INTRODUCTION
The most important priority in the surgical management of mid and distal rectal cancers is adequate oncologic clearance. It is generally accepted that this is achievable by total mesorectal excision, although in Japan extended pelvic lymphadenectomy is also used in selected cases. Total mesorectal excision involves precise excision of the entire rectum and para-rectal lymph nodes en-block, within an oncologic package termed the ‘mesorectal envelope’. A very low local recurrence rate of 3%-6% (associated with improved 5 years survival) has proven to be repeatable, where the surgeon is adequately trained and the technique is correctly practised[1-3]. Restoration of bowel continuity after total mesorectal excision is possible most of the time, without compromise of oncologic clearance. The reported mortality of 7%-8% is comparable to the 2.2%-8% following the alternative abdominoperineal resection[4-6]. Furthermore, local recurrence rates following low anterior resection (7%-14.7%) are similar to that of abdominoperineal resection (12%-18.8%)[4-6].
Confusion in the literature can be minimized by defining clearly the terminology relating to the restoration of bowel continuity after rectal cancer surgery. The type of procedure is defined by the anatomical site of anastomosis rather than the position of the cancer, as commonly described at colonoscopy. The term “high” anterior resection refers to a colorectal anastomosis performed at the level of between the sacral promontory and the anterior peritoneal reflection. The level of the anastomosis is normally measured to be about 8 to 16 cm from the anal verge, depending upon the patient’s body build. The term “low” anterior resection refers to a colorectal anastomosis performed at between distal to the anterior peritoneal reflection and proximal to the anorectal junction. This is normally measured to be about 5 to 8 cm above the anal verge, again depending upon the patient’s body build. The term “ultra-low” or “extended” anterior resection refers to a colorectal or more usually, a coloanal anastomosis at the level of the anorectal junction. This is the type of anastomosis that is performed after proper total mesorectal excision and incision of the Waldeyer’s fascia posterior to the rectum. The latter technical step allows the rectum to be mobilized/“freed” both anteriorly and proximally from the pelvis, allowing for transection of the rectum safely at the anorectal junction. The level of this anastomosis is normally measured to be about 3-5 cm from the anal verge. Such distal anastomoses have much higher risk of anastomotic dehiscence and consideration should be given to temporary defunctioning by either a colostomy or ileostomy. The term “intersphincteric dissection” refers to the special situation where a very distal rectal cancer is excised with clear oncological resection margins by including an en-block excision of the internal anal sphincter and anal mucosa, to the level of the dentate (pectinate) line. The last part of the procedure is usually performed transanally. Reconstruction is done by a pull-through procedure with hand-sewn anastomosis of the colon to the distal anus, again by the transanal route.
Subsequent discussion will be along (1) methods of anastomosis after resection of the rectal cancer, (2) types of operations i.e. straight colorectal or coloanal anastomosis, colonic J-pouch anal anastomosis, coloplasty anastomosis & colonic side-to-end anastomosis, and (3) anterior resection syndrome & management.
ANASTOMOTIC METHODS
Following successful resection of the rectal cancer with total mesorectal excision, bowel continuity can be restored by coloanal/distal rectal anastomosis performed using various techniques.
Stapled anastomosis
The most common method of performing the coloanal/distal rectal anastomosis is with the use of a circular intraluminal stapling instrument introduced transanally. It is technically challenging to insert a hand-sewn purse-string suture to the transected rectal stump and to secure the shoulder of the transanally inserted stapling device, as the transected anorectal junction tends to retract into the pelvic floor. The exposure is often further obscured by a hypertrophied bladder which is commonly found in the older male patients with bladder outlet obstruction problems such as benign prostatic hypertrophy. Hence, the double cross stapled technique is the most commonly employed method particularly in the West where patients are usually of bigger size[7]. The double cross stapled technique consists of stapling and transecting the anorectal junction distal to the cancer. Prior to this, it is important to irrigate the rectum with tumoricidal agents to reduce the risks of cancer recurrence from exfoliated tumour cell implantation. Then the spike of a transanally introduced intraluminal stapling device is passed through the middle of the transected rectal stump. The anvil of the stapling instrument is secured around the proximal cut edge of the colon with a purse-string suture. Then the anvil is re-approximated to the spike, followed by closing and firing of the stapling instrument to achieve an anastomosis. A method of enabling the intraluminal circular stapling device to be introduced from the abdomen, rather than from the anus by performing a hand-sewn purse-string suture to the anorectal stump has been reported. This will be discussed in detail later, under “methods to preserve anal sphincter function”.
