Published online Dec 21, 2006. doi: 10.3748/wjg.v12.i47.7642
Revised: October 25, 2006
Accepted: November 7, 2006
Published online: December 21, 2006
AIM: To investigate whether manual acupuncture at representative acupoints in different parts of the body can modulate responses of gastric motility in rats and regular effects in different acupoint stimulation.
METHODS: The gastric motor activity of rats was recorded by the intrapyloric balloon. The changes of gastric motility induced by the stimulation were compared with the background activity in intragastric pressure and/or waves of gastric contraction recorded before any stimulation. Morphological study was also conducted by observing the Evans dye extravasation in the skin after mustard oil injection into the intragastric mucous membrane to certify cutaneous innervations of blue dots related to gastric segmental innervations.
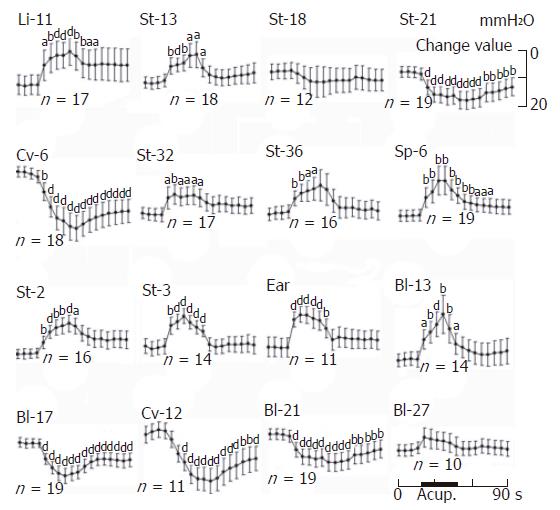
RESULTS: In all six rats that received mustard oil injections into intragastric mucosa, small blue dots appeared in the skin over the whole abdomen, but mainly in peri-midline upper- and middle- abdomen and middle-back, a few in thigh and groin. It may speculate that cutaneous innervations of blue dots have the same distribution as gastric segmental innervations. Acu-stimulation in acupoints of head-neck, four limbs, upper chest-dorsum and lower-dorsum induced markedly augmentation of gastric motility (acupoints on head-neck such as St-2: n = 16, 105.19 ± 1.36 vs 112.25 ± 2.02 and St-3: n = 14, 101.5 ± 1.75 vs 109.36 ± 1.8; acupoints on limbs such as Sp-6: n = 19, 100.74 ± 1.54 vs 110.26 ± 3.88; St-32: n = 17, 103.59 ± 1.64 vs 108.24 ± 2.41; St-36: n = 16, 104.81 ± 1.72 vs 110.81 ± 2.74 and Li-11: n = 17, 106.47 ± 2.61 vs 114.77 ± 3.77, P < 0.05-0.001). Vigorous inhibitory regulations of gastric motility induced by acu-stimulation applied in acupoints on whole abdomen and middle-dorsum were significantly different as compared with the controls before acu-stimulation (abdomen acupoints such as Cv-12: n = 11, 109.36 ± 2.09 vs 101 ± 2.21; Cv-6: n = 18, 104.39 ± 1.42 vs 91.83 ± 3.22 and St-21: n = 12, 107 ± 2.97 vs 98.58 ± 2.81; acupoints on middle-dorsum such as Bl-17: n = 19, 100.63 ± 1.4 vs 92.21 ± 2.07 and Bl-21: n = 19, 103.84 ± 1.48 vs 97.58 ± 2.16, P < 0.05-0.001).
CONCLUSION: Regular regulatory effects of facilitation and inhibition on gastric motility appear to be somatotopically organized in the acupoints of whole body, and the effective regularity of site-special acupoints on gastric motility is involved in segmental innervations between stomach and acupoints.
