Published online Jun 7, 2007. doi: 10.3748/wjg.v13.i21.2945
Revised: March 20, 2007
Accepted: March 26, 2007
Published online: June 7, 2007
AIM: To explore whether preoperative chemoradiation therapy improves survival of patients with pancreatic cancer undergoing resectional surgery.
METHODS: Forty-seven patients with a malignant pancreatic tumor localized in the head or uncinate process of the pancreas underwent radical pancreatico-duodenectomy. Twenty-two received chemoradiation therapy (gemcitabine and radiation dose 50.4 Gy) before surgery (CRR) and 25 patients underwent surgery only (RO). The study was non-randomised. Patients were identified from a prospective database.
RESULTS: The median survival time was 30.2 mo in the CRR group and 35.9 mo in the RO group. No statistically significant differences were found in subclasses according to lymph node involvement, TNM stages, tumor size, or perineural invasion. The one, three and five year survival rates were 81%, 33% and 33%, respectively, in the CRR group and 72%, 47% and 23%, respectively, in the RO group. In ductal adenocarcinoma, the median survival time was 27 mo in the CRR group and 20 mo in the RO group. No statistically significant differences were found in the above subclasses. The one, three and five year survival rates were 79%, 21% and 21%, respectively, in the CRR group and 64%, 50% and 14%, respectively, in the RO group. The overall hospital mortality rate was 2%. The morbidity rate was 45% in the CRR group and 32% (NS) in the RO group.
CONCLUSION: Major multicenter randomized studies are needed to conclusively assess the impact of neoadjuvant treatment in the management of pancreatic cancer.
- Citation: Vento P, Mustonen H, Joensuu T, Kärkkäinen P, Kivilaakso E, Kiviluoto T. Impact of preoperative chemoradiotherapy on survival in patients with resectable pancreatic cancer. World J Gastroenterol 2007; 13(21): 2945-2951
- URL: https://www.wjgnet.com/1007-9327/full/v13/i21/2945.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i21.2945
Pancreatic carcinoma is the sixth most common cause of cancer death worldwide. In 2004, 878 new pancreatic cancers were diagnosed in Finland, and in 2003, 915 patients died of pancreatic cancer (http://www.cancerregistry.fi). The prognosis for patients with pancreatic carcinoma remains extremely poor, with only 0.4% of patients without surgical treatment surviving five years[1]. Between 1990 and 1996, the Finnish Cancer Registry recorded 4922 pancreatic cancer patients, 89 of whom survived for at least five years. Reviewing this series of patients revealed that 45 (49%) of the surviving patients did not have pancreatic ductal adenocarcinoma (PDAC) and, further, 18 patients had no histological verification of their disease. In 26 patients recorded as having histologically proven PDAC, re-evaluation of histological specimens confirmed PDAC in only 10 patients. Therefore, the five year survival rate for PDAC was thus far less than 1%[2].
Only 20% of patients with early stage pancreatic carcinoma are amenable to resection treatment. However, even then the risk for relapse is high, with only 0% to 24% of patients surviving for 5 years[3-5]. Yet, surgery is the only curative treatment for pancreatic cancer. In a recent study, adjuvant chemotherapy yielded survival benefit after surgery[6], whereas adjuvant chemoradiotherapy had an adverse effect on survival[7]. In another study, postoperative gemcitabine significantly delayed the development of recurrent disease after complete resection of pancreatic cancer compared with observation alone, but there was no difference in overall survival between the adjuvant chemotherapy with gemcitabine and the observation group[8].
Neoadjuvant (preoperative) chemoradiation offers several advantages as compared with adjuvant chemoradiation[9,10]: (1) radiotherapy is more effective with intact vascularization, (2) preoperative chemoradiotherapy may reduce cancer cell seeding during tumor manipulation, (3) the potential retardation of postoperative recovery will not postpone neoadjuvant therapy, and (4) the effect of neoadjuvant therapy is identifiable in histopathological examination of the operative specimen.
In a randomized study, gemcitabine improved survival in inoperable pancreatic cancer in comparison with 5-fluorouracil (5-FU)[11]. Gemcitabine has also been shown to exert an effect in 5-FU-refractory pancreatic cancer[12].
We have already reported our first results on gemcitabine and concomitant irradiation in patients undergoing pancreaticoduodenectomy for locally advanced pancreatic cancer[13]. In this study, we compare the outcome of radically operated patients receiving neoadjuvant therapy (gemcitabine-radiotherapy) with that of patients surgically treated without neoadjuvant therapy during same time period.
