Published online Sep 28, 2006. doi: 10.3748/wjg.v12.i36.5870
Revised: February 15, 2006
Accepted: February 26, 2006
Published online: September 28, 2006
AIM: To investigate the expression of nucleoporin 88 (Nup88) in hepatitis B virus (HBV) and C virus (HCV)-related liver diseases.
METHODS: We generated a new monoclonal Nup88 antibody to investigate the Nup88 protein expression by immunohistochemistry (IHC) in 294 paraffin-embedded liver specimens comprising all stages of hepatocellular carcinogenesis. In addition, in cell culture experiments HBV-positive (HepG2.2.15 and HB611) and HBV-negative (HepG2) hepatoma cell lines were tested for the Nup88 expression by Western-immunoblotting to test data obtained by IHC.
RESULTS: Specific Nup88 expression was found in chronic HCV hepatitis and unspecific chronic hepatitis, whereas no or very weak Nup88 expression was detected in normal liver. The Nup88 expression was markedly reduced or missing in mild chronic HBV infection and inversely correlated with HBcAg expression. Irrespective of the HBV- or HCV-status, increasing Nup88 expression was observed in cirrhosis and dysplastic nodules, and Nup88 was highly expressed in hepatocellular carcinomas. The intensity of Nup88 expression significantly increased during carcinogenesis (P < 0.0001) and correlated with dedifferentiation (P < 0.0001). Interestingly, Nup88 protein expression was significantly downregulated in HBV-positive HepG2.2.15 (P < 0.002) and HB611 (P < 0.001) cell lines as compared to HBV-negative HepG2 cells.
CONCLUSION: Based on our immunohistochemical data, HBV and HCV are unlikely to influence the expression of Nup88 in cirrhotic and neoplastic liver tissue, but point to an interaction of HBV with the nuclear pore in chronic hepatitis. The expression of Nup88 in nonneoplastic liver tissue might reflect enhanced metabolic activity of the liver tissue. Our data strongly indicate a dichotomous role for Nup88 in non-neoplastic and neoplastic conditions of the liver.
- Citation: Knoess M, Kurz AK, Goreva O, Bektas N, Breuhahn K, Odenthal M, Schirmacher P, Dienes HP, Bock CT, Zentgraf H, Hausen AZ. Nucleoporin 88 expression in hepatitis B and C virus-related liver diseases. World J Gastroenterol 2006; 12(36): 5870-5874
- URL: https://www.wjgnet.com/1007-9327/full/v12/i36/5870.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i36.5870
Hepatocellular carcinoma (HCC) ranks fifth of the most common cancers worldwide and its incidence is rising in the Western world[1]. Due to the high mortality associated with HCC it is the third leading cause of cancer death worldwide[1]. The majority of HCCs develop due to chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) infection. However, the precise molecular etiology of HBV- and HCV- related HCC development remains poorly understood.
Bidirectional nuclear-cytoplasmic transport activity of oncogenes and tumorsuppressors through the nuclear pore is profoundly disturbed in cancer cells[2]. It is assumed that transport through the nuclear pore is necessarily a crucial event in the development and maintenance of the tumor phenotype[3]. Nucleoporin 88 (Nup88) is a protein located at the nuclear membrane and is involved in the bidirectional nuclear-cytoplasmic transport of proteins by forming nuclear pore complexes (NPC) with other nucleoporins[4,5]. Nup88 is associated in a dynamic subcomplex with CAN/Nup214, which has been implicated in nuclear protein import, nuclear mRNA export, and cell cycle regulation[6]. Recently, overexpression of Nup88 has been demonstrated in a wide range of premalignant lesions and malignant tumors by using a polyclonal antibody[7-9]. Thus, Nup88 has been suggested as a putative marker of malignant transformation. In addition, it has been shown that overexpression of Nup88 is involved in the tumorigenesis and aggressiveness of colorectal cancers[10]. Overexpression of Nup88 is also linked to the enhanced metastatic potential of melanoma cells and it has been shown that Nup88 expression is up-regulated in primary foreskin melanocytes upon UV-A irradiation[11,12]. In the present study we generated a new monoclonal Nup88 antibody and analyzed the expression of Nup88 in HBV- and HCV- related diseases of hepatocellular carcinogenesis in a large patient cohort and correlated major findings to cell culture experiments.
