Published online May 7, 2005. doi: 10.3748/wjg.v11.i17.2666
Revised: May 28, 2004
Accepted: June 17, 2004
Published online: May 7, 2005
AIM: To investigate the capability of multidetector CT (MDCT) to diagnose HCC-associated arterioportal shunt (APS).
METHODS: Two hundred and eighty-two patients with HCC received both thin-slice and enhancement MDCT scanning at early hepatic arterial phase, late hepatic arterial phase and portal venous phase, and digital subtract angiography (DSA) examination. Images were analyzed jointly by two experienced radiologists blinded to the opposite examination results, including the existence or not of APS, shunt locations, types and degrees of APS, with or without thrombosis.
RESULTS: There were 56 APS associated with HCC, including 48 central, seven peripheral and one mixed, or 42 severe, seven moderate, seven mild APS. Forty-one severe, seven moderate and central APS were all revealed with MDCT and DSA. Seven mild and peripheral APS were all displayed with MDCT; only five of them displayed DSA, two faint shunt APS associated with massive HCC were missed. One mixed APS was demonstrated as severe combined with mild shunt with both MDCT and DSA.
CONCLUSION: MDCT could diagnose not only DSA revealed APS, but also missed mild and peripheral APS with DSA due to faint shunt associated with massive HCC, is a simple, effective and noninvasive new technique for diagnosis of HCC-associated APS.
- Citation: Luo MY, Shan H, Jiang ZB, Liang WW, Zhang JS, Li LF. Capability of multidetector CT to diagnose hepatocellular carcinoma-associated arterioportal shunt. World J Gastroenterol 2005; 11(17): 2666-2669
- URL: https://www.wjgnet.com/1007-9327/full/v11/i17/2666.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i17.2666
Hepatocelluar carcinoma (HCC) can easily invade portal vein to form the direct communication between hepatic artery or its branches and portal vein, resulting in arterioportal shunt (APS). APS may cause and/or aggravate portal hypertension and consequently, splenomegaly, stubborn ascites and diarrhea, esophagogastric varices and bleeding, and hepatic encephalopathy, accelerating intrahepatic dissemination and extrahepatic metastasis of carcinoma cells. Transcatheter hepatic angiography (including digital subtract angiography, DSA) is always the gold standard for diagnosis of HCC-associated APS, but has many disadvantages. Multidetector CT (MDCT) could contribute to the diagnosis of HCC-associated APS due to its fast scanning, improved image resolution and quality[1,2]. The purpose of this study was to investigate the capability of MDCT to diagnose HCC-associated APS in contrast with DSA.
Two hundred and eighty-two patients (247 men and 35 women, range 26-76 years, mean age 48.8 years) with HCC were included in the present study. The diagnosis of HCC was based on the results of percutaneous needle biopsy (n = 26) or laboratory testings, including elevated serum alpha-fetoprotein level, in combination with imaging appearance and follow-up images (n = 256) according to the diagnostic criteria for HCC formulated by Chinese National Association of Anticancer Committee.
MDCT scanning was performed with a LightSpeed QX/i MDCT scanner (General Electronic Medical System, Milwaukee, USA). Multidetector row helical technique was applied to the scanning in the cranial to caudal direction. Plain scanning of the liver was carried out, followed by enhancement scanning of 2.5 mm axial section performed at 15, 25 and 65 s after injection of contrast medium for early hepatic arterial phase, late hepatic arterial phase and portal venous phase image acquisition respectively. Contrast medium (1.5 mL/kg; Ultravist 300 mg I/mL, Schering Pharmacy, Guangzhou, China; or Iopamiro 300 mg I/mL, Bracco SPA, Milano, Italy ) was administered to each patient, with a power injector at a rate of 3.5 mL/s through a catheter placed in the peripheral vein of the antecubital fossa.
DSA was performed using a TOSHIBA 1000 MAX (Toshiba Corporation, Tokyo, Japan) within 2 wk of MDCT examination. A catheter was introduced via the right femoral artery by the Seldinger technique. The celiac (34 patients) or selective hepatic (248 patients) DSA was carried out with a catheter placed in the celiac trunk or in the proper hepatic artery and 40 mL contrast medium (Ultravist 300 mg I/mL, Schering Pharmacy, Guangzhou, China; or Iopamiro 300 mg I/mL, Bracco SPA, Milano, Italy ) was injected with a power injector at a rate of 5.0 mL/s. Serial anterior-posterior images were obtained at 1 of every 2 s for the first 8 s and a slower rate thereafter.