The data were collected from 47 consecutive patients who underwent pancreaticoduodenectomy for cure with extended lymphadenectomy for pancreatic carcinoma between January 1999 and January 2002 at the Helsinki University Central Hospital, Helsinki, Finland.
Among the 47 patients, 22 received chemoradiation therapy (CRT) before surgery and these patients formed the chemoradiaton and resection (CRR) group. During the same period 25 patients were surgically treated without neoadjuvant therapy forming the resection only (RO) group. The study was non-randomised. The preoperative chemoradiotherapy was offered to patients living in the Helsinki metropolitan area, but patients who lived in areas further away, where neoadjuvant chemoradiotherapy was not available were operated on directly without neoadjuant therapy. The independent, hospital Committee of Ethics approved the chemoradiation protocol. The patients received both oral and written information about the trial and patients signed informed consent forms before enrollment in the study. Patients were studied with whole body CT with 2 mm slices, with magnetic resonance imaging and with endoscopical ultrasound before the neoadjuvant therapy. Endoscopic retrograde cholangiopancreatography (ERCP) was performed for all patients in the CRR group and for 22/25 patients in the RO group. Percutaneous transhepatic cholangiography (PTC) was done once in both groups in combination with ERCP. Insertion of a biliary stent was done when indicated. A cytological specimen was also obtained using brush or needle aspiration.
The inclusion criteria for neoadjuvant therapy were: (1) adenocarcinoma located in the head or uncinate process of the pancreas or in the ampullary region, with a diameter of less than 5 cm and without evidence of local or metastatic spread of the disease, (2) WHO performance status less than 2, (3) good co-operation, and (4) no other malignancy or serious illness. The radiotherapy dose was constant whereas gemcitabine was given as a 30-min infusion twice weekly before irradiation at three dose levels, which were 20, 50 and 100 mg/m2. The targeted irradiation volume included the tumor, possible surrounding oedema, and 1 cm margin. The tumor radiation dose in planning target volume was 50.4 Gy (ICRU). The therapy was given in 28 fractions of 1.8 Gy per day, five days per week. The median number of gemcitabine-cycles was 10 and everyone received the whole planned radiotherapy. During the study the maximal tolerated dose of gemcitabine was found to be 50 mg/m2. Dose reduction of gemcitabine was needed in 65% of patients. Toxicity and tolerance of concomitant gemcitabine and radiotherapy treatment in these patients has been reported previously[12].
Following CRT, patients were given a 4-wk break for recovery of blood counts and nutrition, and new staging CT and MRI scans were performed.
The CT or MRI criteria for unresecability of tumor were metastatic disease or tumor encircling the superior mesenteric artery. Portal vein resection and reconstruction due to tumor invasion was performed when needed.
Patients underwent pancreaticoduodenectomy without neoadjuvant therapy. Preoperative CT and MRI scans were performed, and a biliary stent was inserted if the patient was jaundiced.
In both groups, the operation was begun with a diagnostic laparoscopy to rule out metastatic or locally advanced disease. Radical pancreaticoduodenectomy was performed with extended lymphadenectomy and removal of retroperitoneal tissue. In the case of tumor infiltration into the portal vein, resection of the vein and reconstruction with autologous venal graft was performed. All operations were performed by one of two surgeons, however, the vast majority of the patients were operated on by surgeon T.K.
Frozen sections of the resection margins of the pancreas and common hepatic duct were collected during operations. All specimens were delivered as fresh, the resection margins were stained and the specimen was fixed in formalin for 1-2 d. The entire tumor area was sectioned in 2-3 mm slides. One macro slide was taken from the largest macroscopically identified tumor area. Lymph nodes and resection margins were also sectioned. All tissues were embedded in paraffin and stained according to the Herovici-van Gieson and Alcian blue-PAS methods. One pathologist (P.K.) examined all specimens. The tumor stage was defined according to the AJCC 2002[14].
After radical surgery, the patients were followed using a routine follow-up program. Liver tests and CA19-9 were measured. Radiological examinations were performed only if the patients developed symptoms, such as pain or ascites, or elevation of the CA19-9 value was observed. In the case of recurrence of the disease, an oncologist was consulted and the patient was offered chemotherapy.
Fishers exact test (F) was used to test differences between categorical variables and Student’s t-test or the Wilcoxon-Mann-Whitney test (W) was used to assess differences between means of continuous variables. Normality of continuous variables was tested with the Kolmogorov-Smirnov test. Kaplan-Meier analyses and log rank tests were used to assess survival. This study was designed to detect 25 mo increase in median survival time, assuming 20 patients in each group, power of 0.80, significance level of 0.05 and median survival time of 15 mo in the surgically treated only group. Accrual time was 48 mo and follow-up time after the end of the recruitment was 48 mo. P < 0.05 was considered statistically significant (NS: not significant).
