Published online Jan 7, 2006. doi: 10.3748/wjg.v12.i1.21
Revised: July 8, 2005
Accepted: July 20, 2005
Published online: January 7, 2006
AIM: To investigate the relationship between the inhibited growth (cytotoxic activity) of berberine and apoptotic pathway with its molecular mechanism of action.
METHODS: The in vitro cytotoxic techniques were complemented by cell cycle analysis and determination of sub-G1 for apoptosis in human gastric carcinoma SNU-5 cells. Percentage of viable cells, cell cycle, and sub-G1 group (apoptosis) were examined and determined by the flow cytometric methods. The associated proteins for cell cycle arrest and apoptosis were examined by Western blotting.
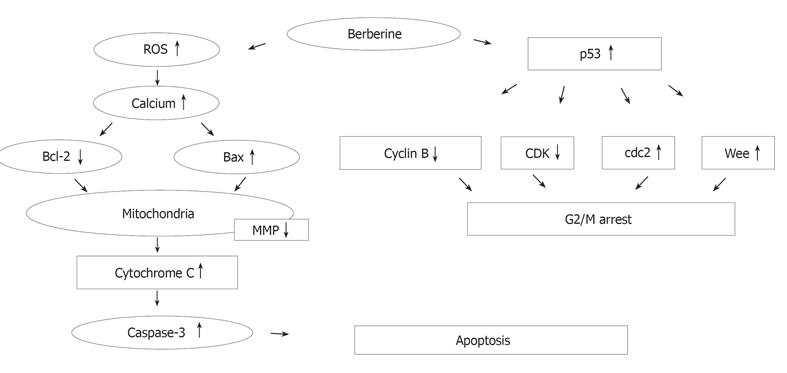
RESULTS: For SNU-5 cell line, the IC (50) was found to be 48 μmol/L of berberine. In SNU-5 cells treated with 25-200 μmol/L berberine, G2/M cell cycle arrest was observed which was associated with a marked increment of the expression of p53, Wee1 and CDk1 proteins and decreased cyclin B. A concentration-dependent decrease of cells in G0/G1 phase and an increase in G2/M phase were detected. In addition, apoptosis detected as sub-G0 cell population in cell cycle measurement was proved in 25-200 μmol/L berberine-treated cells by monitoring the apoptotic pathway. Apoptosis was identified by sub-G0 cell population, and upregulation of Bax, downregulation of Bcl-2, release of Ca2+, decreased the mitochondrial membrane potential and then led to the release of mitochondrial cytochrome C into the cytoplasm and caused the activation of caspase-3, and finally led to the occurrence of apoptosis.
CONCLUSION: Berberine induces p53 expression and leads to the decrease of the mitochondrial membrane potential, Cytochrome C release and activation of caspase-3 for the induction of apoptosis.
- Citation: Lin JP, Yang JS, Lee JH, Hsieh WT, Chung JG. Berberine induces cell cycle arrest and apoptosis in human gastric carcinoma SNU-5 cell line. World J Gastroenterol 2006; 12(1): 21-28
- URL: https://www.wjgnet.com/1007-9327/full/v12/i1/21.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i1.21
The growth of tumor cells not like normal cells is uncontrolled. It is a strategy to change biological properties of cancer cells that lead to apoptosis of killing cancer cells in order to reach chemotherapeutic function for anticancer drugs. Apoptosis is a physiological mode of cell death, which can be selectively triggered by cells in response to the stimuli[1]. Therefore, the induction of apoptosis is a key factor for anticancer drugs.
Berberine (5,6-dihydro-9,10-dimethoxybenzo[g]-1,3-benzodioxole[5,6-a]quinolizinium), a kind of alkaloid, was initially isolated from a Chinese herbal medicine and used as an antibiotic long ago; and it has effeets against many bacterial species[2,3]. In the past years, berberine has subsequently been examined for anticancer activity following evidence of antineoplastic properties[3-5]. It has also been shown that berberine interacts with nucleic acids especially DNA[6] in vitro. Berberine exhibits the ability to induce apoptosis in human cancer cells[5,7] and promyelocytic leukemia HL-60 cells can form berberine complexes with DNA[8].