Hand-sewn
Due the technical difficulties in manipulating tissue deep in the pelvis described above, the classical method of hand suturing with ‘parachuting’ stitches is seldom used in present times. Nonetheless, it is important that the surgeon who performs rectal surgery has the skills to perform a hand-sewn anastomosis on rare occasions when the stapling devices fail.
Pull-through hand-sewn coloanal
The circumstances for performing this type of anastomosis have already been discussed above under “introduction-interspincteric dissection”. In addition, this technique is an option in the obese patient with a narrow pelvis and in circumstances where an anastomosis needs to be salvaged after a stapling instrument mishap.
Stapled instrument malfunctioning
Aspects of salvaging the situation when the stapling instruments malfunction have already been described above, under “hand-sewn” and “pull-through hand-sewn coloanal anastomosis”. It is not often possible to re-do the double cross stapled anastomosis, especially when the original rectal transection has been very distal. A very distal anastomosis can usually be accessed more easily for suture repair from the anus. In addition, the transanal route can be used to insert a purse-string for a repeated stapled anastomosis where the defect has been major. An appropriate defunctioning stoma would be essential.
Defunctioning stoma
It is well recognized that after total mesorectal excision, a distal colorectal/anal anastomosis at the level of the anorectal junction has a much higher risk of anastomotic dehiscence than more proximal colorectal anastomoses. Most of the time, these anastomotic problems are subclinical. There is a strong case for a defunctioning stoma to reduce the complications of an anastomotic leak even in routine circumstances. A defunctioning loop ileostomy is technically easier to fashion and to close, with less risk of damaging the marginal vessels than a colostomy. This has become the preferred technique by most surgeons in the West. However, a colostomy is preferable where the bowel preparation has been inadequate because residual faeces distal to a defunctioning stoma will continue to contaminate the anastomosis and hence will not reduce the complications of an anastomotic leak. For the same reasons, a colostomy would be preferable when the anastomosis is compromised or in places where hot weather would cause excessive dehydration from an ileostomy. Traditionally, the defunctioning stoma is closed 12 wk later when the intraperitoneal adhesions would be more easily managed. This is provided that a contrast study confirms that the anastomosis is intact. In recent years, a case has been made for barrier agents against adhesions such as hyaluronate carboxymethylcellulose membranes (Seprafilm) to be placed around the stoma to enable earlier closure.
TYPES OF OPERATIONS
Using the above methods to re-anastomos the bowel, 4 types of operations normally result i.e.‘straight’ colorectal or coloanal anastomosis, colonic J-pouch anal anastomosis, coloplasty anastomosis & colonic side-to-end anastomosis.
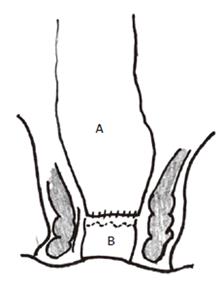
Low anterior resection ‘straight’ anastomosis (Figure
1)
Figure 1 Straight coloanal anastomosis with colon (A) anastomosed directly to anorectum (B).
A straight anastomosis results from a direct end-to-end anastomosis of the colon to the anorectum. After segmental bowel resection (including right hemicolectomy, left hemicolectomy and anterior resection) for colorectal cancer, most patients (58%-78%) have a satisfactory 1 to 2 bowel movements/d[8]. However after low anterior resection, up to one-third of patients have 3 or more bowel movements/d. At times, patients can be troubled with having up to 14 stools a day[9,10]. Other patients may have defecation problems after anterior resection; excessive stool frequency or defecation problems under these circumstances has been termed as the “anterior resection syndrome”.
Reasons for poor bowel function after ‘straight’ anastomosis
Multiple regression analysis on factors influencing postoperative bowel function showed that stool frequency at 1 year was independently predicted by the level of anastomosis and less importantly, the rectal sensation[11]. This suggests that rectal reservoir function is important in controlling stool frequency. After ultra-low anterior resection with a straight colo-rectal/anal anastomosis, it has been shown that stool frequency depends upon the amount of rectum resected[9,12-14]. When the level of anastomosis is less than 4-4.5 cm from the anal verge (ie. at approximately the anorectal junction), there is an increased risk of poor bowel function[11,15]. After end-to-end coloanal (straight) anastomosis at the level of the anorectal junction, the normally compliant rectum that has been removed is replaced by a less compliant segment of descending or sigmoid colon. The replacement colon is physiologically less suitable for storing/regulating feces[11]. Not surprisingly, the results include excessive stool frequency and possibly fecal incontinence associated with the increased stool frequency. Another cause of post low anterior resection fecal incontinence is anal sphincter injuries, which occurs in up to 28 percent of patients following transanal insertion of a stapling instrument[16]. This aspect will be discussed later (in “methods to preserve anal sphincter function”).