Of the 47 patients enrolled in the study, 22 (9 females 13 males) belonged to the CRR group. Their mean age was 65 (49-83) years. 25 patients (13 females and 12 males) belonged to the RO group. Their mean age was 63 (43-76) years. There were no differences between the groups regarding age and sex, status of lymph nodes, tumor stage or tumor size. After chemoradiotherapy, 8 patients were judged as inoperable for laparotomy: 4 patients had metastases to the liver, 2 patients had a poor general condition and 1 patient had a fractured femur and died from a pulmonary embolism before the operation. In addition, one patient refused the operation. All these patients were excluded from the series.
The CRR group included 15 ductal adenocarcinomas, 4 ampullary adenocarcinomas, 2 cystadenocarcinomas and one invasive malignant intraductal papillary mucinous tumor (IPMT) of the pancreas. Thirteen patients with ductal adenocarcinoma were treated by pancreticoduodenectomy. For two patients, total pancreatectomy was performed due to positive resection margins after partial resection. One portal resection was performed due to tumor invasion to the portal vein. The two patients with cystadenocarcinoma were also treated with pancreaticoduodenectomy. In the patient with IPMT, total pancreaticoduodenectomy was performed. Ampullary tumors were treated with pancreaticoduodenectomy.
The RO group included 14 patients with ductal adenocarcinomas, 4 with ampullary adenocarcinomas, 4 with cystadenocarcinomas and 3 with invasive malignant IPMTs. All patients with ductal adenocarcinomas and ampullary tumors were treated with pancreaticoduodenectomy. One portal resection was performed due to tumor invasion to the portal vein. One patient with cystadenocarcinoma was treated with pancreaticoduodenectomy, and three patients with total pancreatectomy. One patient with IPMT underwent pancreaticoduodenectomy. However, after investigation of the paraffin specimens, an intraluminal noninvasive component was found close to the resection border and the operation was completed with total pancreatectomy six months later. The two remaining patients with IPMT were treated with pancreaticoduodenectomy. Table 1 gives the distribution of the stages of tumors for both groups.
| Characteristic | CRR | RO | P |
| n | 22 | 25 | NS |
| Age | 65 (49-83) | 63 (43-76) | NS |
| Sex (male) | 13 (59%) | 12 (48%) | NS |
| Tumor site | NS | ||
| Head | 20 (91%) | 20 (80%) | |
| Body | 3 (12%) | ||
| Tail | |||
| Diffuse | 2 (9%) | 2 (8%) | |
| Tumor size (mm) | 24 (10-41) | 29 (10-150) | NS |
| Tumor type | NS | ||
| Ampullary cancer | 4 (18%) | 4 (16%) | |
| Ductal adenocarcinoma | 15 (68%) | 14 (56%) | |
| Cystadenocarcinoma | 2 (9%) | 4 (16%) | |
| Invasive IPMT | 1 (5%) | 3 (12%) | |
| Tumor stage | NS | ||
| IA | 4 (18%) | 0 | |
| IB | 4 (18%) | 5 (20%) | |
| IIA | 7 (32%) | 9 (36%) | |
| IIB | 7 (32%) | 11 (44%) | |
| Lymph node positive | 7 (32%) | 11 (44%) | NS |
One patient in the CRR group died of multiorgan failure on the 43rd postoperative day (overall hospital mortality rate 2%). In this patient, duodenal perforation with abscess formation due to a biliary stent was found in the primary operation. The patient was reoperated on the same day because of bleeding, and again on the 10th postoperative day because of leakage of the gastrojejunal anastomosis and peritonitis.