Cell cycle studies showed that berberine induces rapid apoptosis in a subpopulation (S phase) of the cells[8]. It is also reported that berberine has dose-dependent effects of berberine on G2/M phase and apoptosis in Balb/c 3T3 cells[9]. However, the effects of berberine on human gastric cells are still unclear. Therefore, the purpose of the present study was to find out the molecular mechanism of berberine underlying human gastric cancer cell line (SNU-5).
Berberine, propidium iodide (PI), Tris-HCl, trypan blue, ribonuclease-A and Triton X-100 were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Potassium phosphates, dimethyl sulfoxide (DMSO), and TE buffer were purchased from Merck Co. (Darmstadt, Germany). Iscove’s modified Dulbecco’s medium, glutamine, fetal bovine serum (FBS), and penicillin-streptomycin, trypsin-EDTA were obtained from Gibco BRL (Grand Island, NY, USA).
SNU-5 cell line (human gastric carcinoma; 33 years, female) was obtained from the Food Industry Research and Development Institute (Hsinchu, Taiwan). Cells were immediately placed into 75 cm ×75 cm ×75 cm tissue culture flasks and grown at 37 °C under a humidified 50 mL/L CO2 and 950 mL/L air in 800 mL/L Iscove's modified Dulbecco’s medium supplemented with 200 mL/L FBS, 10 g/L penicillin-streptomycin (1 MU/L penicillin and 10 g/L streptomycin) and 10 g/L glutamine as described previously[10].
SNU-5 cells were plated in 12-well plates at a density of 5×105 cells/well and grown for 24 h. Various concentrations of berberine were added to the cells for final concentrations of 0, 25, 50, 100, and 200 μmol/L, while only DMSO (solvent) was added for the control regivnen and grown for a different period of time at 37 °C, was added 50 mL/L CO2 and 950 mL/L. The trypan blue exclusion and flow cytometry protocols were used as previously described for determining cell viability[7].
About 5×105 SNU-5 cells/well in 12-well plates were incubated with berberine (0, 25, 50, 100, and 200 μmol/L) for different time periods before the cells were harvested by centrifugation. The cells were fixed gently (drop by drop) in 700 mL/L ethanol (in PBS) in ice overnight at -20 °C and then re-suspended in PBS containing 40 g/L PI, 0.1 g/L RNase (Sigma) and 0/10 g/L Triton X-100. After 30 min at 37 °C in the dark, the cells were transferred to the tube, analyzed with flow cytometry (Becton-Dickinson, San Jose, CA, USA) equipped with an argon laser at 488 nm. Then cell cycle and apoptosis were determined and analyzed[7,10].
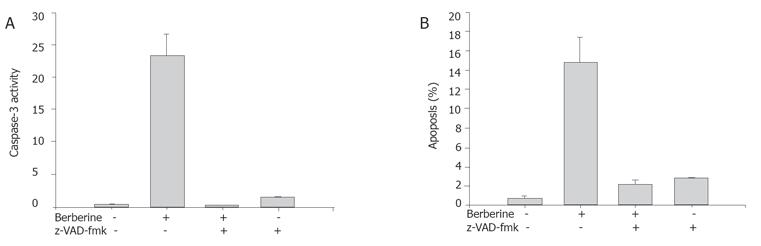
In order to further examine whether caspase-3 activation was involved in apoptosis triggered by berberine, SNU-5 cells were pretreated with the cell permeable broad-spectrum caspase inhibitor z-VAD-fmk 3 h prior to the treatment with 100 μmol/L berberine. Apoptosis and caspase-3 activity were then determined as described above.
The level of ROS in the SNU-5 cells was examined and quantitated by flow cytometry (Becton Dickinson FACS Calibur), using 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA, Sigma). SNU-5 cells were treated with or without berberine (100 mmol/L) for 0, 0.5, 1, 1.5, 2, 4, 6, and 12 h to detect the changes of ROS. The cells were harvested and washed twice, re-suspended in 500 mL of 2,7-dichlorodihydrofluorescein diacetate (10 µmol/L) and incubated at 37 °C for 30 min and analyzed by flow cytometry as described previously[11].