Diagnostic criteria for APS were the earlier enhancement of main portal trunk and/or its first-order branches than that of superior mesenteric or splenic vein, or stronger opacification of main portal trunk and/or its first-order branches than that of superior mesenteric vein or splenic vein; or earlier enhancement of the second-order and smaller branches of portal veins than that of the main portal trunk, or stronger opacification of the second-order and smaller branches of portal veins than that of main portal trunk[3].
According to the location of shunt, APS was divided into three types. The central APS was the shunt located in porta hepatis with earlier enhancement and/or stronger opacification of main portal trunk and/or the first-order branches at early hepatic arterial phase. The peripheral APS was the shunt located in peripheral liver parenchyma with earlier enhancement and/or stronger opacification of the second-order and smaller branches of portal vein, and transient patchy or wedge-shaped enhancement peripheral to HCC foci at late hepatic arterial phase. The mixed APS showed both central and peripheral findings.
According to the time of appearance of APS on images, APS was divided into three degrees. The severe APS showed opacification of main portal trunk and/or the first-order branches with enhancement of hepatic arterial and its branches at the early hepatic arterial phase, without enhancement or with early enhancement of HCC foci. The moderate APS demonstrated opacification of main portal trunk and/or the first-order branches, with middle or late enhancement of HCC foci at late hepatic arterial phase. The mild APS revealed opacification of the second-order and smaller branches of portal veins at late hepatic arterial phase, with transient patchy or wedge-shaped enhancement peripheral to HCC foci.
Images were analyzed jointly by two experienced radiologists blinded to the opposite examination results, and inconsistency in the image analysis was resolved by consensus, including the existence or non-existence of APS, shunt locations, types and degrees of APS, with or without thrombosis.
There were 56 APS in 282 patients with HCC, including 48 central, 7 peripheral and 1 mixed, or 42 severe, 7 moderate and 7 mild APS.
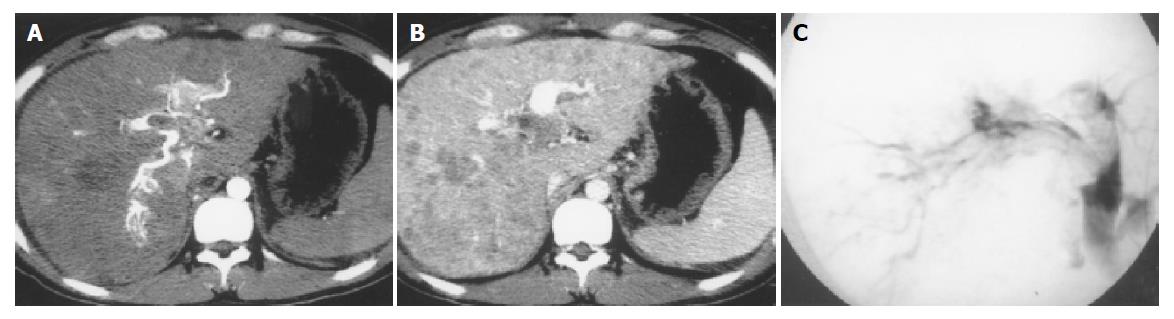
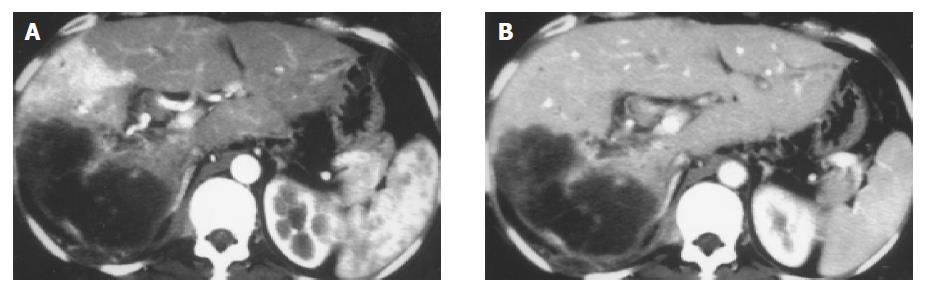
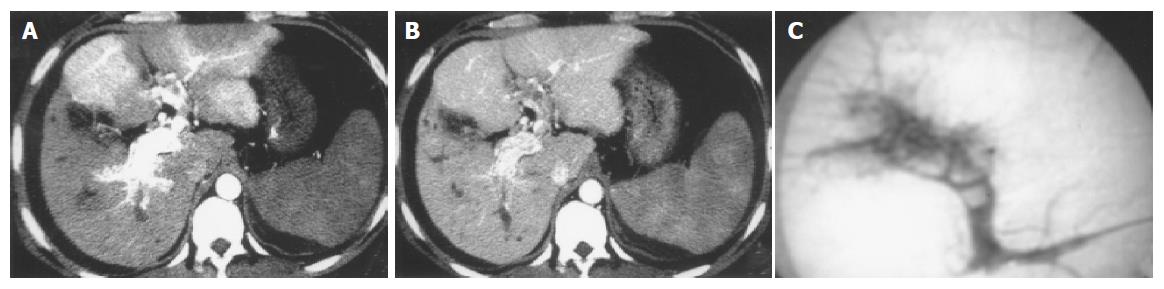
Forty-one severe, seven moderate and central APS were all revealed with MDCT and DSA (Figure 1). Seven mild and peripheral APS were all displayed with MDCT; only five of them were showed with DSA, two faint shunt APS associated with massive HCC were missed (Figure 2). One mixed APS was demonstrated as severe combined with mild shunt with both MDCT and DSA (Figure 3). The results of APS examined with MDCT and DSA are shown in Table 1.
| Examination modality | Shunt pattern | Shunt degree | ||||
| Central | Peripheral | Mixed | Severe | Moderate | Mild | |