Defecation problems may also occur in about 28% of patients after low anterior resection. This risk is similar to that after sigmoid colectomy (25%), which is substantially higher than the risk after more proximal bowel resections, such as right hemicolectomy (ranging from 4% to 15%)[8]. This suggests that the sigmoid colon may have a major role in expelling and evacuating stools[17], which would be consistent with the more muscular nature of this segment of large bowel. Resection causes discontinuity of colonic muscles and intrinsic nerves and, hence, disruption of coordinated colonic mass movement[18]. In addition, division of the lateral ligaments of the rectum at ultra-low anterior resection may denervate the rectum and lead to significant constipation[19].
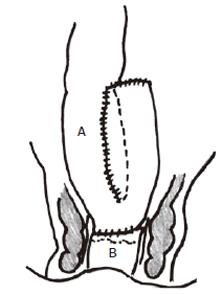
Colonic J-pouch anal anastomosis (Figure
2)
Figure 2 Colonic J-pouch-anal anastomosis with constructed colonic pouch (A) anastomosed to anorectum (B).
The anterior resection syndrome symptoms have lead to various strategies to improve bowel function. To date, most of these strategies have focused on the proximal aspect of the anastomosis. This consists of various methods to better retain stool contents in the neorectum including the colonic J-pouch, coloplasty and colonic side-to-end anastomosis. The evolution of these techniques has been based on physiological considerations, which are hence discussed prior to the technique and results.
Physiology: When colonic J-pouches were first introduced, the aim was to maximize the neo-rectal compliance and volume by constructing a double barreled configuration with limbs sizes measuring up to 15 cm[10,13,14,20-22]. Randomized controlled trials comparing this 15 cm colonic pouch technique to direct straight colo-rectal/anal anastomoses have confirmed improved stool frequency[23-27]. Proctometrographic measurements have shown improved rectal volumes (i.e. increased neorectum reservoir capacity) and rectal compliance (increased ability of the neorectum to retain high pressures)[20,27]. However, these advantages came at the expense of severe evacuation problems[27-30].
Meanwhile, a smaller 6 cm colonic J-pouch which is conveniently constructed from a single longitudinal firing of a linear cutting stapling instrument, was found to be effective in improving stool frequency, but with less rectal evacuation problems[31,32]. This has since been confirmed by studies done in other centres[26,33], and also in a randomized controlled trial comparing 6 cm with 9 cm colonic J-pouches[34]. An interesting finding was that although there was improved function, no differences have been found in the rectal physiology (volume of initial sensation, maximum tolerable volume and compliance) measured at 1-year between small colonic J-pouch and straight coloanal anastomosis patients, in a randomized controlled trial[32].
Compared to stationary anorectal manometric techni-ques, continuous ambulatory manometric monitoring has the advantage of monitoring pressure changes in the anus and rectum during prolonged periods and in a more physiologically normal environment. Using such techniques, patients with the smaller J-pouch were found to have a better tolerance to higher rectal pressures without increased stool frequencies, compared to straight anastomosis patients in a randomized prospective trial[35]. The anorectal pressure gradient was also better preserved[3]; which had been described previously to be related to bowel frequency[24,36]. Defecation in patients with colonic J-pouches may be related to large contraction waves (not corresponding to mass movement complexes) previously detected on ambulatory anorectal manometry, in patients with large colonic J-pouches[36]. These large contraction waves were not observed after prolonged monitoring in patients with smaller 7 cm pouches, which might well explain the less severe rectal evacuation problems in these patients.