A total of 294 tissues were investigated for Nup88 expression by immunohistochemistry (IHC). One hundred and forty-one specimens were assessed on a liver tissue microarray (TMA) consisting of premalignant dysplastic nodules (DNs: n = 33) and hepatocellular carcinomas (HCCs: n = 108). Twenty-eight DNs and 43 HCCs were infected with HCV, 16 DNs and 17 HCCs were infected with HBV, and 11 DNs and 8 HCCs were coinfected with HBV and HCV. In addition, liver biopsies (n = 153) collected at the Institute of Pathology at Cologne were tested for Nup88 expression. These included cases of chronic HBV hepatitis (n = 24) and chronic HCV hepatitis (n = 38) with mild inflammatory activity, 53 cases of liver cirrhosis (HBV-positive, n = 24; HCV-positive, n = 24; alcohol induced, n = 5) and 18 cases of normal liver biopsies and 20 cases of mild unspecific chronic hepatitis (UCH), i.e. patients who underwent liver biopsies in the context of clinically elevated transaminases in the absence of HBV/HCV or any other known liver diseases.
Mouse monoclonal antibodies (mAbs) to protein Nup88 were obtained by immunization with the peptide representing the amino acid sequence (aa 1-263) deduced from the human cDNA sequence of Nup88 (NCBI, accession number: NP002523). As an immunogen the peptide was used to conjugate to keyhole limpet hemocyanin (KLH). For screening the carrier protein conjugated to the peptide was bovine serum albumin (BSA). Screening was performed using ELISA and Western immunoblotting following standard protocols. The mAbs were raised according to the method of Köhler and Milstein[13].
Nup88 IHC was established on paraffin-embedded and formalin-fixed tissues. Colon adenomas and carcinomas (n = 3), breast carcinomas (n = 2) and lymph nodes with Hodgkin’s disease (n = 3) were used as positive controls[8,9]. After deparaffinization and dehydration endogenous peroxidase was blocked by 1% H2O2 in methanol for 30 min. Without further pretreatment the slides were incubated with the mAb (clone 7, 1:750) in diluent buffer (Zymed Laboratories Inc., San Francisco, USA) at 4°C overnight. The Envision System HRP mouse (DakoCytomation, Carpintera, USA) was applied to signal amplification for 30 min. For signal detection 3,3’- diaminobenzidine (DAB; Sigma Chemical Co., St. Louis, USA) was used. Slides were weakly counterstained with hematoxylin. Staining intensity and nuclear accumulation of Nup88 were evaluated by four independent observers and scored as: 0 = no signal; 1 = weak, pale nuclear staining; 2 = moderate staining; and 3 = strong staining. The HBV- and HCV-status was assessed by serology. Biopsies without serological information were tested for HBcAg and surface antigen (HBsAg) by IHC (see below) or by HCV-specific RT-PCR[14]. Immunohistochemical double staining for HBcAg and Nup88 was performed on tissue specimens from mild chronic HBV hepatitis patients. In brief, the specimens were first treated with anti-HbcAg rabbit serum (DAKO, Hamburg, Germany) at a dilution of 1:400 for one hour at 37°C. After treatment with the secondary antibody for 30 min at room temperature, fast red alkaline phosphatase (Dako) was used as a chromogen. The Nup88 IHC was carried out as a second step according to the above protocol.
