Published online Dec 7, 2005. doi: 10.3748/wjg.v11.i45.7218
Revised: August 24, 2005
Accepted: August 27, 2005
Published online: December 7, 2005
A 42-year-old Japanese man with liver cirrhosis by hepatitis C virus (HCV) had successful interferon therapy in May 1991. Since then, serum HCV-RNA and liver function tests had been negative. He had continued to drink more than 100 g/d of alcohol as before. In June 2003, a 5-cm tumor was found in the posterior segment of the liver. The tumor was curatively resected and the surgical specimen showed a well-differentiated hepatocellular carcinoma (HCC). Non-cancerous lesions of the liver revealed fibrosis at stage F3 with minimal to mild inflammation of grade A1. Heavy drinking may retard the dissolution of fibrosis and accelerate HCC development in patients with sustained virological response.
- Citation: Ito Y, Yamamoto N, Nakata R, Kato Y, Iori M, Sakai K, Takemura T, Tateishi R, Yoshida H, Kawabe T, Omata M. Delayed development of hepatocellular carcinoma during long-term follow-up after eradication of hepatitis C virus by interferon therapy. World J Gastroenterol 2005; 11(45): 7218-7221
- URL: https://www.wjgnet.com/1007-9327/full/v11/i45/7218.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i45.7218
Hepatocellular carcinoma (HCC) is the third leading cause of death in Japan. Hepatitis C virus (HCV) infection is apparently a major cause of HCC[1-6]. Patients with chronic HCV infection seem destined for progression from milder forms of hepatitis to cirrhosis and, eventually, to HCC. The more advanced is the fibrosis of the liver, the higher is the risk of HCC development. This risk is surprisingly high in cirrhotic patients, the incident rate being about 7% per year[7]. Previously, those patients had no choice but to pass uneasy days in fear of HCC development. It was believed that liver fibrosis is an irreversible change and liver cirrhosis is an incurable disease. Recently, however, it has been shown that the risk of HCC is reduced in HCV-infected patients by interferon therapy, even in cirrhotic patients[8-13]. Interferon therapy can not only improve inflammation, but also ameliorate fibrosis and reduce the risk of HCC. More recently, it has been considered that HCC rarely develops long after HCV eradication[14,15].
Although interferon therapy has been employed for more than a decade, it is not clear how long the risk of HCC lasts among patients who have achieved sustained virological response (SVR). Recently, we had a case with HCC developing 12 years after HCV eradication. This case seems worthy of noting the details, and the data might be helpful for managing patients after HCV eradication.
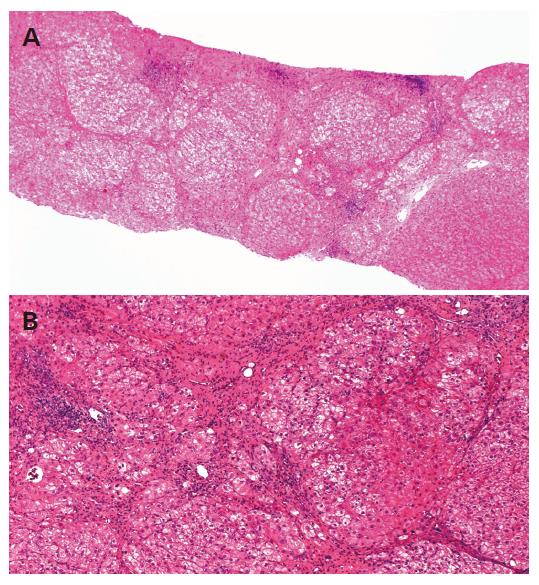
A 42-year-old Japanese man had abnormal liver function tests at a health check-up in 1987. Serum ALT and AST were moderately elevated, 80 and 79 IU/L, respectively. He had no history of blood transfusion or drug abuse, but had been consuming more than 100 g/d of alcohol for 20 years. The liver biopsy specimen showed liver cirrhosis, diagnosed later as A1F4 according to the Classification by Desmet[17]. Alcohol hyaline was not found in the specimen (Figure 1).
In May 1991, he underwent successful interferon therapy after a diagnosis of HCV infection using a total dose of 390 MU of natural interferon-alpha (Sumiferon®, Sumitomo Pharmaceuticals Co., Ltd., Osaka, Japan). Serum HCV-RNA had been negative ever since, and liver function tests, including ALT and AST, had also been normalized. He had continued to drink alcohol heavily as before.