To elicit this further, a scintigraphy study protocol was designed to attempt to follow liquid faeces colonic transit with technetium TC99m tin-colloid and solid faeces colonic transit with I131 implanted microcapsules ingested at the same time[37]. More technetium TC99m tin-colloid was distributed into the liquid colonic contents at the distal colon at 24 h in 6 cm colonic J-pouch patients, than in those with straight coloanal anastomosis. This may be related to a backward stacking up effect from factors such as reversed peristalsis in the colonic J-pouch, accounting for the less frequent stools in those patients. The retention of I131 in the solid stools were not different between colonic J-pouch and straight coloanal anastomosis patients, which may explain the rarity of severe evacuation problems (likely to involve solid stools) in patients with smaller 6 cm colonic J-pouches.
The barostat is a computerized pump that inflates a rectal balloon at controlled and reproducible rates of pressures, and volumes, providing a more accurate technique for assessing rectal physiology. The only study using barostat measurements on colonic J-pouches to date showed that there were no differences at 6 mo between 6 cm colonic J-pouch and straight coloanal anastomosis patients[38]. At 2 years, there was a trend for improved rectal sensation and maximum tolerable volume (as assessed with the phasic program) in both the types of patients. The phasic program assesses afferent sympathetic nerve function and hence these findings may be related to recovery of function in these nerves. Significant improvements in rectal compliance in straight coloanal anastomosis patients at 2 years had previously been documented in a cohort study, using traditional proctometrographic techniques[23]. In addition, enlargements of the colonic J-pouch size have been measured radiologically over a 2 years period[39]. All these changes may be responsible for the long term adaptation seen in patients after straight and colonic J-pouch colorectal/anal anastomoses.
Construction of 6 cm colonic J-pouch: Mobilization of the splenic flexure allows the descending colon to be used for the construction of the J-pouch. Very often this is necessitated by the sigmoid colon being badly affected by diverticulosis. Using a diseased sigmoid colon might compromise the pouch function and the anastomosis integrity. Where the sigmoid colon is healthy and of adequate length, it has been used instead of descending colon with no significant differences in stool frequency, incontinence, urgency, use of pads, need for anitidiarrhoeal drugs, sensation of incomplete evacuation and anorectal physiologic results at 1-year follow-up[40]. However, the descending colon has the advantage of being less muscular and more distensible than the sigmoid colon, which might improve the mid-term functional results[38]. A comparison of results across studies which use descending and sigmoid colon at 2-year follow-up suggests that the descending colon adapts better[38].
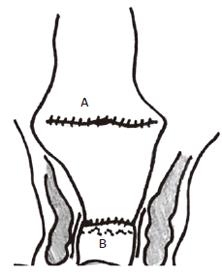
Figure 3 Coloplasty anastomosis with coloplasty in colon (A) anastomosed to anorectum (B).
A coloplasty is designed to ‘interrupt antegrade colonic peristalsis’ and as an option when the pelvis is too narrow to permit a bulky colonic J-pouch anal anastomois[41]. It is similar to a pyloroplasty or stricturoplasty, and had initially been tried out in pigs[42,43] prior to testing out in patients[44,45].
Construction of coloplasty: A 7 cm longitudinal incision is made between the taenia along the anti-mesenteric side of the descending colon, starting 4 cm above the distal cut end. The incision is closed transversely with a continuous single layer of seromuscular absorbable suture. The coloplasty ‘pouch’ is then anastomosed to the stapled anorectal stump by a double cross stapling technique with the coloplasty facing anteriorly.
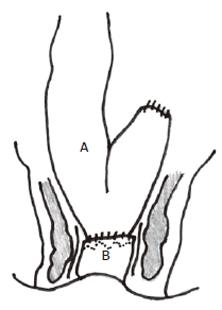
Side-to-end anastomosis (Figure
4)
Figure 4 Side-to-end anastomosis with side of colon (A) anastomosed to end of anorectum (B).
The side-to-end anastomosis that was first described by Baker JW in 1950[45], has been revisited recently as another option to improve postoperative bowel function. There are 2 variations: (1) anastomosis performed by introducing the intraluminal stapler from the anus or (2) anastomosis done entirely from the abdomen alone. Using the former variation, Machado M et al[46] performed a randomized controlled trial which showed that the side-to-end anastomosis was functionally comparable to the 8 cm colonic J-pouch. The only difference was better neorectal evacuation in < 15 min in the colonic J-pouch group, at 6 mo. The second variation of the side-to-end anastomosis hinges upon the concept of preserving anal sphincter function per se (as compared to improving proximal bowel motiliy or reservoir function), and hence that is discussed in further detail.
Methods to preserve anal sphincter function: After low anterior resection, faecal continence may be compromised in 13%-80% of patients[5,9,10]. It is known that some damage to the anal sphincters can occur after low anterior resection[35,47,48]. Anal pressures are significantly impaired and the rectosphincteric inhibitory reflex recovered only in a few patients at 2 years after low anterior resection, regardless of any reservoir construction[38]. The rectosphincteric inhibitory reflex requires an intact reflex pathway based upon the anal sphincter muscles and intrinsic rectal innervation. Ambulatory anorectal manometric studies have shown that after low anterior resection, patients who complained of soiling while passing flatus had lower minimal anal pressures[35]. The latter is very likely related to injury to the internal anal sphincter during transanal stapling instrument insertion[24,35,36].
Horgan et al[49] monitored the anal pressures of patients on the operating table undergoing anterior resections. They found that the anal pressures were maintained at the division of the inferior mesenteric artery, full mobilization of the rectum and mesorectum, and anal transection. The anal pressures only decreased significantly after transanal introduction of the intraluminal circular stapler, suggesting direct injury during anal insertion of the instrument. Molloy et al[50], found that anal resting pressures in dogs was significantly lower after transanal introduction of intraluminal circular stapler than handsewn colorectal anastomoses. However, it was likely that nerve injury during rectal mobilization was also important in dogs, because there was significant impairment of anal pressures after both types of anastomoses. A randomized controlled trial showed that direct injuries to the internal anal sphincter occurred after transanal insertion of the stapler but not with the biofragmentable anastomotic ring (where the anastomosis was performed entirely intra-abdominally) after high anterior resection[51]. The nature of the injury was documented with endoanal ultrasound in a follow-up randomized controlled clinical study to be the result of anal sphincter injuries[16]. In order to avoid transanal introduction of the intraluminal stapling device, the second variation of the side-to-end anastomosis was devised to enable a stapled anastomosis performed entirely from the abdomen. Huber FT et al[52]. reported on a randomized controlled trial which compared this variation of side-to-end anastomosis (with 3-4 cm blind end) with the 6 cm colonic J-pouch.
COLONIC J-POUCH-ANAL ANASTOMOSIS
Complications
The better-vascularized end-to-side anastomosis with a J-pouch has been shown to reduce the risks of anastomotic dehiscence, compared to the straight colo-anal/rectal anastomosis[23]. This will be discussed in detail in the section on ‘coloplasty’.
Function
Early function (1 year): Patients with a small 6 cm colonic J-pouch-anal anastomois have a median of 3 bowel movements a day compared with a median of 6 a day for patients with straight anastomoses, at 1 year after surgery[31,32]. Urgency to defecate is less troubling in colonic J-pouch patients. However, a frequent sensation of incomplete neorectal evacuation was more common after the small colonic J-pouch-anal anastomosis, but most of these patients do not require suppositories, laxatives or enemas to evacuate.
Late function (2 years): At 2 years of follow-up, patients with 6 cm colonic J-pouch and those with straight coloanal anastomoses have similar bowel frequency at about 1 bowel movement a day[38]. The need for anti-diarrhoeal medications is minimal in both groups. In the only other randomized controlled trial with long-term follow-up, Lazorthes et al[24] reported no improvements in their straight coloanal anastomosis patients at 2 years. This may well be due to using the sigmoid colon for some of their coloanal anastomoses. As the sigmoid colon is less distensible, it is less likely to adapt successfully in the long-term as a storage reservoir for faeces. This would account for the no difference in bowel function between colonic pouch and straight anastomosis patients, reported in the other available data from cohort studies[23,53]. Dehni et al[54] reported that stool frequency remained superior in the pouch patients after 5 years but conceded that they had difficulty assessing stool frequency because of significant stool fragmentation in their patients.
Bowel continence has been reported by some to be better in colonic J-pouch patients than in those with straight coloanal anastomoses[11,23,25,26]. However, the differences are often minor, like less likelihood of soiling with passing flatus[38]. At 2 years, studies to date have confirmed no differences in continence with either anastomoses[23,38,41,43]. At this stage, unless there is excessive stool frequency, it is likely that significant faecal incontinence is related more to anal sphincter injuries than to neorectal reservoir function (see below).
Bowel evacuation is improved at 2 years with straight coloanal anastomosis patients, but major evacuation problems remain minimal with colonic J-pouch patients[38]. A randomized controlled trial comparing the function of 6 cm and 9 cm colonic J-pouches at 2 years showed that fewer 6 cm pouch patients required laxatives and enemas for severe constipation[34]. Stool fragmentation/clustering has been defined as multiple evacuations over a 1-2 h period associated with persistent sensation of rectal fullness. More straight anastomosis patients had persistent long-term stool fragmentation[53,54]. As this phenomenon has not been confirmed in the only other large randomized controlled trial to date, these findings well may be related to cultural and dietary factors[38]. The results to date suggest that the small 6-7 cm colonic J-pouch-anal/rectal anastomosis is the procedure of choice because of early improved bowel function and less risk of anastomotic complications.
COLOPLASTY ANAL ANASTOMOSIS
Complications
A randomized controlled trial comparing colonic J-pouch with coloplasty ‘pouch’-anal anastomosis showed significantly more anastomotic leaks in the latter (15.9%)[41]. Seven percent were clinical and 9% were radiologic, found at routine barium enema prior to ileostomy closure. All the leaks were at the anterior of the coloanal anastomoses, below the site of the coloplasty. Anastomotic leaks were not significantly associated with postoperative chemotherapy or radiotherapy (P = 0.417). A 5% incidence of clinical anastomotic leak had been reported in the only other series on coloplasty pouch patients (nonrandomized) to date[45]. The lower incidence of anastomotic leak after J-pouch may be due to better proximal anastomotic blood supply, as shown by the laser Doppler technique. This better blood supply at the critical anastomotic site was related to the J-pouch being anastomosed side-to-end to the anal canal, compared with the straight coloanal, which is an end-to-end anastomosis. Hence, higher leak rates after coloplasty may be due essentially to the coloanal anastomosis being made end-to-end, as in the straight coloanal anastomoses. The reported coloplasty leak rates were comparable to the straight anastomosis leak rates. An additional possibility to consider would be some compromise of blood supply to the colonic anastomotic end as a result of the coloplasty. This may account for all the clinical and radiologic leaks occurring anteriorly at the coloanal anastomosis, just distal to the coloplasty.
Function
The differences between the early functional results of the small colonic J-pouch and coloplasty techniques are subtle[41]. J-pouch patients had significantly less stool fragmentation, which required returning to the toilet at least once within 15 min of evacuation. However, both groups of patients reported increased stool fragmentation at 1 year. It is known that patients may have difficulty in differentiating stool fragmentation from increased bowel frequency. No differences were found with other stool evacuation problems. However, coloplasty patients had significantly better stool deferment time and less nocturnal liquid stool leakage. No differences were found with other stool incontinence symptoms and with continence scoring. The only other published study on the coloplasty technique compared 20 patients with historical controls consisting of 16 J-pouch and 17 straight coloanal anastomoses[45]. It confirmed no significant differences between coloplasty and colonic J-pouch patients when stool frequency, use of antidiarrheal medication, and continence were compared. Patients’ perceptions as measured by the Fecal Incontinence Quality of Life scale also showed no difference between the small colonic J-pouch and coloplasty techniques[41].
Physiology
Anorectal manometric findings did not show any significant differences in the function of small colonic J-pouch and coloplasty patients[41]. Colonic pouch reservoir function, as measured by the rectal volume of initial sensation, maximum tolerable volume, and compliance, was not different between the groups. This was consistent with the findings of Mantyh et al[45], mentioned above. Z’graggen et al[42] also found no differences in maximum tolerable volume and compliance between J-pouch and coloplasty in their experimental surgery on 15 pigs. Although experimental surgery construction of a coloplasty may provide a 40% increase in volume[42], it is more than likely that in the clinical situation motility factors such as disruption of colonic propulsion as a result of the coloplasty on the antimesenteric surface may be more important[44].
At this time because of higher risks of anastomotic complications, coloplasty cannot be recommended except for special circumstances when a bulky J-pouch cannot be brought through a narrow pelvis for anastomosis to the anorectal junction. Nonetheless, further studies with variations in coloplasty design and longer follow-up may reveal other advantages in the technique. However, due caution, including the use of defunctioning stoma and careful postoperative observation, should be exercised.
MANAGEMENT OF ‘ANTERIOR RESECTION’ SYNDROME
Conservative
Traditionally, management of poor bowel function has been managed expectantly having excluded other causes particularly tumour recurrence and pelvic sepsis. It is now known the colonic adaptation can take up to 18 mo to occur after ultra-low anterior resection with total mesorectal excision[38]. The patient is advised to take adequate soluble fibre in the diet and to avoid foods which aggravate the bowel dysfunction. Those with increased stool frequency may be prescribed diphenoxylate, codeine and/or bile salt binding agents to help control the symptoms. Patients with rectal evacuation problems may be prescribed regular laxatives and enemas. On rare occasions, patients fail to respond to conservative treatment with persisting debilitating bowel function lasting beyond 18 mo. Under such circumstances, anorectal biofeedback therapy and/or postanal sphincter repair may need to be considered. Resort to a stoma would be only needed in very exceptional circumstances only, where patients are managed in a specialist colorectal surgical centre.
Anorectal biofeedback
Biofeedback has been shown to be effective in treating certain types of faecal incontinence[55]. This is a specific form of behaviour modification that aims to control bodily function. Biofeedback has been reported to be successful in managing patients who have stool frequency and/or incontinent problems after anterior resection[56]. At a mean follow-up of 10.6 mo, 90% success with no regressions or complications was found. Anorectal physiologic tests done before and after biofeedback show minimal increase in anal pressures. It is possible that biofeedback works by improving the anal sphincteric coordination, rectal sensation, rectal liquid retention and/or anal canal sensation. Although patient-biofeedback therapist relationship may be vital, none of the patients received any formal psychiatric counseling. Biofeedback has also been reported to be 90% successful in managing intractable constipation following low anterior resection[57]. Intractible constipation after low anterior resection is likely to result from resection of the sigmoid colon, which is the main propulsive organ of the large intestine[17]. Again, the results of pre and post-biofeedback anorectal physiologic tests are inconclusive, suggesting that similar factors outlined previously may play an important role in bringing about the positive changes in bowel function.
Postanal sphincter repair
Treatment options are limited for persistent intractable excessive stool frequency and incontinence after low anterior resection for rectal cancer. Fortunately, this is quite rare, but such patients treated successfully by postanal sphincter repair have been reported[58]. In such cases, it is essential that anorectal physiologic tests and endoanal ultrasound findings are consistent with internal anal sphincter injuries, which are known to occur with transanal insertion of stapling instruments. After postanal sphincter repair, stool frequency was reported reduced from a mean 5.7 to 1.7 stools per day[58]. Fecal incontinence requiring pads in all patients was improved to full continence in 67% and minor incontinence for flatus in 33%. Continence score improved from a mean 13.7 to 1.3. Mean follow-up was 3.2 years. With the recent advent of bulking agents implanted intersphincterically by injection, another option for managing internal sphincter injuries in patients after low anterior resection is now available. Clinical studies are awaited.
LAPAROSCOPIC ULTRA-LOW ANTERIOR RESECTION
Laparoscopic colonic cancer surgery has been proven to be at least as safe and effective as traditional open surgery. However, data is still sparse with regard to the laparoscopic management of rectal cancer. It is now technically possible to perform the mobilization of the left colon and total mesorectal excision by a laparoscopic technique. The anorectal junction can be stapled and transected with an endoscopic linear cutter stapler. The specimen can then be extracted through a plastic drape protected 4-5 cm muscle splitting transverse incision, which can be used eventually for the temporary defunctioning stoma. A colonic J-pouch or coloplasty can be performed extracorporeally and pneumoperitoneum reconstituted to perform an intracorporeal end-to-end double cross stapled anastomosis, with the intraluminal stapling device introduced transanally. Further data and refinement of the technique is awaited.
In conclusion, at present, the small colonic J-pouch-anal anastomosis is the most widely accepted method of restoring colonic anal continuity after a total mesorectal excision. A straight colorectal anastomosis is preferred where the anastomosis is more than 4-6 cm above the anal verge. In these circumstances, there is adequate residual rectum to provide the neccessary rectal reservoir capacity. Besides, performing a colonic J-pouch-rectal anastomosis at this proximal level is likely to result in rectal evacuation problems. Where a coloanal anastomosis at the anorectal junction needs to be considered in a heavily built patient with a narrow pelvis, a coloplasty can be considered. The other methods of restoring bowel continuity are best kept for special circumstances such as stapling gun misfiring, where their unique technical features will help salvage the operation, where others are not possible. A laparoscopic technique is likely to be the method of choice in the near future.