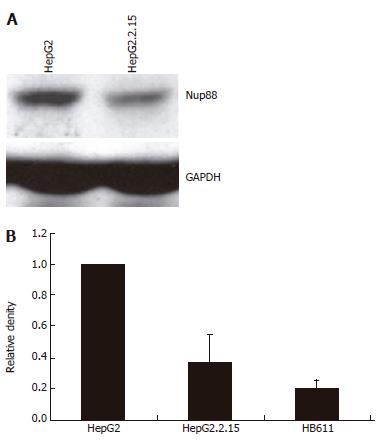
To analyze Nup88 expression in relation to HBV in cell culture, Nup88 and HbcAg expressions were tested in a hepatoma cell line. The HBV-negative HepG2 hepatoma cell line and its HBV-positive counterpart HepG2.2.15 were used[15]. The latter contains the full length HBV genome and has been shown to produce infectious virions[15]. Western immunoblots were performed as previously described using 75 g/L polyacrylamid gel[17]. The monoclonal Nup88 antibody (clone 7, 1:2000 dilution) detected the 88 kDa Nup88 protein, the monoclonal HBcAg antibody (1:1000 dilution) and the 23 kDa HBcAg protein. Equal amounts of protein were loaded on the gel as tested by Bradford assay, Ponceau staining and GAPDH-Western blotting (Sigma, Germany; 1:1000) (Figure 3B).
For statistical analysis of the immunohistochemistry data of the TMA, chi-square test was applied. Densitometric evaluation of Western blot results from 6 independent experiments using EASY plus Rev 4.16 were expressed as mean ± SE. Results were compared using Student’s t-test. P < 0.05 was considered statistically significant.
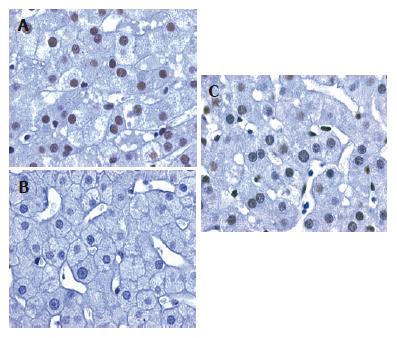
In order to determine the Nup88 expression in viral hepatocellular carcinogenesis by IHC we first evaluated the efficacy of the new mAb Nup88 using different positive controls, i.e. colon carcinomas, breast carcinomas and lymph nodes with Hodgkin’s disease[8,9]. All positive controls demonstrated specific nuclear staining for Nup88 in the tumor cells whereas adjacent non-neoplastic tissue revealed no Nup88 expression. Of interest, no or only very weak Nup88 expression was detected in the 18 cases of normal liver biopsies, but in all 20 cases with mild reactive changes (mean staining score 2.4) (Figure 1A). In these cases Nup88 expression was found in most hepatocytes and occasionally in sinusoidal non-parenchymal liver cells. In contrast, in mild chronic HBV infection Nup88 expression was patchy, remarkably weaker or missing (n = 24, mean staining score 1.25) as compared to normal liver biopsies (Figure 1B). Stage-matched mild chronic HCV-infected liver samples (n = 38) revealed a moderate nuclear Nup88 expression (mean staining score 1.94; Figure 1C). No difference was observed in Nup88 expression between HBV- and HCV-related cirrhosis (mean staining score HBV-cirrhosis 1.91; HCV-cirrhosis: 2.04).
In chronic HBV-related hepatitis with mild inflammatory activity HBcAg expression was patchy and restricted to single clusters of hepatocytes. To determine whether Nup88 was co-expressed with HBcAg in mild chronic HBV-related hepatitis we analyzed the relation between Nup88 and HBcAg expressions in mild chronic HBV-related hepatitis (n = 5) by IHC on serial sections and by IHC double staining. IHC staining for Nup88 and HBcAg on serial sections revealed a mutually exclusive staining of Nup88 or HBcAg. This inverse correlation between Nup88 and HBcAg expression patterns was confirmed by IHC double staining in which hepatocytes expressing HBcAg showed negative Nup88 expression and vice versa (data not shown).