- Citation: Li YQ, Zhu B, Rong PJ, Ben H, Li YH. Effective regularity in modulation on gastric motility induced by different acupoint stimulation. World J Gastroenterol 2006; 12(47): 7642-7648
- URL: https://www.wjgnet.com/1007-9327/full/v12/i47/7642.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i47.7642
Acupuncture therapy, originated and prospering in China and prevailing in Asia, has been used empirically in clinical practice for several millennia. It has long been accepted that acupuncture at certain points on the body, can have analgesic and therapeutic effects in the treatment of various diseases[1]. This therapy has drawn the attention of many investigators and become a research subject of international interest around the world. Although a large number of previous clinical studies support the efficacy of acupuncture for treating gastrointestinal symptoms and/or diseases[2-4], little is known about the underlying mechanism(s). Motility of the digestive tract encompasses the phenomena of myoelectrical activity, contractile activity, tone, compliance, and transit. In the functional gastrointestinal disorders, various types of dysmotility have been documented repeatedly, and most likely reflect dysfunction at one or more levels of the brain-gut axis. It has been demonstrated that the somatosensory inputs from the skin and/or muscle are involved in the control of various autonomic functions[5-7]. A series of investigations regarding somato-autonomic reflexes has also been carried out focusing on gastrointestinal function. In some of those investigations, there is good evidence indicating the importance of cutaneo-sensory inputs in the autonomic control of gastrointestinal motility. In anesthetized rats, for instance, it has been shown that the cutaneo-gastric reflexes mediate the inhibition and the stimulation of gastric motility via sympathetic and parasympathetic efferents, respectively[7-9]. It was shown that the cutaneo-sensory stimulation induced by pinching abdominal skin of rats inhibits gastric motility by increasing sympathetic activity. On the other hand, cutaneo-sensory stimulation induced by pinching the hindlimb enhances gastric motility by increasing vagal activity[8].
It is generally believed that acupuncture at different acupoints show different effects. Previous studies suggest the site-specific inhibitory or stimulatory effects of acupuncture on gastric motility[8,10,11]. The Pc-6 (neiguan) at wrist and St-36 (zusanli) at hindlimb are the common acupoints used for treating gastric symptoms such as nausea and vomiting[12,13], suggesting that acupuncture at these acupoints may stimulate gastric motility. In contrast, acupuncture on the abdomen has been used for treating abdominal pain[2-4], suggesting that acupuncture at this point may inhibit gastric motility and/or reduce gastrospasm. Acupuncture at St-36 and Pc-6 has been shown to enhance the gastric migrating motor complex in conscious dogs[14] and in anesthetized rats[10]. It has been also shown that manual acupuncture on the abdomen inhibits gastric motility[10] and induced gastric relaxations via the somato-sympathetic pathway[15] in anesthetized rats. Meanwhile, it was also existent in inter-contradictive results[16-19]. Recent investigations have suggested that the modulations of acupuncture on visceral functional activities can demonstrate totally different facilitative/inhibitory effects on gastric motility in diverse site-acupoints given. Therefore, the present studies were to investigate whether manual acupuncture at representative meridian-acupoints in different parts of the body can modulate responses of gastric motility, and what are regular effects in different acupoint stimulation given.
All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of China Academy of Chinese Medical Sciences. Experiments were performed on 48 adult male Sprague-Dawley rats weighing between 250 and 300 g. The animals were maintained on a 12:12-h light-dark cycle (8:00-20:00) with free access to food and water. The rats were fasted overnight with free access to water in proxima luce and anesthetized with an intraperitoneal injection of urethane (1.0-1.2 g/kg). The trachea was cannulated but not immobilized only to keep respiratory tract unobstructed and a catheter was inserted into one of the jugular veins for infusion of the necessary solutions or anesthetics. The abdomen was opened by midline section, and a small longitudinal incision was made in the duodenum about 2-3 cm from the pylorus. A small balloon made of flexible condom rubber was inserted via incision of the duodenum into the pyloric area of rat and kept in position by tying the connecting catheter to the duodenum, and another catheter (inner diameter of 1 mm) was also inserted into the same hole by incision in order to drain digestive juices secreted from stomach. Then the cut skin of the abdomen was closed by suture before experiments. The balloon was filled with warm-water about 0.2-0.5 mL, which gave about 80-150 mmH2O pressures. Pressure in the balloon was measured by a transducer (TP-400T, Nihon Kohden) for low pressure through a thin polyethylene tube (1.5 mm in outer diameter) and then input on a polygraph (RM-6000, Nihon Kohden) amplifier and led into a data acquisition system (Power-Lab) for further analysis. Demi-fasting gastric motor activity was recorded as a control for at least 1 h before either the acupuncture or somatic stimulation.
The changes of gastric motility induced by the stimulation were compared with the background activity in intragastric pressure and/or waves of gastric contraction recorded before any stimulation. If the changes of gastric motility during stimulation were 20% more or less than the background activity, the response was then considered to have an excitatory or inhibitory regulation, respectively. Systemic blood pressure was continuously monitored from a common carotid artery and heart rate was continuously monitored, rectal temperature kept constantly around 37°C by means of a feedback-controlled heating blanket.
