INTRODUCTION
Liver metastasis of breast cancer usually presents cancer nodules scattered in the liver and the radiological diagnosis is not difficult. However, rarely, it does not form space-occupying lesions and manifests itself as multifocal occlusion of intrahepatic branches of the portal and/or hepatic veins by tumor thrombi. Such circulatory disturbances can result in deformity of the liver, mimicking cirrhosis[1-4]. In these cases, since radiological findings are uneven hepatic circulation and hepatic deformity, the diagnosis is difficult and sometimes misleading[5-8]. We report a case of hepatic metastasis of breast carcinoma, which diffusely spread in the liver without radiologically detectable lesions or obvious hepatic deformity. It was successfully diagnosed by laparoscopy-assisted liver biopsy. We have discussed its pathogenesis based on the previous reports.
CASE REPORT
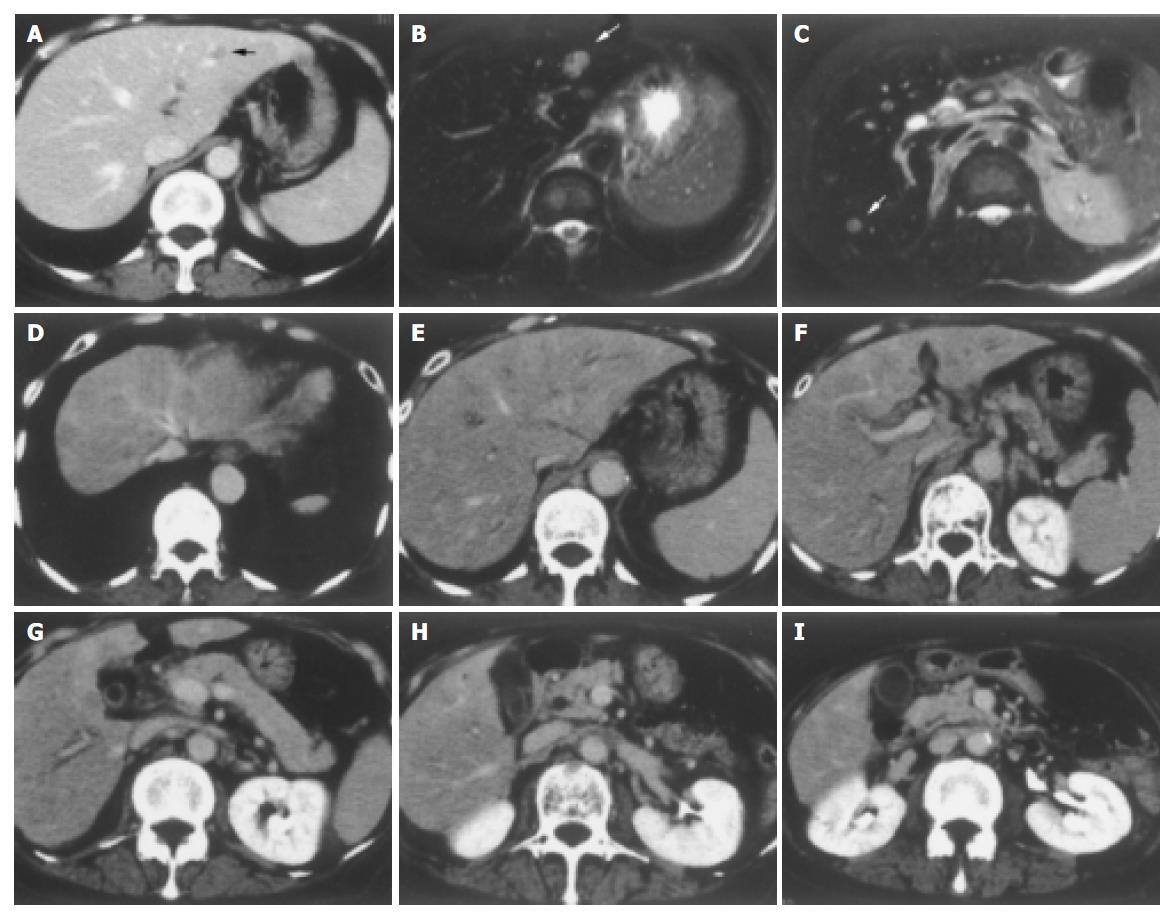
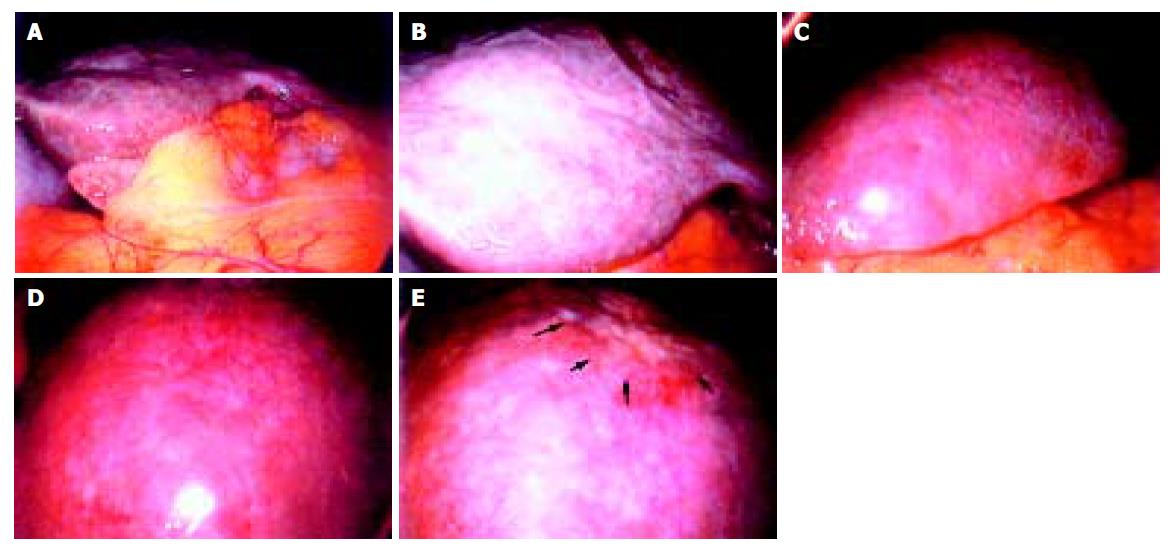
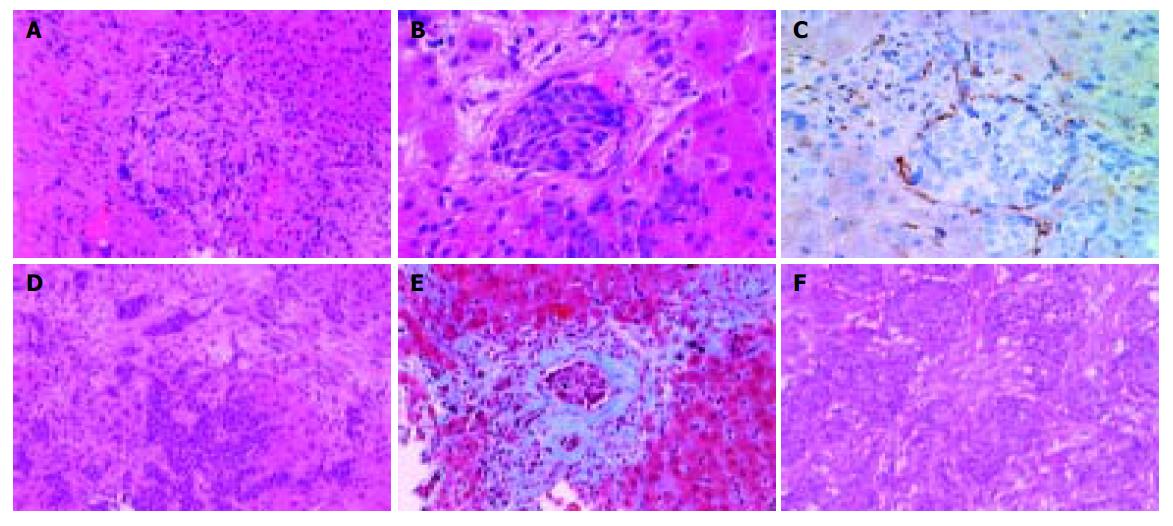
A 68-year-old woman was admitted to our hospital complaining of general malaise. She had received left partial mastectomy on August 2, 2002. The tumor was in stage I and was histologically invasive ductal carcinoma of scirrhous type. An enhanced computed tomography (CT) scan on April 2, 2003 revealed a metastatic lesion in liver S2 (Figure 1A), and an enhanced magnetic resonance image with Ferridex on May 19, 2003 showed metastatic lesions in S2 and S6 (Figures 1B and 1C). From April to December 2003, combination chemotherapy was administered every month, using 900 mg of cyclophosphamide