To evaluate the impact of the presence of HBV on Nup88 expression in cell culture the expression of Nup88 was assessed in hepatoma cell lines, i.e. the HBV-negative HepG2 and HBV-positive HepG2.2.15 and HB611 cell lines, by Western blot analysis. As expected, in HBV-negative HepG2 cells (Figure 2A) Nup88 expression could be clearly demonstrated. In contrast, Nup88 expression was significantly reduced in the HBV-positive HepG2.2.15 (P < 0.002) and HB611 (P < 0.001) hepatoma cell lines compared to HBV-negative HepG2 cells (Figure 2A and B).
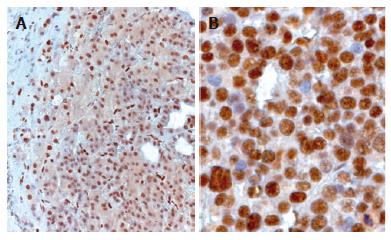
Nup88 was highly expressed in neoplastic hepatocytes of DN (Figure 3A) and HCC, but no significant differences were observed by IHC in Nup88 expression between HBV- or HCV- associated HCCs and DNs. Most if not all HCCs revealed a strong nuclear expression of Nup88. The strongest staining intensity was observed in undifferentiated HCC (Figure 3B). The high overexpression of Nup88 correlated with the degree of tumor cell dedifferentiation (P < 0.0001). In contrast, a weaker Nup88 expression was observed in DNs. By comparing Nup88 expression intensity between DNs and different grades of HCC, a significant increase was observed (P < 0.0001).
HCC is a major global health problem[17]. The most common cause of HCC worldwide is chronic infection with HBV and HCV[18]. Although the link of virus-related hepatocellular carcinogenesis has been well established, the underlying molecular pathogenesis is still poorly understood. In the present study we investigated the Nup88 expression in chronic viral hepatitis and virus-associated hepatocellular carcinogenesis. Nup88 is one of the known components of nucleoporins forming the nuclear pore complex (NPC) which is involved in nuclear transport. By using a polyclonal antiserum it has recently been shown that Nup88 is highly expressed in premalignant and malignant conditions[7-11]. Here, we introduce a new monoclonal anti-Nup88 antibody which specifically detects Nup88 in Western-immunoblotting. In addition, this is the first monoclonal antibody to specifically detect Nup88 in paraffin-embedded and formalin-fixed tissues by IHC. In line with recently published data, our Nup88 antibody can also specifically detect neoplastic colorectal, mammary and lymphoma cells[8,9]. Nup88 expression has recently been suggested as a marker for premalignant or malignant transformation. Therefore, the expression of Nup88 in non-neoplastic hepatocytes is remarkable. According to our results the expression of Nup88 in non-neoplastic liver tissue might reflect an elevated metabolic activity of cells that ensures the nuclear import and export of transcription factors[2]. This hypothesis is supported by the finding of nuclear Nup88 expression in viral hepatitis as well as in HCC. In both cases hepatocytes exhibit an increased metabolic activity due to inflammation or malignant transformation. Until today Nup88 expression has only been described in the context of malignant transformation or tumor progression. Thus, our data might indicate additional functions of Nup88 expression in non-neoplastic liver tissue.