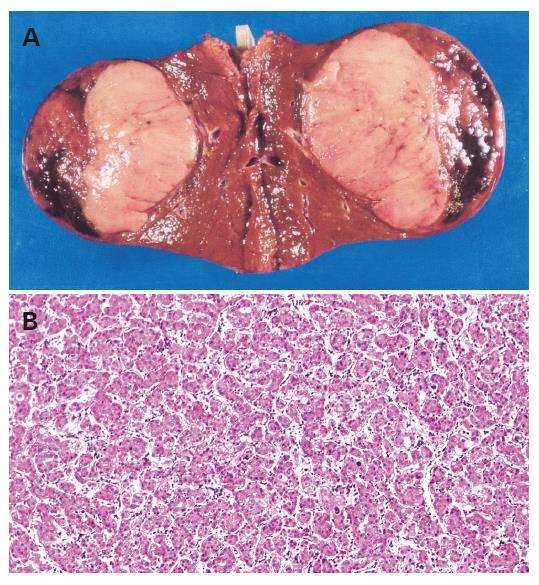
His follow-up had included blood tests and ultrasound examinations every 4-6 mo. In June 2003, ultrasonography and CT scan demonstrated a tumor of 5 cm in diameter in the posterior segment of the liver (Figure 2). Blood tests were normal except for elevated desgamma carboxy prothrombin (DCP, 44 mAu/mL, normal <40 mAu/mL). Angiography confirmed a hypervascular nodule, suggesting HCC.
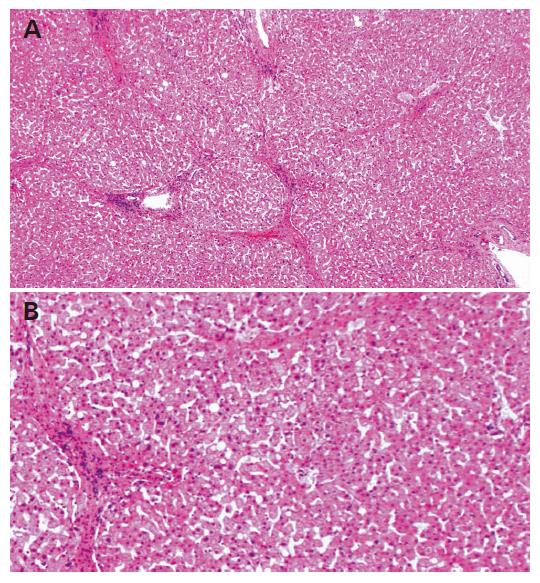
The tumor was curatively resected and the surgical specimen showed a well-differentiated HCC (Figure 3). Non-cancerous lesions revealed minimal to mild inflammation and severe fibrosis of A1F3 (Figure 4).
The patient had recurrence of multiple HCCs, 12 mo after the surgery. Chemoembolization was performed repeatedly, and he was in remission up to September 2004.
Interferon therapy has been shown to reduce the risk of HCC development among the patients with chronic HCV infection. Their risk is decreased to one-third and their survival is also significantly improved when they obtain SVR[17,18].
In patients achieving SVR, fibrosis is gradually dissolved and the risk for HCC development is reduced. Our concern is how long their risk of HCC lasts. There have been few documented cases of HCC development for more than 10 years after the interferon therapy, with more than 10 years having passed since its introduction. At that point, the possibility of HCC development is also believed to be notably decreased.
The present case developed HCC, 12 years after HCV eradication, perhaps the latest developed HCC case on record. It may be helpful for the management of post-eradication patients to review the details of this case in terms of the potential of HCC development.
The present patient had been showing normal aminotransferase values, including ALT and AST, and negative results for serum HVC RNA for 12 years after receiving interferon treatment. Although the histological findings of the liver were improved, great amounts of septa were still observed in the surgical specimen, suggesting F3. In patients with this status, the HCC risk is appraised at 5% per year. The dissolution of fibrosis may have been retarded in the present case. Shiratori et al[19] reported that the F values regress at a rate of -0.282/year among patients with SVR that is based on the method of Poynard[20]. The regression rate in the present case was from F4 to F3 for over the course of 12 years, a meager -0.083/year compared to the reported rate.