and 110 mg of epirubicin for the first 4 mo and then reducing cyclophosphamide to 500 mg and epirubicin to 60 mg. In February 2004, the patient was admitted to our hospital, complaining of general fatigue and appetite loss and showing abnormal liver function test results. After admission, she also manifested drowsiness, disorientation and insomnia. The patient was 154 cm in height and weighed 54 kg. Visible mucosa showed slight jaundice and no anemia. There was no sign of palmar erythema or vascular spider. Superficial lymph nodes were not palpable. There were two sites of skin eruption at the left anterior chest, which had already been diagnosed as skin metastasis by biopsy. There was leg edema but no hepatosplenomegaly. The blood test showed abnormalities in liver function, coagulation disturbance, and thrombo-cytopenia. Specifically, the results were as follows: platelet count, 9.3×104/μL; prothrombin time, 82%; fibrin degradation product, 15.4 μg/mL; C-reactive protein, 5.7 mg/dL; lactate dehydrogenase, 810 IU/L; aspartate aminotransferase, 219 IU/L; alanine aminotransferase, 240 IU/L; alkaline phosphatase, 1826 IU/L; γ-glutamyltr-anspeptidase, 960 IU/L; total bilirubin 4.97 mg/dL; ammonia, 252 μg/dL; total protein, 5.6 g/dL; albumin, 3.1 g/dL. Dynamic study with CT scan demonstrated uneven enhancement effect in the liver in late phase, suggesting uneven hepatic blood supply. At this time, the metastatic tumors had become undetectable (Figures 1D-1I) and hepatic deformity was not obvious. To clarify the exact cause of liver function abnormalities, laparoscopy was