Reduced Nup88 expression in mild chronic HBV hepatitis compared to normal liver tissue and liver samples with mild uncharacteristic changes may contribute new insights to the understanding of HBV and NPC interaction. Recently, it has been shown that the HBV core protein binds to the NPC[20,21]. However, the target domain of the anti-NPC antibody used in this study has not been exactly defined within the NPC, which consists of many different nucleoporins[4,5]. We tempt to speculate that the low Nup88 detection in mild chronic HBV-associated hepatitis is due to an interaction of HBV with Nup88. The mechanism of this interference is not clear. However, it could occur directly by protein-protein interaction and therefore masking the Nup88 epitope or HBV may be able to downregulate Nup88 at the transcriptional or protein level. Another hypothesis is that HBV, most likely the core antigen, might compete for NPC-components like CAN/Nup214. This is supported by the mutual exclusiveness of Nup88 and HBcAg expression in mild chronic HBV-associated hepatitis. In line with these data we have demonstrated in cell culture experiments that Nup88 expression is significantly reduced in HBV-positive HepG2.2.15 and HB611 cells compared to HBV-negative HepG2 cells. Moreover, recent work has identified the NPC-filament protein CAN/Nup214 as a docking site for incoming adenovirus type 2 (Ad2) capsid proteins[22]. Therefore we hypothesize that NPC components like CAN/Nup214 and/or Nup88 might serve as docking sites for different viruses, e.g. HBV. Currently we are investigating not only this hypothesis in our laboratories by generating monoclonal Nup214 antibodies but also the possible transcriptional regulation of Nup88 by HBV. It is of interest that this phenomenon seems to be restricted to mild chronic HBV hepatitis and is not observed in later stages of hepatocellular carcinogenesis. Both increased nuclear Nup88 expression and elevated intensity correlate with the dedifferentiation of HCC and might be of interest in histopathological diagnostics. Although Nup88 expression in tumor cells is a marker for malignant transformation, it is highly overexpressed especially in undifferentiated HCC. Increased Nup88 expression has also been found in the colorectal adenoma/carcinoma sequence and overexpression of Nup88 mRNA has been shown to be associated with high aggressiveness of breast cancer[3,9,10]. In contrast to Emterling and coworkers[10] we were not able to determine in our patient cohort whether the expression of Nup88 is of relevance with the survival of HCC patients, although undifferentiated HCCs tend to have a worse clinical outcome.
In summary, Nup88 is expressed in non-neoplastic and malignant hepatocytes, indicating that Nup88 plays a dichitomous role in non-neoplastic and neoplastic conditions of the liver. In addition, Nup88 is significantly overexpressed in poorly differentiated HCCs. The markedly reduced Nup88 expression in mild chronic HBV hepatitis and significantly less Nup88 expression in HBV-positive HepG2.2.15 and HB611 cells strongly point to an interference of HBV with the nuclear pore.
The authors are indebted to the excellent technical support of Mrs. Marion Müller and Mrs. Katharina Petmecky.
S- Editor Wang GP L- Editor Wang XL E- Editor Bi L
| 1. | Block TM, Mehta AS, Fimmel CJ, Jordan R. Molecular viral oncology of hepatocellular carcinoma. Oncogene. 2003;22:5093-5107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 374] [Cited by in F6Publishing: 395] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 2. | Kau TR, Way JC, Silver PA. Nuclear transport and cancer: from mechanism to intervention. Nat Rev Cancer. 2004;4:106-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 336] [Cited by in F6Publishing: 351] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 3. | Agudo D, Gómez-Esquer F, Martínez-Arribas F, Núñez-Villar MJ, Pollán M, Schneider J. Nup88 mRNA overexpression is associated with high aggressiveness of breast cancer. Int J Cancer. 2004;109:717-720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Vasu SK, Forbes DJ. Nuclear pores and nuclear assembly. Curr Opin Cell Biol. 2001;13:363-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 205] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 5. | Suntharalingam M, Wente SR. Peering through the pore: nuclear pore complex structure, assembly, and function. Dev Cell. 2003;4:775-789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 349] [Cited by in F6Publishing: 356] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 6. | Fornerod M, van Baal S, Valentine V, Shapiro DN, Grosveld G. Chromosomal localization of genes encoding CAN/Nup214-interacting proteins--human CRM1 localizes to 2p16, whereas Nup88 localizes to 17p13 and is physically linked to SF2p32. Genomics. 1997;42:538-540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Martínez N, Alonso A, Moragues MD, Pontón J, Schneider J. The nuclear pore complex protein Nup88 is overexpressed in tumor cells. Cancer Res. 1999;59:5408-5411. [PubMed] [Cited in This Article: ] |