The patient had been consuming alcohol since the successful interferon therapy, perhaps accounting for the fact that the hepatic fibrosis had not been dissolved as expected. Alternatively, a long time after eradication, the regression rate in patients may not be as high as in those previously reported. As little data is available regarding regression or HCC development in patients with more than 10 years of follow-up, further studies will be needed.
In addition, alcohol itself may accelerate HCC development. Alcohol intake of 41-80 g/d and over 80 g/d is reported to increase the risk of HCC in HCV-infected patients by two- and four-fold, respectively[21]. Alcohol may also facilitate the growth of HCC, as the doubling time of tumor volume was reported as 148 d in abstemious patients and 78 d in imbibing patients[22].
The degree of liver fibrosis, male gender, higher age, and alcohol intake are well-known risk factors for HCC in chronic HCV-infected patients. The present patient met all these conditions, although little is known as to whether they serve as risk factors in patients with SVR. However, alcohol may have played a role in the development of HCC in this case. If he had stopped consuming alcohol, his liver fibrosis would likely have been improved to a greater extent, and he might have escaped from HCC entirely.
In conclusion, heavy drinking may retard the dissolution of fibrosis and accelerate HCC development in patients with SVR. A larger population of similar cases needs to be studied.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Ikeda K, Saitoh S, Koida I, Arase Y, Tsubota A, Chayama K, Kumada H, Kawanishi M. A multivariate analysis of risk factors for hepatocellular carcinogenesis: a prospective observation of 795 patients with viral and alcoholic cirrhosis. Hepatology. 1993;18:47-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 444] [Cited by in F6Publishing: 400] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 2. | Takano S, Yokosuka O, Imazeki F, Tagawa M, Omata M. Incidence of hepatocellular carcinoma in chronic hepatitis B and C: a prospective study of 251 patients. Hepatology. 1995;21:650-655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 256] [Cited by in F6Publishing: 222] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 3. | Bruix J, Barrera JM, Calvet X, Ercilla G, Costa J, Sanchez-Tapias JM, Ventura M, Vall M, Bruguera M, Bru C. Prevalence of antibodies to hepatitis C virus in Spanish patients with hepatocellular carcinoma and hepatic cirrhosis. Lancet. 1989;2:1004-1006. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 552] [Cited by in F6Publishing: 548] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 4. | Colombo M, Kuo G, Choo QL, Donato MF, Del Ninno E, Tommasini MA, Dioguardi N, Houghton M. Prevalence of antibodies to hepatitis C virus in Italian patients with hepatocellular carcinoma. Lancet. 1989;2:1006-1008. [PubMed] [Cited in This Article: ] |
| 5. | Di Bisceglie AM, Order SE, Klein JL, Waggoner JG, Sjogren MH, Kuo G, Houghton M, Choo QL, Hoofnagle JH. The role of chronic viral hepatitis in hepatocellular carcinoma in the United States. Am J Gastroenterol. 1991;86:335-338. [PubMed] [Cited in This Article: ] |
| 6. | Wietzkebetaraun P, Meier V, Braun F, Ramadori G. Combination of "low-dose" ribavirin and interferon alfa-2a therapy followed by interferon alfa-2a monotherapy in chronic HCV-infected non-responders and relapsers after interferon alfa-2a monotherapy. World J Gastroenterol. 2001;7:222-227. [PubMed] [Cited in This Article: ] |
| 7. | Shiratori Y, Omata M. Predictors of the efficacy of interferon therapy for patients with chronic hepatitis C before and during therapy: how does this modify the treatment course? J Gastroenterol Hepatol. 2000;15 Suppl:E141-E151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Chemello L, Cavalletto L, Casarin C, Bonetti P, Bernardinello E, Pontisso P, Donada C, Belussi F, Martinelli S, Alberti A. Persistent hepatitis C viremia predicts late relapse after sustained response to interferon-alpha in chronic hepatitis C. TriVeneto Viral Hepatitis Group. Ann Intern Med. 1996;124:1058-1060. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 76] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Hoofnagle JH, Mullen KD, Jones DB, Rustgi V, Di Bisceglie A, Peters M, Waggoner JG, Park Y, Jones EA. Treatment of chronic non-A,non-B hepatitis with recombinant human alpha interferon. A preliminary report. N Engl J Med. 1986;315:1575-1578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 704] [Cited by in F6Publishing: 694] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 10. | Davis GL, Balart LA, Schiff ER, Lindsay K, Bodenheimer HC, Perrillo RP, Carey W, Jacobson IM, Payne J, Dienstag JL. Treatment of chronic hepatitis C with recombinant interferon alfa. A multicenter randomized, controlled trial. Hepatitis Interventional Therapy Group. N Engl J Med. 1989;321:1501-1506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1213] [Cited by in F6Publishing: 1190] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 11. | Di Bisceglie AM, Martin P, Kassianides C, Lisker-Melman M, Murray L, Waggoner J, Goodman Z, Banks SM, Hoofnagle JH. Recombinant interferon alfa therapy for chronic hepatitis C. A randomized, double-blind, placebo-controlled trial. N Engl J Med. 1989;321:1506-1510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 900] [Cited by in F6Publishing: 889] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 12. | Wietzkebetaraun P, Meier V, Braun F, Ramadori G. Combination of "low-dose" ribavirin and interferon alfa-2a therapy followed by interferon alfa-2a monotherapy in chronic HCV-infected non-responders and relapsers after interferon alfa-2a monotherapy. World J Gastroenterol. 2001;7:222-227. [PubMed] [Cited in This Article: ] |
| 13. | Azzaroli F, Accogli E, Nigro G, Trere D, Giovanelli S, Miracolo A, Lodato F, Montagnani M, Tamé M, Colecchia A. Interferon plus ribavirin and interferon alone in preventing hepatocellular carcinoma: a prospective study on patients with HCV related cirrhosis. World J Gastroenterol. 2004;10:3099-3102. [PubMed] [Cited in This Article: ] |
| 14. | Yamaura T, Matsumoto A, Rokuhara A, Ichijo T, Tanaka E, Hanazaki K, Kajikawa S, Kiyosawa K. Development of small hepatocellular carcinoma in a patient with chronic hepatitis C after 77 months of a sustained and complete response to interferon therapy. J Gastroenterol Hepatol. 2002;17:1229-1235. [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Toyoda H, Kumada T, Tokuda A, Horiguchi Y, Nakano H, Honda T, Nakano S, Hayashi K, Katano Y, Nakano I. Long-term follow-up of sustained responders to interferon therapy, in patients with chronic hepatitis C. J Viral Hepat. 2000;7:414-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Yoshida H, Shiratori Y, Moriyama M, Arakawa Y, Ide T, Sata M, Inoue O, Yano M, Tanaka M, Fujiyama S. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study Group. Inhibition of Hepatocarcinogenesis by Interferon Therapy. Ann Intern Med. 1999;131:174-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 833] [Cited by in F6Publishing: 857] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 17. | Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513-1520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1582] [Cited by in F6Publishing: 1461] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 18. | Yoshida H, Arakawa Y, Sata M, Nishiguchi S, Yano M, Fujiyama S, Yamada G, Yokosuka O, Shiratori Y, Omata M. Interferon therapy prolonged life expectancy among chronic hepatitis C patients. Gastroenterology. 2002;123:483-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 184] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 19. | Shiratori Y, Imazeki F, Moriyama M, Yano M, Arakawa Y, Yokosuka O, Kuroki T, Nishiguchi S, Sata M, Yamada G. Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann Intern Med. 2000;132:517-524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 547] [Cited by in F6Publishing: 569] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 20. | Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825-832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2199] [Cited by in F6Publishing: 2083] [Article Influence: 77.1] [Reference Citation Analysis (0)] |
| 21. | Tagger A, Donato F, Ribero ML, Chiesa R, Portera G, Gelatti U, Albertini A, Fasola M, Boffetta P, Nardi G. Case-control study on hepatitis C virus (HCV) as a risk factor for hepatocellular carcinoma: the role of HCV genotypes and the synergism with hepatitis B virus and alcohol. Brescia HCC Study. Int J Cancer. 1999;81:695-699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 4] [Reference Citation Analysis (0)] |
| 22. | Matsuhashi T, Yamada N, Shinzawa H, Takahashi T. Effect of alcohol on tumor growth of hepatocellular carcinoma with type C cirrhosis. Intern Med. 1996;35:443-448 DOI : 10.2169/internalmedicine.35.443. [Cited in This Article: ] |