Cutaneous stimulation was obtained by pinching a localized skin area of about 5 mm2 with a forceps, pinching area distributed in forelimb, lateral abdomen, dorsum and hindlimb. And an additional hot water bath (50°C) on the tail was given. Manual acupuncture with a needle of 0.3 mm in diameter was inserted into the skin and underlying muscles at different acupoints of the body, and it was rotated clockwise and anti-clockwise at 2 Hz for 30 s. Based on the comparative anatomical localization in rats as compared with that in human, selected points included 29/45 cephalocaudal acupoints in stomach-Meridian, and Li-11 (quchi) acupoint of Large-intestine-meridian in forelimb, Cv-6 (qihai), Cv-12 (zhongwan) acupoints of conception-vessel in abdomen, Bl-13 (feishu, T3 spinal segment), Bl-17 (geshu, T7), Bl-21 (weishu, T12), Bl-27 (xiaochangshu, S1) acupoints of Bladder-meridian in dorsum, Sp-6 (sanyingjiao) of spleen-meridian in hindlimb and the auriculo-concha, non-acupoint in tail (the location of acupoints is shown in Figure 2, Figure 3, Figure 4).
This experiment was performed in ten SD rats including six for mineral oil of injection in intragastric mucous membrane and four for the control of saline injection. Fifteen minutes after the surgery was completed, Evans blue dye (Sigma) dissolved in sterile water (5 mg per 100 mL) was injected through the arterial catheter. Ten minutes after the dye injection, 0.3-0.5 mL (volume depending on injected points of the stomach) of 10% mustard oil (Sigma, dissolved in mineral oil) was injected into the intragastric mucosa and visceral peritoneum covering the stomach in several areas, in order to induce a chemical inflammation of the gastric mucous membrane. Skin color changes were observed for 1-2 h, and the area of dye extravasation was sketched on the body charts for assessment. Dye extravasation was quantified by counting the number of blue dots in the skin over frontal and dorsal body areas. In control rats, a similar volume of physiological saline was injected into the intragastric mucous membrane. Evans blue dye injection was the same as mustard oil experimental animals.
The rats were kept in supine position; gastric motor activity was first analyzed visually to detect cyclic waves of contractions. When gastric background activity is well recorded, the response of gastric motor to skin stimulation and acupuncture is tested separately. A standard protocol was employed for the determination of effects of acupuncture and somatic stimuli on the gastric motility response. A gastric background activity was recorded for 5-10 min followed by a test of their responses to manual acupuncture at various acupoint or natural stimulation on the skin was applied for 30 s. After the acupuncture or somatic stimulation the gastric motility response was recorded for another 5-10 min. After the series of experiments was finished, rats were sacrificed under an overdose deep anesthesia (urethane, > 2 g/kg ip).
The data obtained before and after intervention in the same group was compared statistically by an independent t test. P < 0.05 was considered as a statistical significance. All data are expressed as mean ± SE.
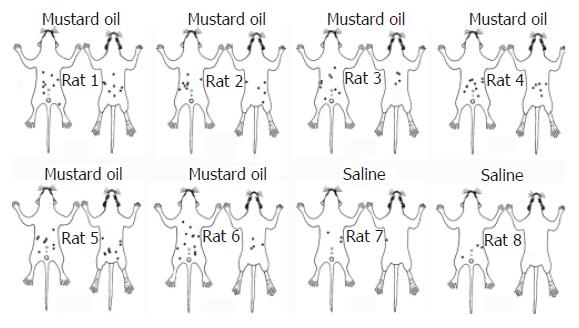
Injection of mustard oil into intragastric mucous membrane resulted in massive mucous inflammation evidenced on histological examination, which showed that the intragastric mucosa had become edematous, dilated blood vessels and ulceration of the endogastric lining. In all six rats that received mustard oil injections into intragastric mucosa (Figure 1), small blue dots appeared in the skin over the whole abdomen, but mainly in peri-midline upper- and middle- abdomen, middle-back, a few in thigh and groin. The number and distribution of blue dots varied considerably among rats. The dots started to appear about 20 min after injection of mustard oil, and the majority of dots were visible within 50 min. The dots were very small, usually ranging from 1-3 mm in diameter. However, several dots appeared in the state of stick-like in length of 3-6 mm (Figure 1). In contrast, two of four control rats that received saline injections into the intragastric mucous membrane showed no skin color changes at all. The remaining two control rats showed only 3-5 small blue dots over the middle-abdomen (Figure 1), perhaps this extravasation restricted to abdominal skin in some of the control rats might be associated with the abdominal surgical incision. It may speculate that cutaneous innervations of blue dots had same distribution as gastric segmental innervations.