| MDCT | 48 | 7 | 1 | 42 | 7 | 7 |
| DSA | 48 | 5 | 1 | 42 | 7 | 5 |
Thirty-two patients with central APS had thromboses in portal veins, including 18 patients with thromboses in main portal trunks and the first-order branches, 11 patients with thromboses in the right first-order branches and 3 patients with thromboses in the left first-order branches.
Transcatheter hepatic angiography (including DSA) is always the gold standard for diagnosis of APS in the past, can reveal the shunt locations, types and degrees of APS. However, it has many disadvantages. Firstly, introduced via femoral artery by the Seldinger technique, a catheter is placed in the celiac trunk or proper hepatic artery to perform celiac or selective hepatic angiography, it is an invasive examination and may result in some pain and complications; secondly, it may fail due to anatomic variation of hepatic artery; thirdly, APS in segment 2 or 3 may be missed because of the unsatisfied opacification of hepatic artery; fourthly, mild and peripheral APS may be missed due to faint shunt, specially for mild and peripheral APS associated with massive HCC; finally, it may be restricted by equipment conditions, examination technology and cost. A proportion of patients that could not undergo transcatheter hepatic angiography, could have missed diagnosis by APS and lost the treatment opportunity in clinical practice[4,5]. In our series, only five of seven mild and peripheral APS were displayed with DSA, two were missed due to faint shunt associated with massive HCC.
Study result of Itai et al[6], indicated that in contrast with transcatheter hepatic angiography, conventional CT could reveal 82.35% central APS diagnosed with transcatheter hepatic angiography; 32% peripheral APS, which displayed as transient wedge-shaped enhancement peripheral to HCC foci. Chen et al[3], reported that biphasic enhancement scanning of single detector CT could completely display central APS and its shunt degrees confirmed with transcatheter hepatic angiography.
MDCT is a new breakthrough in medical imaging examination technology. It can perform multislice image data acquisition simultaneously, greatly reduces the time of volume scanning. In addition, image quality is improved due to increased image resolution and clarity. MDCT could therefore offer thin-slice and dynamic enhancement scanning of liver at early hepatic arterial phase, late hepatic arterial phase and portal venous phase, with comprehensive revealing of the characteristics of blood supply of liver and other viscus in upper abdomen and hemodynamic changes of APS, providing a convenient, fast and noninvasive new technology for examination of HCC-associated APS[1,2]. In our study, MDCT diagnosed not only 41 moderate, seven severe and central, five mild and peripheral, one severe combined with mild and mixed APS revealed with DSA, but also two mild and peripheral APS missed with DSA due to faint shunt associated with massive HCC.