The level of Ca2+ in the SNU-5 cells was determined and quantitated by flow cytometry (Becton Dickinson FACS Calibur), using the Indo 1/AM (Calbiochem; La Jolla, CA, USA). Cells were treated with or without berberine (100 mmol/L) for 0, 0.5, 1, 1.5, 2, 4, 6, and 12 h to detect the changes of Ca2+ concentrations. The cells were harvested and washed twice, and re-suspended in Indo 1/AM (3 mg/L) and incubated at 37 °C for 30 min and analyzed by flow cytometry as described previously[12].
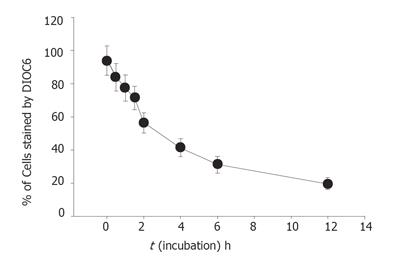
The level of mitochondrial membrane potential in the SNU-5 cells was determined by flow cytometry (Becton Dickinson FACS Calibur), using DiOC6 (4 mol/L) (Calbiochem, Inc., La Jolla, CA, USA). Cells were treated with or without various concentrations (0, 25, 50, 100, and 200 mmol/L) of berberine for 1, 2, 4, 6, 12, 24 h to detect the changes of mitochondrial membrane potential. The cells were harvested and washed twice, re-suspended in 500 mL of DiOC6 (4 mol/L) and incubated at 37 °C for 30 min and analyzed by flow cytometry[11].
The caspase-3 activity and apoptosis in the SNU-5 cells were determined by flow cytometry (Becton Dickinson FACS Calibur), using PhiPhiLux-G2D2 (4×10-4 mmol/L) (OncoImmunin, Inc., MD, USA). Cells were treated with or without various concentrations (0, 25, 50, 100, and 200 mmol/L) of berberine and co-treated with or without caspase-3 inhibitor (z-VAD-fmk ) for 24 h to detect the changes of caspase-3 activity and apoptosis. The cells were harvested and washed twice, re-suspended in 50 mL PhiPhiLux-G2D2 of (4×10-4 mmol/L) and incubated at 37 °C for 30 min and analyzed by flow cytometry as described previously[11].
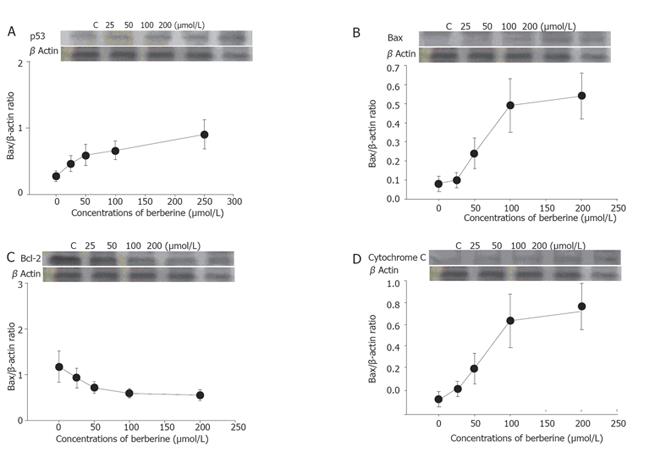
The total protein was collected from SNU-5 cells treated with or without various concentrations of berberine for 48 h before CDK1, Wee1, Cdc25, p53, JNK, Bcl-2, Bax, and cytochrome C were measured by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot as described previously[12,13].