Gastric motor characteristics were observed in 38 rats under normal anesthetic condition. When the intrapyloric balloon pressure was increased from 0 to about 80-200 mmH2O levels, the rhythmic wave of contractions in pyloric area were observed in 32 rats, at least in the periodic course during recording. Others showed no cyclic contraction during recording. Among the total, the contractile amplitude of rhythmic wave was about 5-50 mmH2O, whereas strong contractions exceeded to 300 mmH2O. When the pressure in an intragastric balloon was maintained at about 100 mmH2O by expanding the volume of the balloon with warm-water, rhythmic contractions occurred at a rate of 4-6 per minute. With regard to gastric motor characteristics, it was noteworthy both the changes of intragastric pressure and rhythmic contraction in present study. Generally, the intragastric pressure represents the index of gastric tone motility and rhythmic contraction represents then gastric peristalsis induced by circular muscle contractions, similar to slow-wave of gastric motor activity.
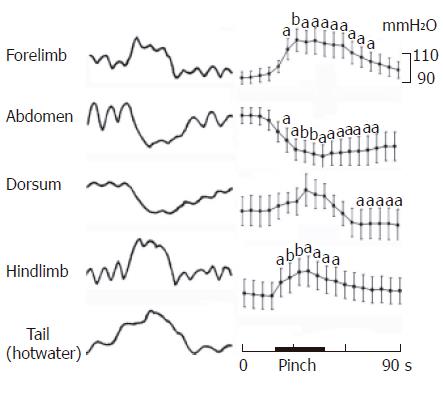
Effects of noxious mechanical or hot cutaneous stimulation of various sites on gastric motility were studied in 14 rats. As illustrated in Figure 2, pinching of the abdominal skin always produced strong inhibition (10/11 rats, 100.09 ± 2.73 vs 87.82 ± 3.68, P < 0.05) in the pyloric pressure; the maximum response of decrease was obtained about 10-s after the onset of stimulation. Pinching the middle dorsal skin in some, but not all cases, produced slight or moderate inhibition or only post-inhibition (n = 11, 102.3 ± 3.36 vs 100 ± 3.33, P < 0.05). Interestingly, pinching in fore- or hind-limb induced mainly moderate increase of gastric motility (n = 8, 104.56 ± 1.29 vs 113.78± 5.37, P < 0.05; n = 9, 97.13 ± 4.63 vs 104.88 ± 5.03, P < 0.05; respectively), meanwhile, hot water bath (50°C) on the tail could produce moderate augmentation of gastric motility.
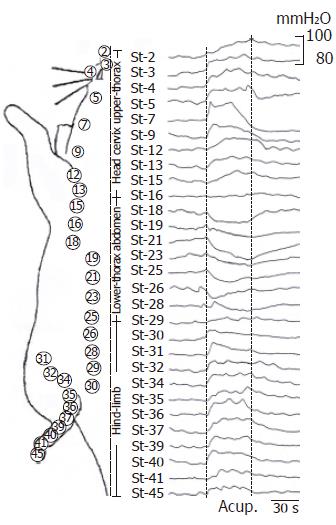
Figure 3 shows the gastric motility in response to majority craniocaudal stomach-meridian acupoint stimuli was systemically observed in four rats. Manual acu-stimulation in 9 acupoints of face (St-2, St-3, St-4, St-5 and St-7), neck (St-9) and cephalic-chest (St-12, St-13 and St-15), induced significantly moderate increase of gastric motility. This facilitation could be observed about 2-4 s after application and continued to augment during 30 s acu-stimulation. On seven acupoints of lower-chest (St-18) and whole-abdomen (St-19, St-21, St-23, St-25, St-26 and St-28), acu-stimulation produced moderate-strong suppressive responses on gastric motility, which started to suppress about 2 s delay of onset and lasted throughout the period of application of the acu-stimulation. The inhibitory magnitude on gastric motility was arranged from fadein to fadeout among these cephalocaudal acupoints. Acu-stimulation in hindlimb (St-30, St-31, St-32, St-34, St-35, St-36, St-37, St-39, St-40, St-41 and St-45) evoked moderate augmentation of gastric motility. However, in between acupoints of St-16 (in the 5th intercostal space) and St-30 (slightly above the inguinal groove), acupuncture could not bring about clear response of excitation/inhibition on gastric movement in most cases. It was noteworthy that, from the above observations, manual acu-stimulation applied in the Stomach-meridian acupoints from the lower-breast to the groin suppressed generally the gastric motor activity, whereas acu-stimulation in other acupoints of Stomach-meridian (i.e. face, neck, cephalic-chest and hindlimb) brought about exciting responses in different magnitudes on gastric motility. These results suggested the effective regularity of site-special acupoint on gastric motility. Next study focused at acupoint stimuli in different meridians and different parts of the body, and compared their effects on responses of gastric motility.