performed. It showed an irregular and deformed liver surface with multiple indentations and shallow linear depressions (Figures 2A-2D). A wide scar was observed on the surface of S2 possibly at the site where the metastatic tumor existed before chemotherapy (Figure 2E, arrowheads). Laparoscopic liver biopsy from a wide scar lesion showed residual cancer cells scattered in a wide fibrous band in one area (Figure 3A). In another area intraportal tumor thrombi were clearly demonstrated by CD31 immun-ohistochemical staining (Figures 3B and 3C). Cancer cells showed desmoplastic change around them (Figure 3D), which extended toward the sinusoids (Figure 3E). Because of its similarity to the histology of original breast cancer (Figure 3F), we concluded that the hepatic functional abnormalities were due to the uneven blood supply caused by intraportal invasion of the breast cancer. Drowsiness, disorientation and insomnia, along with high level of serum ammonia, were suggestive of hepatic encephalopathy. Lactulose and branched-chain amino acid supplement were given but failed to improve the symptoms, and serum ammonia level stayed over 200 μg/dL. The patient presented a rapid downhill course and died of respiratory failure due to lymphangitis carcinomatosa on March 20, 2004.
Figure 1 A: Enhanced CT scan on April 2, 2003.
A metastatic lesion in liver S2 (arrowhead). B and C: Enhanced magnetic resonance image with Ferridex on May 19, 2003. Metastatic lesions in S2 (B, arrowhead) and S6 (C, arrowhead). D-I: Dynamic study by CT scan on admission. Uneven enhancement effect of the liver in late phase.