Student’s t-test was used in statistical analysis between berberine-treated and control groups. P<0.05 was considered statisticahy significant
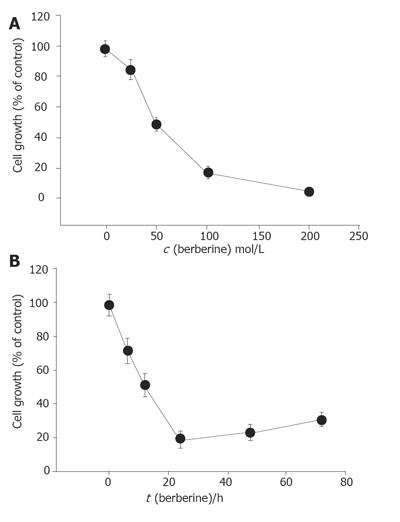
Percentage of cell growth was significantly different between the berberine-treated group and control groap. The effects of berberine on SNU-5 cells were dose-dependent (Figure 1A). Increasing the time of incubation led to the decrease of percentage of cell growth (Figure 1B). Apparently the effects of berberine on SNU-5 cells also were time dependent.
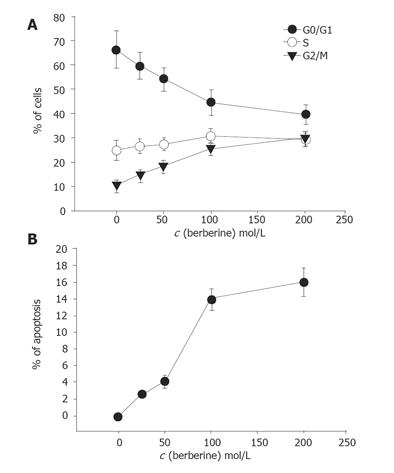
First, we studied the cell cycle and occurrence of apoptosis induced by berberine. Cell cycle and apoptosis were detected by PI staining and annexin V method after 48 h of continuous exposure to berberine before analyzed by flow cytometry (Figures 2A and 2B). As shown in Figure 2, berberine induced G2/M arrest and apoptosis in a concentration- and time-dependent manner.
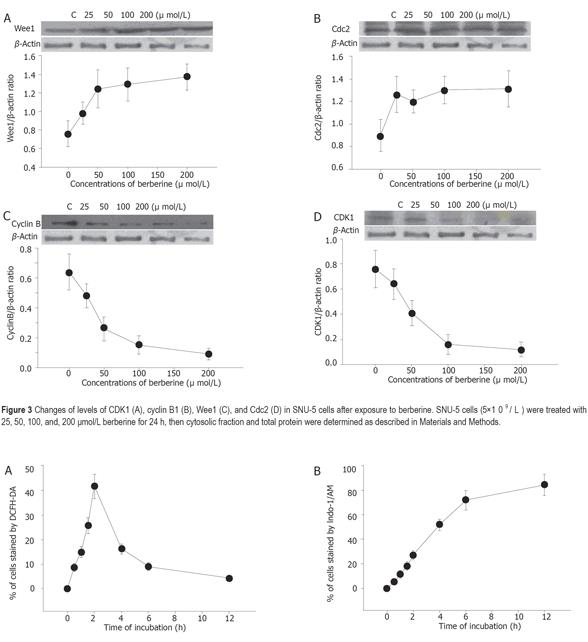
Berberine increased the expression of Wee1 and CDC25C (Figures 3A and 3B) but decreased the expression of cyclin B and CDK1 (Figures 3C and 3D) as detected by western blotting.
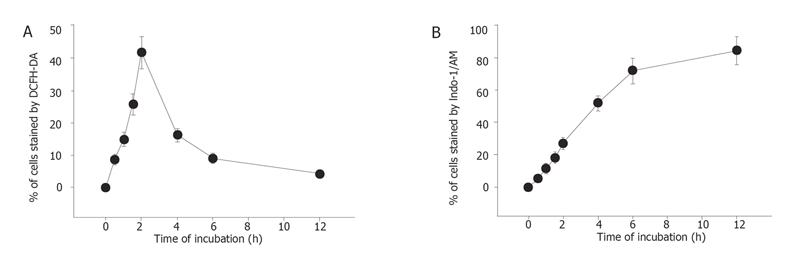
Percentage of ROS was significantly different between the berberine-treated group and control group. The effects of berberine on taking up of DCFH-DA dye by SNU-5 cells were dose-dependent (Table 1) and time dependent (Figure 4A).
Percentage of Ca2+ concentrations was significantly different between the berberine-treated group and control groap. The effects of berberine on taking up of Indo-1/AM dye by SNU-5 cells were dose and time-dependent (Table 1, Figure 4B).