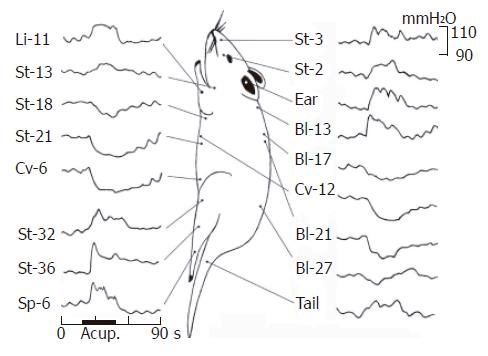
The acu-stimulation at 16 acupoints in the different parts of the body and one non-acupoint in tail on responses of gastric motility was systemically observed. A total of 27 rats were studied. Most of them (22/27) exhibited the rhythmic wave of contractions in the periodic course, and the pyloric pressure maintained at about 80-200 mmH2O. Gastric motility in the most rats showed various responses to acu-stimulation applied to some representative acupoints in several meridians and different parts of the body. An individual example of various kinds of responses was illustrated in manual acu-stimulation applied to acupoints either St-2 (sibai), St-3 (juliao) of Stomach-meridian and the auriculo-concha in face, Li-11 (quchi) in forelimb, St-13 (qihu) in upper-breast, Bl-13 (feishu) in upper-dorsum, Bl-27 (xiaochangshu) in lower-dorsum, or St-32 (futu), St-36 (zusanli), Sp-6 (sanyinjiao) in hindlimb and non-acupoint in tail, which gave rise to slight-moderate facilitation of gastric motility with a rapid onset, followed by a tonic motor that lasted throughout the period of acu-stimulation. These responses were followed in several cases by after-effects of promotion; this was particularly clear when the auriculo-concha, Li-11, St-36 and Sp-6 acupoints were acu-stimulated but was also obvious in the other cases (except from Bl-27). However it was interesting to emphasize that, the effects in both abdomen and middle-dorsum on modulation of gastric motility differed basically from those in the head, upper chest/dorsum and fore-/hind-limbs. The same stimulus applied to the acupoints of St-18 (rugen, lower breast) and St-21 (liangmen), Cv-6 (qihai) and Cv-12 (zhongwan) in the abdomen, and Bl-17 (geshu) and Bl-21 (weishu) in the middle dorsum, resulted in high suppression of gastric tonic motility with a rapid onset, followed by a obvious inhibition of rhythmic wave of contractions. These suppressions lasted throughout the period of acu-stimulation and followed in most cases after inhibition.