Figure 2 Irregular and deformed liver surface with multiple indentations and shallow linear depressions (A and B: right lobe, C and D: left lobe).
A wide scar on the surface of S2 at the site where metastatic tumor existed before chemotherapy (E, arrowheads).
Figure 3 A: Laparoscopic liver biopsy from a wide scar lesion showed residual cancer cells scattered in a wide fibrous band in one area (hematoxylin and eosin (HE) stain).
B and C: In another area intraportal tumor thrombi were clearly demonstrated. Endothelial cells were stained brown by CD31 immunohistochemistry (B, HE stain, C, CD31 immunohistochemical stain). D and E: Cancer cells showed desmoplastic change around them, which extended toward the sinusoids (D, HE stain, E, Masson’s trichrome stain). F: Original breast cancer. Note desmoplastic change around the tumor cells (HE stain).
DISCUSSION
When metastatic breast carcinoma in the liver does not form space-occupying lesions, its radiological diagnosis is difficult. There are some case reports on diffuse infiltration of breast cancer cells found at autopsy, which was not diagnosed even with the most advanced modalities like CT scan, ultrason-ography and magnetic resonance imaging[5-8]. In the present case, radiological diagnosis was difficult because dynamic study by CT scan only demonstrated uneven hepatic blood supply, which is also seen in chronic or acute liver injury. Indeed, definitive diagnosis was made only by laparoscopy-assisted liver biopsy.
Carcinomatous involvement of the liver mimicking cirrh-osis is a rare complication of metastatic carcinoma, most frequ-ently observed with scirrhous carcinoma of the breast[1-4]. Borja et al[1], reported a case of metastatic breast cancer, which presented various clinical manifestations of liver failure grossly characterized by a distorted liver surface caused by multifocal portal obstruction with cancer cells. Busni et al[9], first introduced the term hepar lobatum carcinomatosum (HLC), which was defined as an acquired hepatic deformity consisting of an irregularly lobulated hepatic contour caused by intravascular infiltration of metastatic carcinoma. To our knowledge, only nine cases of HLC have been reported in the literature to date[9-13].
In the present case, although radiological examinations revealed neither distorted nor irregularly lobulated liver surface, laparoscopy presented a slightly irregular and deformed liver surface with multiple indentations and shallow linear depressions. A biopsy sample from the wide fibrous scar revealed the intraportal infiltration of cancer cells surrounded by fibrous tissues. As speculated by Gravel et al[13], multifocal carcinomatous obstruction of portal veins caused circulatory deficiency leading to multiple scattered and linear depressions on the liver surface, which were interpreted as fibrous scarring. In addition, desmoplastic change caused by carcinoma cells may have also contributed to the formation of fibrous scar tissue. These pathologic conditions are basically the same as those of HLC, and therefore, our case can be categorized as the same entity, though it was not so far advanced in terms of hepatic deformity.
Combination chemotherapy with cyclophosphamide and epirubicin was performed in this case. Various chemoth-erapeutic agents were given to eight out of the nine previously reported HLC patients, but most of the agents, including cyclophosphamide and doxorubicin (the same group as epirubicin) are not known to induce fibrogenesis by themselves or even synergistically. Hence, some authors[10-14] speculate that fibrosis in HLC is not caused by direct effect of chemotherapeutic agents, but is actually scar contraction after tissue collapse by chemotherapy-induced tumor necrosis or regression. In the present case, this condition occurred after chemotherapy which caused the disappearance of metastatic liver tumors. Furthermore, a wide scar was observed at the site where the metastatic tumor existed before chemotherapy. These findings also support the possibility of chemotherapy as another important but indirect cause of fibrous scarring.
In this case, drowsiness, disorientation and insomnia, and a high level of serum ammonia were suggestive of hepatic encephalopathy. However, lactulose and branched-chain amino acid supplementation were not effective, and serum ammonia level stayed over 200 μg/dL. In addition, leg edema with low serum albumin level mimicked the clinical manife-station of cirrhosis. These clinical symptoms and laboratory data indicate that rapidly progressive and diffuse circula-tory disturbance due to carcinomatous obstruction of portal veins can cause some symptoms of hepatic failure[15-18].