The overall morbidity rate was 38%. In the CRR group, ten patients (45%) had complications. There were five cases of intra-abdominal infections, which were treated conservatively, and one case of pneumonia. Intra-abdominal infection was recorded if the CRP had increased and the broad spectrum antibiotic had been changed after the 5th postoperative day and pneumonia was excluded. Two patients suffered from ileus. The central venous catheter of one patient was accidentally inserted into the pleural space. In the RO group, eight patients (32%) had complications. Two patients were reoperated because of bleeding with gastrointestinal anastomosis. Three patients had minor wound infections and one had pneumonia. In one patient, rupture of the intima of the common hepatic artery was suspected due to lack of pulse and immediate reconstruction with venous graft was performed. One patient suffered a stroke during the postoperative convalescence period. Information on complications is presented in Table 2.
| CRR | RO | |
| Infection | ||
| Wound infection/prolonged antibiotic therapy | 1,25 | 13 |
| Pneumonia | 1 | 1 |
| Ileus/delayed gastric emptying | 2 | 0 |
| Postoperative bleeding and reoperation | 21 | 2 |
| Postoperative intestinal haemorraghia: no reoperation | 1 | 1 |
| Central venous catheter in pleural place | 1 | 0 |
| Stroke during the postoperative time | 0 | 11 |
| Vascular injury: occlusion of hepatic artery: immediate reconstruction | 0 | 1 |
| Reoperation: broken drain | 11 | 0 |
| Leakage of gastroenteral anastomosis | 21 | 0 |
| Death | 21 | 0 |
| Complicated patients rate | 10/22 | 8/25 |
The length of hospital stay was 15 ± 1.6 d (mean +SE) in the CRR group and 11.8 ± 0.9 d (NS) in the RO group. Blood loss during operation was 2200 ± 300 mL (range 700-4300 mL) in the CRR group and 2400 ± 400 mL (range 300-8500 mL; NS) in the RO group.
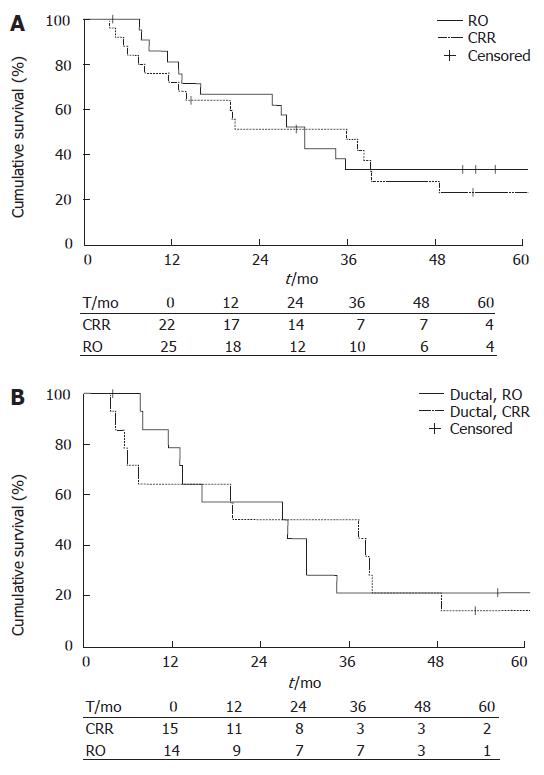
Survival rates were calculated for all types of pancreatic cancers and for the subgroups of ductal adenocarcinoma. The median survival time was 30.2 mo (95% CI 25.46-34.94) in the CRR group (n = 22), and 35.9 mo (95% CI 10.51-61.29) in the RO group (n = 25). No statistical differences were found by log Rank analyses. 1-y survival was 81% ± 8.6 (SE) in the CRR group and 72% ± 9.0 in the RO group, 3-year survival rates were 33 ± 10 and 47% ± 10 and 5 year survival rates were 33% ± 10.3 and 23% ± 9.0, respectively (Figure 1A).