At a quantitative level, the facilitative/inhibitory responses on the gastric motility were evaluated before 20 s, during 30 s and after 40 s of acu-stimulation. The mean values are shown in Figure 5. Acu-stimulation in acupoints of head-neck, limbs upper chest-dorsum and lower-dorsum induced markedly augmentation of gastric motility (acupoints on head-neck such as St-2: n = 16, 105.19 ± 1.36 vs 112.25 ± 2.02 and St-3: n = 14, 101.5 ± 1.75 vs 109.36 ± 1.8; acupoints on limbs such as Sp-6: n = 19, 100.74 ± 1.54 vs 110.26 ± 3.88; St-32: n = 17, 103.59 ± 1.64 vs 108.24 ± 2.41; St-36: n = 16, 104.81 ± 1.72 vs 110.81 ± 2.74 and Li-11: n = 17, 106.47 ± 2.61 vs 114.77 ± 3.77, P < 0.05-0.001 respectively). Notable inhibitory responses of gastric motility induced by acu-stimulation applied in acupoints of whole abdomen and middle-dorsum were significantly different as compared with the control before acu-stimulation (abdomen acupoints such as Cv-12: n = 11, 109.36 ± 2.09 vs 101 ± 2.21; Cv-6: n = 18, 104.39 ± 1.42 vs 91.83 ± 3.22 and St-21: n = 12, 107 ± 2.97 vs 98.58 ± 2.81; acupoints on middle-dorsum such as Bl-17: n = 19, 100.63 ± 1.4 vs 92.21 ± 2.07 and Bl-21: n = 19, 103.84 ± 1.48 vs 97.58 ± 2.16, P < 0.001 respectively). Acu-atimulation in the tail of non-acupoint could also increase gastric motor. Such regular regulatory effects both of facilitation/inhibition on gastric motility did appear to be somatotopically organized in the acupoints of the whole body and suggested the effective regularity of site-special acupoints on gastric motility.
Recent studies have begun exploring the effects of acupuncture on gastrointestinal physiology and its potential for the treatment of gastrointestinal diseases[2,20-22]. However, despite its well-known effects on nausea and vomiting, relatively little attention has been paid to its potential for the management of disorders of gastrointestinal motility[13,14]. The purpose of this part of the study was to investigate the cutaneous distributions in association with gastric segmental innervations. Discomfort and pain were the sensory experiences most commonly evoked from visceral organs. Many forms of visceral pain were felt in the regions of the body other than the organ whose stimulation caused pain (referred pain, or viscero-somatic reflex).
Stomachal innervation has clear anatomical data, but recent study has further developed. Retrograde axonal transport of horseradish peroxidase (HRP) was applied to the ventral surface of the cat stomach. Hino et al[23] investigated the number, size and distribution of HRP-positive cells in spinal ganglia. The unexpected finding was the wide distribution of these cells from T3 down to L3. Spinal ganglion cells innervating the stomach of the rats were demonstrated using the somatopetal HRP transport technique. After injection of the tracer into the anterior wall of the stomach, labelled neurons were observed bilaterally within spinal ganglia T4-L1. They were most numerous in ganglia T8-T10[24]. The distribution of sensory neurons innervating the peritoneum was studied using axonal transport of fluoro-gold. The tracer was injected into parietal peritoneum and visceral peritoneum covering the stomach and small intestine. Many neurons in the nodose ganglia in addition to somata in the dorsal root ganglia from T4 to T13 were labeled when the tracer was placed on the peritoneum lining the stomach, small intestine or caecum[25].
In agreement with gastric (-intestinal) segmental innervations, chemical gastric inflammation with mustard oil in rats results in neurogenic plasma extravasation in the skin over whole abdomen and middle- and lower-back; neuroanatomically, these somatic territories belong to T4-L1 dermatomes[26]. It may speculate that cutaneous innervations of blue dots distribution were similar to the gastric segmental innervations.
Our results showed that, noxious mechanical (pinch) stimulation applied to limbs or tail as well as noxious heat (hot water bath at 50°C on the tail) induced gastric excitation. It was remarkable that (electro-) acu-stimulation applied to the acupoints of six in face, two in neck, one in forelimb, five in upper chest-dorsum and one in hindlimb, which were distributed in the remote somite (hetero-segment) with gastric segmental innervation, produced a facilitative response of gastric motility followed by the activities increasing slightly in vagal and/or inhibiting in sympathetic nerves innervated to stomach.
It is generally believed that acupuncture at different points (acupoints) shows different effects. Previous studies presented the site-specific inhibitory or stimulatory effects of acupuncture on gastric motility[10]. During 2-3 Hz of electroacupuncture on St-36 acupoint, there was a significant increase in the percentage of normal frequency. The percentage of normal frequency in the post-acupuncture period was also increased[16,17]. The pericardium Pc-6 (neiguan) at wrist and stomach St-36 are the common acupoints used for treating gastric symptoms such as nausea and vomiting[12,13]. On the basis of our studies and current results, it is conceivable therefore, that acupuncture at total acupoints, with exception in homo-somite with gastric segmental innervation, may encourage gastric motility and cure some disorders of gastroparesis[27], gastroadynamics or gastroatonia.