Percentage of mitochondrial membrane potential (MMP) was significantly different between the berberine-treated group and control group. Apparently the effects of berberine on the levels of MMP determined by the take up of the DiOL6 dye in SNU-5 cells were dose and time-dependent (Table 1, Figure 5).
The results indicate the caspase inhibitor that berberine increased caspase-3 activity in a dose- and time-dependent manner (Figure 6A). The SNU-5 cells were pretreated with the cell permeable broad-spectrum caspase inhibitor (z-VAD-fmk) 3 h prior to the treatment with berberine. The z-VAD-fmk decreased the caspase-3 activity. After treated with berberine and z-VAD-fmk in SNU-5 cells, inhibition of berberine-mediated caspase-3 activation was accompanied with the marked attenuation of berberine-induced apoptotic cell death (Figure 6B).
Berberine (25-200 µmol/L) was cytotoxic to SNU-5 cells in a dose- and time-dependent manner. The IC50 for SNU-5 cells was 48 µmol/L (Figure 7). It is slightly different in HL-60 cells[8]. But the sensitivity of murine leukemia L1210 cells to the berberine is higher than that of HeLa cells[14]. Our results also showed that berberine induced ROS in a dose-dependent manner. It may be due to the cell death induced by ROS. Although it was demonstrated that berberine can decrease the intracellular ROS[5], such variable effects are not uncommon. However, cells after treated with berberine for 48 and 72 h slightly increased their viability. They therefore may lead to the resistance to berberine due to the expression of multidrug-resistant transporters (mdr) because berberine can modulate expression of mdr1 gene product (pgp-170) that leads to reduced response to paclitaxel in digestive track cancer cells[15].
Berberine arrests cells in S- and G2/M-phase of the cell cycle, but the former effect is transient. However, G2/M arrest is obvious. Apparently this effect is dose-dependent. Although it was reported that berberine could induce G0/G1 cell cycle arrest in murine L1210 cells[14], it was also reported that berberine can induce G2/M cell cycle arrest in Balb/c 3T3 cells[4,16], suggesting that berberine induces cell cycle arrest depending on cell types. Therefore, the mechanism of berberine is not the same in all cell types. Western blot results from the present studies also demonstrated that berberine inhibits the levels of cyclin B and CDK1 but increases the levels of Wee1 and Cdc2, which may be the factors for G2/M arrest in SNU-5 cells. It has been reported that increases of Cdc2 activity are in response to drug-induced G2/M arrest[16,17]. Formation of Cdc2-cyclin B complex is necessary for G2/M transition and cells to enter mitosis[16]. Our result demonstrated that berberine declined cyclin b levels in SNU-5 cells, and that G2/M arrest could be controlled by cyclin B rather than by Cdc2 activation. Cyclin-dependent kinases (Cdks) are the central regulators of cell division cycle. Inhibitors of Cdks ensure proper coordination of cell cycle events and regulate cell proliferation in tissues and organs. Wee1 homologs phosphorylate a conserved tyrosine to inhibit the mitotic cyclin-dependent kinase Cdk1[18]. It was also reported that the induction of Cdc2 phosphorylation due to the increase of Wee1 and Myt1 as well as the reduction of Cdc2 and cyclin B1, is involved in 1,25 [OH] 2VD3-induced G2/M arrest of keratinocytes[16,19]. It has been shown that multisite phosphorylation of either CDK, Cdc2, Wee1, or CDK-activating kinase is sufficient to generate dynamical behaviors including bistability and limited cycles[20,21]. Experimental depletion of Wee1 by a small interfering RNA directed to Wee1 mRNA could alleviate Vpr-induced G(2) arrest and allow normal progression from M into G phase[16].