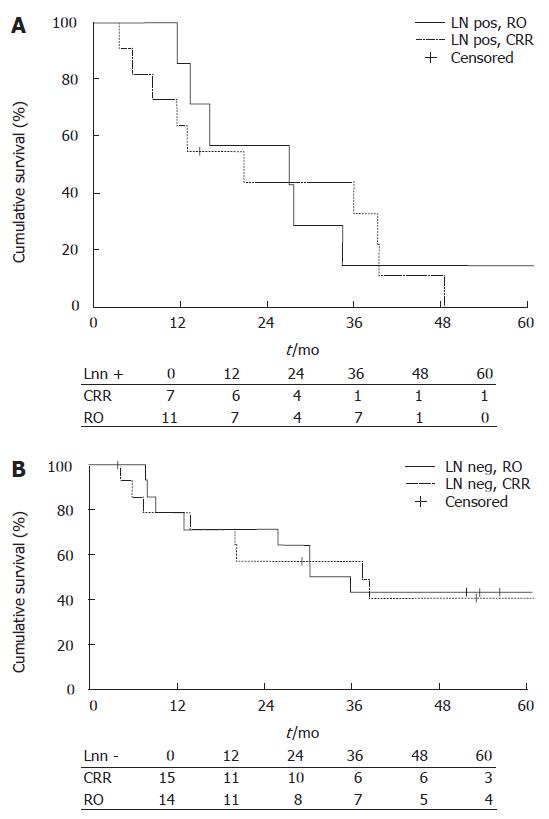
The median survival time of LN negative patients in the CRR group (n = 15) was 30.2 mo (95% CI 17.98-42.42), and in the RO group (n = 14) 37.4 mo (95% CI 7.79-67). The respective values of LN positive patients in the CRR group (n = 7) were 27.0 mo (95% CI 0.0-55.2), and in LN positive patients in the RO group (n = 11) 20.7 mo (95% CI 6.75-34.65). The 1-year survival rate for LN negative patients was 79% ± 11 in the CRR group and 79% ± 11.0 in the RO group, 3-year survival rates were 43% ± 13.2 and 57% ± 13.2, and 5-year survival rates were 43% ± 13.2 and 41% ± 13.6, respectively. In LN positive patients, the 1-year survival rate was 86% ± 13.2 in the CRR group and 64% ± 14.5 in the RO group, 3-year survival rates were 14% ± 13.2 and 33% ± 15 and 5-year survival rates were and 14% ± 13.2 and 0%, respectively. There were no significant differences between the groups (Figure 2). Median survival times for different cancers are given in Table 3.
| CRR median months(95% CI) | RO median months(95% CI) | |
| All cancers | 30.2 (25.5, 34.9) | 35.9 (10.5, 61.3) |
| LNN negative | 30.2 (17.9, 42.4) | 37.4 (7.8, 6.7) |
| LNN positive | 27.0 (0, 55.2) | 20.7 (16.7, 34.6) |
| Ductal adenocancers | 27.0 (5.5, 48.4) | 20.2 (0, 52.0) |
| LNN negative | 30.0 (9.9, 50.5) | 20.2 (19.6, 20.3) |
| LNN positive | 27.0 (0, 55.2) | 39.0 (0, 110.5) |
| Ampullar cancers | 39.4 (5.9, 72.8) | |
| Cystadenocancers | 20.7 (00, 48.2) | |
When the CRR and RO groups were divided according T classes (T1, T2, and T3), no statistical differences were revealed between the groups (data not shown).
The survival calculations were also made according to tumor size, tumor stage and perineural invasion, but no statistically significant differences between the CRR and RO groups were found in these subgroups.
The median survival time was 27.00 mo (95% CI 5.55-48.45) in the CRR group (n = 15) and 20.20 (95% CI 0-52) in the RO group (n = 14, Table 3). The 1-year survival rate was 78.6% ± 11.0 in the CCR group and 64.3 % ± 12.8 in the RO group. The respective 3-year survival rates were 21.4% ± 11.0 and 50.0% ± 13.4 and 5-year survival rates were 21.4% ± 11 and 14.3 % ± 9.4. No statistical differences were found between the groups. (Figure 1B).
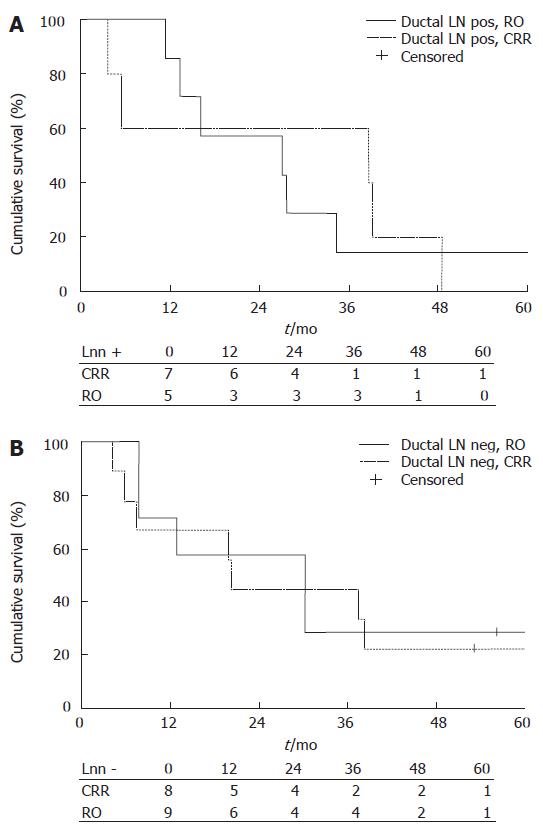
No difference was found between the CRR and RO groups regarding lymph node status. The median survival time of LN negative patients in the CRR group (n = 8) was 30.2 mo (95% CI 9.94-50.46) and in the RO group (n = 9) 20.2 mo (95% CI 19.62-20.78) (Figure 2B). The median survival time of LN positive patients in the CRR group (n = 7) was 27.0 mo (95% CI 0.00-55.23) and in the RO group (n = 5) the median survival time was 38.8 mo (95% CI 0.00-110.51). The 1-year survival rates in LN negative patients were 71% in the CRR group and 67% in the RO group, 3-year survival rates were 29% and 44% and 5-year survival rates were 29% and 22%, respectively. In LN positive patients, the rates were 86% vs 60%, 14% vs 60% and 14% vs 0%, respectively. There were no statistical differences between the groups (Figure 3).