| 8. | Gould VE, Martinez N, Orucevic A, Schneider J, Alonso A. A novel, nuclear pore-associated, widely distributed molecule overexpressed in oncogenesis and development. Am J Pathol. 2000;157:1605-1613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Gould VE, Orucevic A, Zentgraf H, Gattuso P, Martinez N, Alonso A. Nup88 (karyoporin) in human malignant neoplasms and dysplasias: correlations of immunostaining of tissue sections, cytologic smears, and immunoblot analysis. Hum Pathol. 2002;33:536-544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Emterling A, Skoglund J, Arbman G, Schneider J, Evertsson S, Carstensen J, Zhang H, Sun XF. Clinicopathological significance of Nup88 expression in patients with colorectal cancer. Oncology. 2003;64:361-369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Zhang H, Schneider J, Rosdahl I. Expression of p16, p27, p53, p73 and Nup88 proteins in matched primary and metastatic melanoma cells. Int J Oncol. 2002;21:43-48. [PubMed] [Cited in This Article: ] |
| 12. | Zhang H, Rosdahl I. Ultraviolet A and B differently induce intracellular protein expression in human skin melanocytes--a speculation of separate pathways in initiation of melanoma. Carcinogenesis. 2003;24:1929-1934. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. 1975. Biotechnology. 1992;24:524-526. [PubMed] [Cited in This Article: ] |
| 14. | Dries V, von Both I, Müller M, Gerken G, Schirmacher P, Odenthal M, Bartenschlager R, Drebber U, Meyer zum Büschenfeld KH, Dienes HP. Detection of hepatitis C virus in paraffin-embedded liver biopsies of patients negative for viral RNA in serum. Hepatology. 1999;29:223-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 53] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Sells MA, Zelent AZ, Shvartsman M, Acs G. Replicative intermediates of hepatitis B virus in HepG2 cells that produce infectious virions. J Virol. 1988;62:2836-2844. [PubMed] [Cited in This Article: ] |
| 16. | Tsurimoto T, Fujiyama A, Matsubara K. Stable expression and replication of hepatitis B virus genome in an integrated state in a human hepatoma cell line transfected with the cloned viral DNA. Proc Natl Acad Sci USA. 1987;84:444-448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 156] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Kurz AK, Graf D, Schmitt M, Vom Dahl S, Häussinger D. Tauroursodesoxycholate-induced choleresis involves p38(MAPK) activation and translocation of the bile salt export pump in rats. Gastroenterology. 2001;121:407-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 121] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Pisani P, Parkin DM, Bray F, Ferlay J. Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer. 1999;83:18-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 30] [Reference Citation Analysis (0)] |
| 19. | Kao JH, Chen DS. Overview of hepatitis B and C viruses. Infectious causes of cancer. New Jersey: Humana Press 2000; . [Cited in This Article: ] |
| 20. | Bock CT, Schwinn S, Schröder CH, Velhagen I, Zentgraf H. Localization of hepatitis B virus core protein and viral DNA at the nuclear membrane. Virus Genes. 1996;12:53-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Kann M, Sodeik B, Vlachou A, Gerlich WH, Helenius A. Phosphorylation-dependent binding of hepatitis B virus core particles to the nuclear pore complex. J Cell Biol. 1999;145:45-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 184] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 22. | Trotman LC, Mosberger N, Fornerod M, Stidwill RP, Greber UF. Import of adenovirus DNA involves the nuclear pore complex receptor CAN/Nup214 and histone H1. Nat Cell Biol. 2001;3:1092-1100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 233] [Cited by in F6Publishing: 243] [Article Influence: 10.6] [Reference Citation Analysis (0)] |