There seemed to be not much difficulty in the diagnosis of severe or moderate HCC-associated APS, if there is a correct understanding of the MDCT appearance. Mild APS should be differentiated from hepatic perfusion abnormalities of physiological conditions and other pathologic causes[7-11]. Hepatic perfusion abnormalities caused by physiological conditions such as origin of a variety of segment or subsegment of hepatic artery, aberrant biliary bladder vein or gastric vein are only shown as local transient hepatic parenchyma hyperattenuation at hepatic arterial phase with no abnormality at portal vein phase and no HCC foci[12]. Abnormal perfusion in cirrhotic liver had the typical wedge-shaped and homogeneous appearance with or without internal linear branching structures at hepatic arterial phase, returning to isoattenuation or slight hyperattenuation at portal vein phase. APS in hepatic hemangioma manifested as a wedge-shaped or irregular homogenous hyperattenuation in the liver parenchyma adjacent to the tumor at hepatic arterial phase, becoming isoattenuation or slight hyperattenuation at portal vein phase, and hemangioma itself tending to show rapid enhancement at portal vein phase, specially for hemangioma of 2-3 cm in diameter or less[13-15]. Hepatic adenoma and focal nodular hyperplasia appeared as rapid and homogenous enhancement at hepatic arterial phase, being hypoattenuation at delayed time phase. The site of abnormal perfusion associated with thrombosis in portal vein was conformed to respective portal vein distribution. Hepatic metastasis with abundant blood supply, liver infection, Budd-Chiari syndrome, changes after transjugular intrahepatic portosystemic shunt, APS following liver biopsy, abnormal perfusion resulted from acute biliary bladder inflammation or acute pancreatitis all had their own MDCT features, could be differentiated from HCC-associated mild APS combined with clinical materials[16-21].
The formation of HCC-associated APS may bring about some difficulty and danger to treatment, such as cause chemotherapy drug and embolism agent run-off through shunt path, and result in aberrant embolism included pulmonary embolism. However, if hepatic function and general physical condition is good, the embolism therapy is safe and effective after comprehensive being aware of the location and degree of APS, thrombosis in vein and collateral circulation, having active significance for prolonging of life span and elevation of life quality of patients[22]. Our 56 APS were all completely embolized by absolute ethanol combined with spring steel coil (41 moderate, 7 severe and central, 1 severe combined with mild and mixed APS) or absolute ethanol (seven mild and peripheral APS) via 4.0 F catheter or 3.0 F microcatheter superselective embolism under the guidance of MDCT information, without recanalization and recurrence of APS by follow-up MDCT examinations, and their esophagogastric varices and bleeding, stubborn ascites and diarrhea were all brought under timely control, having elevated safety and effect of embolism.
In conclusion, triphasic thin-slice and enhancement scanning of MDCT could diagnose not only DSA revealed APS, but also missed mild and peripheral APS with DSA due to faint shunt associated with massive HCC, is a simple, effective and noninvasive new technique for diagnosis of HCC-associated APS.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Takahashi S, Murakami T, Takamura M, Kim T, Hori M, Narumi Y, Nakamura H, Kudo M. Multi-detector row helical CT angiography of hepatic vessels: depiction with dual-arterial phase acquisition during single breath hold. Radiology. 2002;222:81-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Mortele KJ, McTavish J, Ros PR. Current techniques of computed tomography. Helical CT, multidetector CT, and 3D reconstruction. Clin Liver Dis. 2002;6:29-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Chen JH, Chai JW, Huang CL, Hung HC, Shen WC, Lee SK. Proximal arterioportal shunting associated with hepatocellular carcinoma: features revealed by dynamic helical CT. AJR Am J Roentgenol. 1999;172:403-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Chen JH, Chen WP, Huang CL, Shen WC. Dynamic helical CT as a novel technique for diagnosing hepatic perfusion disorders. Hepatogastroenterology. 1999;46:303-307. [PubMed] [Cited in This Article: ] |