Survival calculations were also made according to tumor size, perineural invasion and tumor stage, but no statistical differences were found between the CRR and RO groups for any subgroup.
At the end of the follow up, two patients with ductal adenocarcinoma in the CRR group were disease free. Their follow up times were 71 and 57 mo and tumor stages were T1N0M0 and T3N0M0, respectively. One patient (T3N1M0) was followed 71 mo and recurrence of disease was detected two months before the end of follow-up. Two of the 14 patients with ductal adenocarcinoma in the RO group were alive, but only one was disease free (follow up 53 mo, T2N0M0). The other (T3N0M0) had local recurrence at 46 mo with the total follow up time being 61 mo.
Patients with adenocarcinoma originating from the ampullary region had a median survival time of 39.4 mo (95% CI 5.95-72.85). In this group, the one year survival rate was 88%, the three year survival rate was 60% and the five year survival rate was 45%. In patients with cystadenocarcinoma (n = 6), the median survival time was 20.70 (95% CI 0.0-48.2), and the one year survival rate was 83%, the three year survival rate was 33%, and the five year survival rate was 33%.
In the present study, the survival time for pancreatic cancer treated by surgery only is comparable to earlier reports[15-18]. We did not show, however, any statistical survival benefit with preoperative chemoradiation therapy. Although chemoradiation is associated with improved overall survival in locally advanced disease, it rarely leads to surgical “downstaging” with consequent potential curative pancreatic resection[18]. In both groups, more than 70% (73% in the CRR group and 71% in the RO group) of the tumors were T3 tumors, i.e. locally advanced tumors, which extend beyond the pancreatic capsule, but do not involve the celiac axis or superior mesenteric artery. In patients with ductal adenocarcinoma, the median survival time in the CRR group (27 mo) was clearly longer than that in the RO group (20 mo), however again, the difference was not statistically significant, perhaps because of the small number of patients. On the other hand, when all cancers were included, neoadjuvant chemoradiation seemed to shorten, albeit not significantly, the survival time (30 vs 36 mo).
We reported earlier acceptable toxicity for preoperative twice-weekly gemcitabine and concomitant irradiation therapy[13]. Others have reported late toxicity in some patients receiving a neoadjuvant gemcitabine-radiotherapy[20,21] regimen, and the finding that gemcitabine increases radiosensitivity of both normal and malignant tissue, including pancreatic cancer, has also been reported[22-24].
Preoperative chemoradiation therapy seemed to lengthen hospital stay after the operation, but the difference was not statistically significant. The rate of complications due to infection was similar, but the infections in the RO group were only minor wound infections, whereas in the CRR group there were five cases of abdominal sepsis and one case of pneumonia, which were treated by antibiotics. This suggests that preoperative chemoradiation therapy may enhance susceptibility to infection. The length of time to carry endobiliary stents was significantly longer in the CRR group than in the RO group, which might predispose to infection, although any major disadvantages of biliary drainage have not been reported[25-28]. Only one patient died of complications after pancreaticoduodenectomy. The trigger for this complication was doubtlessly perforation of the duodenal wall by the biliary stent into the retroperitoneal space with resultant abscess formation. Although the neoadjuvant therapy was generally well-tolerated[13], the patients might have been more fragile and susceptible to complications, with compromised postoperative capacity to recovery.
After operation, no adjuvant therapy was given, but if a recurrence was found, the possibility of chemotherapy or systemic oncological treatment was offered and the patients also consented. The possible effect of the oncological therapy after relapse was not documented.
White et al[29] reported a median survival time of 23 mo after neoadjuvant (5-fluorouracil and radiation) therapy and a 3-year survival rate of 37% (n = 70) and an estimated 5-year survival rate of 23%. These figures are rather similar to our results of 27 mo and 20%. Breslin et al[30] reported a 21 mo survival time (5-fluorouracil, paclitaxel, or gemcitabine and radiation therapy; n = 132), but the median follow up time was only 14 mo. We followed our patients from 48 to 72 mo.