Cell cycle analysis revealed the presence of apoptotic cell death (sub-G1 group) following treatment with berberine. We also did morphological examination which showed cell shrinkage, loss of cell-to-cell contact, membrane blebbing and chromatin condensation elicited by increasing berberine concentration and length of exposure. These results were also confirmed by fluorescence microscopy and flow cytometry. So far, many signals and stimuli have been reported to join the induction of apoptosis, therefore the survival of specific cells is under the control of a wide complex of signals. Especially the caspase activities have been demonstrated to be the regulators of apoptosis[22,23]. Most apoptosis models are involved in caspase activation and two main pathways: caspases-8 and -3 or activation of caspases-9 and -3 activation[24]. The caspases-9 and -3 are involved in the release of cytochrome C from mitochondria which causes the decrease of mitochondrial membrane potential. Our data demonstrated that berberine was able to induce the increase of Ca2+ and mitochondrial membrane potential loss and cytochrome C release in cytosolic fraction of SNU-5 cells, which correlates well with the activation of caspases-9 and -3. Our result also showed that berberine decreased the levels of Bcl-2 which control mitochondrial membrane potential and Bax was increased which promotes the cytochrome C release (Figure 8).
Berberine can decrease apoptosis induced by paclitaxel in human cancer cell lines including gastric cancer cells[25,26], whereas in the present study berberine induced cell cycle arrest and cell death (apoptosis) in SNU-5 cells in a dose- and time-dependent manner. Based on these findings, we suggest that berberine may contribute to the antineoplastic activity of gastric cancer cells. However, the molecular basis of such effects needs for further investigations. Although the exact binding sites or receptors of berbarine on the SNU-5 cells are still unknown, the antineoplastic activity of berberine may provide a basis for further studies.
S- Editor Wang XL and Guo SY L- Editor Elsevier HK E- Editor Wang J
| 1. | Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9960] [Cited by in F6Publishing: 9675] [Article Influence: 186.1] [Reference Citation Analysis (0)] |
| 2. | Ghosh AK, Bhattacharyya FK, Ghosh DK. Leishmania donovani: amastigote inhibition and mode of action of berberine. Exp Parasitol. 1985;60:404-413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 111] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | HANO K, MIMURA F, OKU S, OKU K, KANI S, HAGIHARA A, OKAMURA T, NISHIYAMA T, MIYAZAKI S, HONDA F. [Pharmacological studies on metabolism of cancer tissues. XIII. pharmacological studies on carcinostatic effects of some plant components and their derivatives. I]. Gan. 1957;48:443-445. [PubMed] [Cited in This Article: ] |
| 4. | Hoshi A, Ikekawa T, Ikeda Y, Shirakawa S, Iigo M. Antitumor activity of berberrubine derivatives. Gann. 1976;67:321-325. [PubMed] [Cited in This Article: ] |
| 5. | Zhang RX, Dougherty DV, Rosenblum ML. Laboratory studies of berberine used alone and in combination with 1,3-bis(2-chloroethyl)-1-nitrosourea to treat malignant brain tumors. Chin Med J (Engl). 1990;103:658-665. [PubMed] [Cited in This Article: ] |
| 6. | YAMAGISHI H. Interaction between nucleic acids and berberine sulfate. J Cell Biol. 1962;15:589-592. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Li YC, Tyan YS, Kuo HM, Chang WC, Hsia TC, Chung JG. Baicalein induced in vitro apoptosis undergo caspases activity in human promyelocytic leukemia HL-60 cells. Food Chem Toxicol. 2004;42:37-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Kuo CL, Chou CC, Yung BY. Berberine complexes with DNA in the berberine-induced apoptosis in human leukemic HL-60 cells. Cancer Lett. 1995;93:193-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 145] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Yang IW, Chou CC, Yung BY. Dose-dependent effects of berberine on cell cycle pause and apoptosis in Balb/c 3T3 cells. Naunyn Schmiedebergs Arch Pharmacol. 1996;354:102-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Lin JP, Lu HF, Lee JH, Lin JG, Hsia TC, Wu LT, Chung JG. (-)-Menthol inhibits DNA topoisomerases I, II alpha and beta and promotes NF-kappaB expression in human gastric cancer SNU-5 cells. Anticancer Res. 2005;25:2069-2074. [PubMed] [Cited in This Article: ] |
