-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Yoshida H, Ishiko O, Sumi T, Matsumoto Y, Ogita S. Survivin, bcl-2 and matrix metalloproteinase-2 enhance progression of clear cell- and serous-type ovarian carcinomas. Int J Oncol. 2001;19:537-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Konno R, Yamakawa H, Utsunomiya H, Ito K, Sato S, Yajima A. Expression of survivin and Bcl-2 in the normal human endometrium. Mol Hum Reprod. 2000;6:529-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 115] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Rödel F, Hoffmann J, Grabenbauer GG, Papadopoulos T, Weiss C, Günther K, Schick C, Sauer R, Rödel C. High survivin expression is associated with reduced apoptosis in rectal cancer and may predict disease-free survival after preoperative radiochemotherapy and surgical resection. Strahlenther Onkol. 2002;178:426-435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 70] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Guan J, Chen J, Luo Y, Gao J, Qiu H. [Effects of antisense bcl-2 or survivin on the growth of human neuroblastoma cell line SK-N-MC]. Zhonghua Yixue Zazhi. 2002;82:1536-1540. [PubMed] [Cited in This Article: ] |
| 26. | Sarela AI, Scott N, Ramsdale J, Markham AF, Guillou PJ. Immunohistochemical detection of the anti-apoptosis protein, survivin, predicts survival after curative resection of stage II colorectal carcinomas. Ann Surg Oncol. 2001;8:305-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 93] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Hiroshima K, Iyoda A, Toyozaki T, Supriatna Y, Shibuya K, Shimamura F, Haga Y, Yoshida S, Fujisawa T, Ohwada H. Proliferative activity and apoptosis in thymic epithelial neoplasms. Mod Pathol. 2002;15:1326-1332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Mikami T, Yoshida T, Akino F, Motoori T, Yajima M, Okayasu I. Apoptosis regulation differs between ulcerative colitis-associated and sporadic colonic tumors. Association with survivin and bcl-2. Am J Clin Pathol. 2003;119:723-730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Park JW, Choi YJ, Suh SI, Baek WK, Suh MH, Jin IN, Min DS, Woo JH, Chang JS, Passaniti A. Bcl-2 overexpression attenuates resveratrol-induced apoptosis in U937 cells by inhibition of caspase-3 activity. Carcinogenesis. 2001;22:1633-1639. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Monzó M, Rosell R, Felip E, Astudillo J, Sánchez JJ, Maestre J, Martín C, Font A, Barnadas A, Abad A. A novel anti-apoptosis gene: Re-expression of survivin messenger RNA as a prognosis marker in non-small-cell lung cancers. J Clin Oncol. 1999;17:2100-2104. [PubMed] [Cited in This Article: ] |
| 31. | Smith SD, Wheeler MA, Plescia J, Colberg JW, Weiss RM, Altieri DC. Urine detection of survivin and diagnosis of bladder cancer. JAMA. 2001;285:324-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 212] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 32. | Moore M. Urine detection of survivin and diagnosis of bladder cancer. J Insur Med. 2001;33:202-203. [PubMed] [Cited in This Article: ] |
| 33. | Swana HS, Grossman D, Anthony JN, Weiss RM, Altieri DC. Tumor content of the antiapoptosis molecule survivin and recurrence of bladder cancer. N Engl J Med. 1999;341:452-453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 225] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 34. | Yamamoto T, Tanigawa N. The role of survivin as a new target of diagnosis and treatment in human cancer. Med Electron Microsc. 2001;34:207-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 91] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Sela B. [Survivin: anti-apoptosis protein and a prognostic marker for tumor progression and recurrence]. Harefuah. 2002;141:103-107, 123. [PubMed] [Cited in This Article: ] |
| 36. | Xia C, Xu Z, Yuan X, Uematsu K, You L, Li K, Li L, McCormick F, Jablons DM. Induction of apoptosis in mesothelioma cells by antisurvivin oligonucleotides. Mol Cancer Ther. 2002;1:687-694. [PubMed] [Cited in This Article: ] |
| 37. | Carter BZ, Wang RY, Schober WD, Milella M, Chism D, Andreeff M. Targeting Survivin expression induces cell proliferation defect and subsequent cell death involving mitochondrial pathway in myeloid leukemic cells. Cell Cycle. 2003;2:488-493. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Li F. Survivin study: what is the next wave. J Cell Physiol. 2003;197:8-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 243] [Article Influence: 11.6] [Reference Citation Analysis (0)] |