To our knowledge, the present study is the only one in which chemoradiaton neoadjuvant therapy is compared to operation only without neoadjuvant therapy, performed by the same two surgeons during the same period of time. No benefit of neoadjuvat therapy was observed in this study.
The behavior and prognosis of pancreatic malignancies varies and it is most important to know the histological diagnosis of tumors when the results are reported. Therefore, in our study, a reliable survival time could only be reported for ductal adenocarcinomas. The other groups (ampullary carcinomas, cystadenocarcinomas and IMPT) were too small for accurate and reliable calculations.
The weakness of this study was its unrandomized nature. However, the groups were analyzed and patients with ductal adenocarcinoma did not differ regarding tumor size, age, lymph node involvement, perineural invasion, or sex. The surgical team was the same and the patients were operated on during the same period of time. Also, the number of patients was too small for definitive conclusions. Consequently, major multicenter randomized studies are needed to assess conclusively the impact of neoadjuvant treatment in the management of pancreatic cancer.
S- Editor Liu Y L- Editor Zhu LH E- Editor Lu W
| 1. | Bramhall SR, Neoptolemos JP. Advances in diagnosis and treatment of pancreatic cancer. Gastroenterologist. 1995;3:301-310. [PubMed] [Cited in This Article: ] |
| 2. | Carpelan-Holmström M, Nordling S, Pukkala E, Sankila R, Lüttges J, Klöppel G, Haglund C. Does anyone survive pancreatic ductal adenocarcinoma? A nationwide study re-evaluating the data of the Finnish Cancer Registry. Gut. 2005;54:385-387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 236] [Cited by in F6Publishing: 255] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 3. | Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg. 1996;223:273-279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 539] [Cited by in F6Publishing: 537] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 4. | Shahrudin MD. Carcinoma of the pancreas: resection outcome at the University Hospital Kuala Lumpur. Int Surg. 1997;82:269-274. [PubMed] [Cited in This Article: ] |
| 5. | Sener SF, Fremgen A, Menck HR, Winchester DP. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985-1995, using the National Cancer Database. J Am Coll Surg. 1999;189:1-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 612] [Cited by in F6Publishing: 609] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 6. | Neoptolemos JP, Dunn JA, Stocken DD, Almond J, Link K, Beger H, Bassi C, Falconi M, Pederzoli P, Dervenis C. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358:1576-1585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 817] [Cited by in F6Publishing: 724] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 7. | Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200-1210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1945] [Cited by in F6Publishing: 1818] [Article Influence: 90.9] [Reference Citation Analysis (0)] |
| 8. | Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1779] [Cited by in F6Publishing: 1681] [Article Influence: 98.9] [Reference Citation Analysis (0)] |
| 9. | Spitz FR, Abbruzzese JL, Lee JE, Pisters PW, Lowy AM, Fenoglio CJ, Cleary KR, Janjan NA, Goswitz MS, Rich TA. Preoperative and postoperative chemoradiation strategies in patients treated with pancreaticoduodenectomy for adenocarcinoma of the pancreas. J Clin Oncol. 1997;15:928-937. [PubMed] [Cited in This Article: ] |
| 10. | Chandler NM, Canete JJ, Stuart KE, Callery MP. Preoperative chemoradiation in resectable pancreatic cancer. J Hepatobiliary Pancreat Surg. 2003;10:61-66. [PubMed] [Cited in This Article: ] |
| 11. | Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403-2413. [PubMed] [Cited in This Article: ] |
| 12. | Rothenberg ML. New developments in chemotherapy for patients with advanced pancreatic cancer. Oncology (Williston Park). 1996;10:18-22. [PubMed] [Cited in This Article: ] |
| 13. | Joensuu TK, Kiviluoto T, Kärkkäinen P, Vento P, Kivisaari L, Tenhunen M, Westberg R, Elomaa I. Phase I-II trial of twice-weekly gemcitabine and concomitant irradiation in patients undergoing pancreaticoduodenectomy with extended lymphadenectomy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;60:444-452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | AJCC Cancer Staging Handbook/American Joint Committee on Cancer. -6th Edition Greene FL and others, editors ISBN 0-387-95270-5 Springer. . [Cited in This Article: ] |