| 5. | Chen JH, Huang CL, Hwang JI, Lee SK, Shen WC. Dynamic helical biphasic CT emerges as a potential tool for the diagnosis of proximal arterioportal shunting. Hepatogastroenterology. 1999;46:1791-1797. [PubMed] [Cited in This Article: ] |
| 6. | Itai Y, Furui S, Ohtomo K, Kokubo T, Yamauchi T, Minami M, Yashiro N. Dynamic CT features of arterioportal shunts in hepatocellular carcinoma. AJR Am J Roentgenol. 1986;146:723-727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 55] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Chen WP, Chen JH, Hwang JI, Tsai JW, Chen JS, Hung SW, Su YG, Lee SK. Spectrum of transient hepatic attenuation differences in biphasic helical CT. AJR Am J Roentgenol. 1999;172:419-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Park CM, Cha SH, Kim DH, Choi JA, Cha IH, Kim YH, Chung KB, Suh WH. Hepatic arterioportal shunts not directly related to hepatocellular carcinoma: findings on CT during hepatic arteriography, CT arterial portography and dual phase spiral CT. Clin Radiol. 2000;55:465-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Quiroga S, Sebastià C, Pallisa E, Castellà E, Pérez-Lafuente M, Alvarez-Castells A. Improved diagnosis of hepatic perfusion disorders: value of hepatic arterial phase imaging during helical CT. Radiographics. 2001;21:65-81; questionnaire 288-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 84] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Choi BI, Lee KH, Han JK, Lee JM. Hepatic arterioportal shunts: dynamic CT and MR features. Korean J Radiol. 2002;3:1-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 102] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Luo TY, Shi B, Li YM, Lu FJ, Yuan SW, Yan M, Wu JQ. A study on the transient hepatic abnormal enhancement in the hepatic arterial phase during dynamic contrast-enhanced spiral CT. Zhonghua Fangshexue Zazhi. 2003;37:258-263. [Cited in This Article: ] |
| 12. | Yoon KH, Matsui O, Kadoya M, Yoshigawa J, Gabata T, Arai K. Pseudolesion in segments II and III of the liver on CT during arterial portography caused by aberrant right gastric venous drainage. J Comput Assist Tomogr. 1999;23:306-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Naganuma H, Ishida H, Konno K, Hamashima Y, Komatsuda T, Ishida J, Masamune O. Hepatic hemangioma with arterioportal shunts. Abdom Imaging. 1999;24:42-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Vilgrain V, Boulos L, Vullierme MP, Denys A, Terris B, Menu Y. Imaging of atypical hemangiomas of the liver with pathologic correlation. Radiographics. 2000;20:379-397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 319] [Cited by in F6Publishing: 244] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 15. | Kim KW, Kim TK, Han JK, Kim AY, Lee HJ, Choi BI. Hepatic hemangiomas with arterioportal shunt: findings at two-phase CT. Radiology. 2001;219:707-711. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 72] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Arita T, Matsunaga N, Takano K, Hara A, Fujita T, Honjo K. Hepatic perfusion abnormalities in acute pancreatitis: CT appearance and clinical importance. Abdom Imaging. 1999;24:157-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Lim JH, Lee SJ, Lee WJ, Lim HK, Choo SW, Choo IW. Iodized oil retention due to postbiopsy arterioportal shunt: a false positive lesion in the investigation of hepatocellular carcinoma. Abdom Imaging. 1999;24:165-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Sato M, Ishida H, Konno K, Komatsuda T, Hamashima Y, Naganuma H, Ohyama Y. Longstanding arterioportal fistula after laparoscopic liver biopsy. Abdom Imaging. 1999;24:383-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Quiroga S, Sebastià MC, Moreiras M, Pallisa E, Rius JM, Alvarez-Castells A. Intrahepatic arterioportal shunt: helical CT findings. Eur Radiol. 1999;9:1126-1130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Yamasaki M, Furukawa A, Murata K, Morita R. Transient hepatic attenuation difference (THAD) in patients without neoplasm: frequency, shape, distribution, and causes. Radiat Med. 1999;17:91-96. [PubMed] [Cited in This Article: ] |
| 21. | Lee WK, Stuckey S. Arterioportal fistula following liver biopsy demonstrated by lipiodol computed tomography. Clin Radiol. 2000;55:489-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Luo PF, Chen XM, Zhang LM, Zhou ZJ, Fu L, Wei ZH. The management of arteriovenous shunting in hepatocellular carcinoma. Zhonghua Fangshexue Zazhi. 2002;36:114-117. [Cited in This Article: ] |