| 11. | Lu HF, Sue CC, Yu CS, Chen SC, Chen GW, Chung JG. Diallyl disulfide (DADS) induced apoptosis undergo caspase-3 activity in human bladder cancer T24 cells. Food Chem Toxicol. 2004;42:1543-1552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Chung JG, Yeh KT, Wu SL, Hsu NY, Chen GW, Yeh YW, Ho HC. Novel transmembrane GTPase of non-small cell lung cancer identified by mRNA differential display. Cancer Res. 2001;61:8873-8879. [PubMed] [Cited in This Article: ] |
| 13. | Hahn FE, Ciak , J Antibiotics. Vol.3. Editors: Gottliele D. P D, Shaw J.W. Cocoran. New York: Springer-Verlag 1975; 577. [Cited in This Article: ] |
| 14. | Jantová S, Cipák L, Cernáková M, Kost'álová D. Effect of berberine on proliferation, cell cycle and apoptosis in HeLa and L1210 cells. J Pharm Pharmacol. 2003;55:1143-1149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 124] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 15. | Lin HL, Liu TY, Wu CW, Chi CW. Berberine modulates expression of mdr1 gene product and the responses of digestive track cancer cells to Paclitaxel. Br J Cancer. 1999;81:416-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 85] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Dai X, Yamasaki K, Yang L, Sayama K, Shirakata Y, Tokumara S, Yahata Y, Tohyama M, Hashimoto K. Keratinocyte G2/M growth arrest by 1,25-dihydroxyvitamin D3 is caused by Cdc2 phosphorylation through Wee1 and Myt1 regulation. J Invest Dermatol. 2004;122:1356-1364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Li XK, Motwani M, Tong W, Bornmann W, Schwartz GK. Huanglian, A chinese herbal extract, inhibits cell growth by suppressing the expression of cyclin B1 and inhibiting CDC2 kinase activity in human cancer cells. Mol Pharmacol. 2000;58:1287-1293. [PubMed] [Cited in This Article: ] |
| 18. | Yuan H, Kamata M, Xie YM, Chen IS. Increased levels of Wee-1 kinase in G(2) are necessary for Vpr- and gamma irradiation-induced G(2) arrest. J Virol. 2004;78:8183-8190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Stumpff J, Duncan T, Homola E, Campbell SD, Su TT. Drosophila Wee1 kinase regulates Cdk1 and mitotic entry during embryogenesis. Curr Biol. 2004;14:2143-2148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Martin SJ, O’Brien GA, Nishikoa WK, Mcgahon AJ, Mahboubi A, Saido TC, Green DR. Protease activation during apoptosis: Death by a thousand cuts. J Biol Chem. 1997;270:6425-6431. [Cited in This Article: ] |
| 21. | Yang L, MacLellan WR, Han Z, Weiss JN, Qu Z. Multisite phosphorylation and network dynamics of cyclin-dependent kinase signaling in the eukaryotic cell cycle. Biophys J. 2004;86:3432-3443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Krammer PH. CD95(APO-1/Fas)-mediated apoptosis: live and let die. Adv Immunol. 1999;71:163-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 275] [Cited by in F6Publishing: 257] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 23. | Panaro NJ, Popescu NC, Harris SR, Thorgeirsson UP. Flavone acetic acid induces a G2/M cell cycle arrest in mammary carcinoma cells. Br J Cancer. 1999;80:1905-1911. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Thornberry AT, Rosen A, Nicholson DW. Control of apoptosis by proteases. Apoptosis. San Diego: Acad Press 1997; 155-168. [Cited in This Article: ] |
| 25. | Donaldson KL, Goolsby GL, Kiener PA, Wahl AF. Activation of p34cdc2 coincident with taxol-induced apoptosis. Cell Growth Differ. 1994;5:1041-1050. [PubMed] [Cited in This Article: ] |
| 26. | Wartenberg M, Budde P, De Mareés M, Grünheck F, Tsang SY, Huang Y, Chen ZY, Hescheler J, Sauer H. Inhibition of tumor-induced angiogenesis and matrix-metalloproteinase expression in confrontation cultures of embryoid bodies and tumor spheroids by plant ingredients used in traditional chinese medicine. Lab Invest. 2003;83:87-98. [PubMed] [Cited in This Article: ] |