| 15. | Nitecki SS, Sarr MG, Colby TV, van Heerden JA. Long-term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving? Ann Surg. 1995;221:59-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 428] [Cited by in F6Publishing: 453] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 16. | Yeo CJ, Cameron JL, Lillemoe KD, Sitzmann JV, Hruban RH, Goodman SN, Dooley WC, Coleman J, Pitt HA. Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg. 1995;221:721-731; discussion 731-7333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 705] [Cited by in F6Publishing: 684] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 17. | Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, Sauter PK, Coleman J, Hruban RH, Lillemoe KD. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567-579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1097] [Cited by in F6Publishing: 1094] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 18. | Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Ann Surg. 2003;237:74-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 454] [Cited by in F6Publishing: 438] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 19. | Kim HJ, Czischke K, Brennan MF, Conlon KC. Does neoadjuvant chemoradiation downstage locally advanced pancreatic cancer? J Gastrointest Surg. 2002;6:763-769. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 96] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Ammori JB, Colletti LM, Zalupski MM, Eckhauser FE, Greenson JK, Dimick J, Lawrence TS, McGinn CJ. Surgical resection following radiation therapy with concurrent gemcitabine in patients with previously unresectable adenocarcinoma of the pancreas. J Gastrointest Surg. 2003;7:766-772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Pipas JM, Mitchell SE, Barth RJ, Vera-Gimon R, Rathmann J, Meyer LP, Wagman RS, Lewis LD, McDonnell C, Colacchio TA. Phase I study of twice-weekly gemcitabine and concomitant external-beam radiotherapy in patients with adenocarcinoma of the pancreas. Int J Radiat Oncol Biol Phys. 2001;50:1317-1322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Crane CH, Janjan NA, Evans DB, Wolff RA, Ballo MT, Milas L, Mason K, Charnsangavej C, Pisters PW, Lee JE. Toxicity and efficacy of concurrent gemcitabine and radiotherapy for locally advanced pancreatic cancer. Int J Pancreatol. 2001;29:9-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 71] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | McGinn CJ, Lawrence TS. Recent advances in the use of radiosensitizing nucleosides. Semin Radiat Oncol. 2001;11:270-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Eisbruch A, Shewach DS, Bradford CR, Littles JF, Teknos TN, Chepeha DB, Marentette LJ, Terrell JE, Hogikyan ND, Dawson LA. Radiation concurrent with gemcitabine for locally advanced head and neck cancer: a phase I trial and intracellular drug incorporation study. J Clin Oncol. 2001;19:792-799. [PubMed] [Cited in This Article: ] |
| 25. | Mullen JT, Lee JH, Gomez HF, Ross WA, Fukami N, Wolff RA, Abdalla EK, Vauthey JN, Lee JE, Pisters PW. Pancreaticoduodenectomy after placement of endobiliary metal stents. J Gastrointest Surg. 2005;9:1094-1104; discussion 1104-1105. [PubMed] [Cited in This Article: ] |
| 26. | Gerke H, White R, Byrne MF, Stiffier H, Mitchell RM, Hurwitz HI, Morse MA, Branch MS, Jowell PS, Czito B. Complications of pancreaticoduodenectomy after neoadjuvant chemoradiation in patients with and without preoperative biliary drainage. Dig Liver Dis. 2004;36:412-418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Sewnath ME, Karsten TM, Prins MH, Rauws EJ, Obertop H, Gouma DJ. A meta-analysis on the efficacy of preoperative biliary drainage for tumors causing obstructive jaundice. Ann Surg. 2002;236:17-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 314] [Cited by in F6Publishing: 341] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 28. | Pisters PW, Hudec WA, Lee JE, Raijman I, Lahoti S, Janjan NA, Rich TA, Crane CH, Lenzi R, Wolff RA. Preoperative chemoradiation for patients with pancreatic cancer: toxicity of endobiliary stents. J Clin Oncol. 2000;18:860-867. [PubMed] [Cited in This Article: ] |
| 29. | White RR, Xie HB, Gottfried MR, Czito BG, Hurwitz HI, Morse MA, Blobe GC, Paulson EK, Baillie J, Branch MS. Significance of histological response to preoperative chemoradiotherapy for pancreatic cancer. Ann Surg Oncol. 2005;12:214-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 132] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 30. | Breslin TM, Hess KR, Harbison DB, Jean ME, Cleary KR, Dackiw AP, Wolff RA, Abbruzzese JL, Janjan NA, Crane CH. Neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreas: treatment variables and survival duration. Ann Surg Oncol. 2001;8:123-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 267] [Cited by in F6Publishing: 283] [Article Influence: 12.3] [Reference Citation Analysis (0)] |