In contrast within the remote somite (hetero-segment) with gastric segmental innervations, the present study showed that the mechanical (pinch) stimulation applied at abdomen-dorsum induced gastric inhibition. Interestingly, acu-stimulation applied to the homotopic acupoints in the lower-chest, middle-dorsum and whole abdomen induced an inhibitory response of gastric motility followed by the activities of increasing in sympathetic and/or inhibiting slightly in vagal nerves innervated to stomach. These effects involved in intact preparation of sympathetic (splanchnic) nerves, but did not require intact preparation of bilateral vagal nerves and high spinal cord. Sato et al[10] showed that when it was applied to the abdomen and lower chest region, acupuncture-like stimulation elicited suppressive response on gastric motility. The authors proposed that acupuncture provoked a reflex that had cutaneous and muscle nerves as its afferent pathway, and the sympathetic gastric branches as its efferent pathway; and the acu-stimulation in abdomen inhibited gastric motility by increasing the activity of the efferent nerves of sympathetic gastric branches, abdomen acu-stimulation-induced suppression of gastric motility persisted in spinalized rats. The inhibitory duodenal response by electro-acupuncture stimulation in abdomen was a propriospinal reflex response involving splanchnic excitatory nerves, and cutting the vagal nerve branches innervating the duodenum did not affect the suppressed duodenal response[28].
Acupuncture on the abdomen has been used for treating abdominal pain[2-4], suggesting that acupuncture at this point may inhibit gastric motility and/or reduce gastrospasm or gastrohypertonics. It is conceivable that, the inhibition in gastric motility and the increase in sympathetic activity could appear only in electro-acupuncture stimulation applied to the abdomen and middle-dorsum acupoints, e.g., the homo-segmental somatic territory with gastric innervations. The results of this experiment suggest that, regardless of any meridian-acupoints, manual acupuncture at the acupoints of widespread territory except homo-segmental innervations with gastric innervations, induce a facilitative response on gastric motility; it is effective for the treatment of disturbances of gastrointestinal hypomotility such as gastroparesis, gastroptosis, gastroadynamic, enteroparesis, constipation or gastroatonia. However, acu-stimulation at the acupoints of local territory of homo-segmental innervations with gastric innervations, induces inhibitory response on gastric motility and is effective for the treatment of disturbances of gastrointestinal overmotility such as gastralgia, gastrospasm, diarrhea or enterospasm. In conclusion, acu-stimulation applied to the distant acupoints, such as in face, neck, forelimbs, upper chest-dorsum and hindlimbs, produced a facilitative response on gastric motility; whereas acu-stimulation applied to the local acupoints, such as in lower-chest, middle-dorsum and whole abdomen, induced an inhibitory response on gastric motility.
We gratefully acknowledge Professor XC Yu for his help in preparing this manuscript.
S- Editor Wang GP L- Editor Ma JY E- Editor Lu W
| 1. | Mayer DJ. Biological mechanisms of acupuncture. Prog Brain Res. 2000;122:457-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 70] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Diehl DL. Acupuncture for gastrointestinal and hepatobiliary disorders. J Altern Complement Med. 1999;5:27-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 83] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Hussain Z, Quigley EM. Systematic review: Complementary and alternative medicine in the irritable bowel syndrome. Aliment Pharmacol Ther. 2006;23:465-471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Gu Y. Treatment of acute abdomen by electro-acupuncture--a report of 245 cases. J Tradit Chin Med. 1992;12:110-113. [PubMed] [Cited in This Article: ] |
| 5. | Jansson G. Extrinsic nervous control of gastric motility. An experimental study in the cat. Acta Physiol Scand Suppl. 1969;326:1-42. [PubMed] [Cited in This Article: ] |
| 6. | Kehl H. Studies of reflex communications between dermatomes and jejunum. J Am Osteopath Assoc. 1975;74:667-669. [PubMed] [Cited in This Article: ] |
| 7. | Koizumi K, Sato A, Terui N. Role of somatic afferents in autonomic system control of the intestinal motility. Brain Res. 1980;182:85-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 52] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Kametani H, Sato A, Sato Y, Simpson A. Neural mechanisms of reflex facilitation and inhibition of gastric motility to stimulation of various skin areas in rats. J Physiol. 1979;294:407-418. [PubMed] [Cited in This Article: ] |
| 9. | Sato A, Sato Y, Shimada F, Torigata Y. Changes in gastric motility produced by nociceptive stimulation of the skin in rats. Brain Res. 1975;87:151-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 54] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Sato A, Sato Y, Suzuki A, Uchida S. Neural mechanisms of the reflex inhibition and excitation of gastric motility elicited by acupuncture-like stimulation in anesthetized rats. Neurosci Res. 1993;18:53-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 166] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Tatewaki M, Harris M, Uemura K, Ueno T, Hoshino E, Shiotani A, Pappas TN, Takahashi T. Dual effects of acupuncture on gastric motility in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2003;285:R862-R872. [PubMed] [Cited in This Article: ] |
| 12. | al-Sadi M, Newman B, Julious SA. Acupuncture in the prevention of postoperative nausea and vomiting. Anaesthesia. 1997;52:658-661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 84] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Lin X, Liang J, Ren J, Mu F, Zhang M, Chen JD. Electrical stimulation of acupuncture points enhances gastric myoelectrical activity in humans. Am J Gastroenterol. 1997;92:1527-1530. [PubMed] [Cited in This Article: ] |
| 14. | Qian L, Peters LJ, Chen JD. Effects of electroacupuncture on gastric migrating myoelectrical complex in dogs. Dig Dis Sci. 1999;44:56-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Tada H, Fujita M, Harris M, Tatewaki M, Nakagawa K, Yamamura T, Pappas TN, Takahashi T. Neural mechanism of acupuncture-induced gastric relaxations in rats. Dig Dis Sci. 2003;48:59-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Chang CS, Chou JW, Ko CW, Wu CY, Chen GH. Cutaneous electrical stimulation of acupuncture points may enhance gastric myoelectrical regularity. Digestion. 2002;66:106-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Chou JW, Chang YH, Chang CS, Chen GH. The effect of different frequency electrical acu-stimulation on gastric myoelectrical activity in healthy subjects. Hepatogastroenterology. 2003;50:582-586. [PubMed] [Cited in This Article: ] |
| 18. | Shiotani A, Tatewaki M, Hoshino E, Takahashi T. Effects of electroacupuncture on gastric myoelectrical activity in healthy humans. Neurogastroenterol Motil. 2004;16:293-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Tabosa A, Yamamura Y, Forno ER, Mello LE. A comparative study of the effects of electroacupuncture and moxibustion in the gastrointestinal motility of the rat. Dig Dis Sci. 2004;49:602-610. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Jin HO, Zhou L, Lee KY, Chang TM, Chey WY. Inhibition of acid secretion by electrical acupuncture is mediated via beta-endorphin and somatostatin. Am J Physiol. 1996;271:G524-G530. [PubMed] [Cited in This Article: ] |
| 21. | Tougas G, Yuan LY, Radamaker JW, Chiverton SG, Hunt RH. Effect of acupuncture on gastric acid secretion in healthy male volunteers. Dig Dis Sci. 1992;37:1576-1582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Uvnäs-Moberg K, Lundeberg T, Bruzelius G, Alster P. Vagally mediated release of gastrin and cholecystokinin following sensory stimulation. Acta Physiol Scand. 1992;146:349-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Hino O, Kanafusa K, Tsunekawa K. Quantitative histological study of spinal afferent innervation on the ventral surface of the cat stomach by horseradish peroxidase (HRP) method. Experientia. 1979;35:379-380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Neuhuber W, Niederle B. Spinal ganglion cells innervating the stomach of the rat as demonstrated by somatopetal transport of horseradish peroxidase (HRP). Anat Embryol (Berl). 1979;155:355-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 36] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Tanaka K, Matsugami T, Chiba T. The origin of sensory innervation of the peritoneum in the rat. Anat Embryol (Berl). 2002;205:307-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Takahashi Y, Nakajima Y. Dermatomes in the rat limbs as determined by antidromic stimulation of sensory C-fibers in spinal nerves. Pain. 1996;67:197-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 136] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Ouyang H, Yin J, Wang Z, Pasricha PJ, Chen JD. Electroacupuncture accelerates gastric emptying in association with changes in vagal activity. Am J Physiol Gastrointest Liver Physiol. 2002;282:G390-G396. [PubMed] [Cited in This Article: ] |
| 28. | Noguchi E, Ohsawa H, Tanaka H, Ikeda H, Aikawa Y. Electro-acupuncture stimulation effects on duodenal motility in anesthetized rats. Jpn J Physiol. 2003;53:1-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |