Published online Dec 28, 2007. doi: 10.3748/wjg.v13.i48.6458
Revised: August 27, 2007
Accepted: September 6, 2007
Published online: December 28, 2007
Despite advances in preoperative evaluation and postoperative care, intervention, especially surgery, for relief of obstructive jaundice still carries high morbidity and mortality rates, mainly due to sepsis and renal dysfunction. The key event in the pathophysiology of obstructive jaundice-associated complications is endotoxemia of gut origin because of intestinal barrier failure. This breakage of the gut barrier in obstructive jaundice is multi-factorial, involving disruption of the immunologic, biological and mechanical barrier. Experimental and clinical studies have shown that obstructive jaundice results in increased intestinal permeability. The mechanisms implicated in this phenomenon remain unresolved, but growing research interest during the last decade has shed light in our knowledge in the field. This review summarizes the current concepts in the pathophysiology of obstructive jaundice-induced gut barrier dysfunction, analyzing pivotal factors, such as altered intestinal tight junctions expression, oxidative stress and imbalance of enterocyte proliferation and apoptosis. Clinicians handling patients with obstructive jaundice should not neglect protecting the intestinal barrier function before, during and after intervention for the relief of this condition, which may improve their patients’ outcome.
- Citation: Assimakopoulos SF, Scopa CD, Vagianos CE. Pathophysiology of increased intestinal permeability in obstructive jaundice. World J Gastroenterol 2007; 13(48): 6458-6464
- URL: https://www.wjgnet.com/1007-9327/full/v13/i48/6458.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i48.6458
When mechanical biliary obstruction is diagnosed, surgical, endoscopic or radiologic intervention is usually recommended. However, despite advances in preoperative evaluation and postoperative care, intervention, especially surgery, for relief of obstructive jaundice still carries high morbidity and mortality rates, mainly due to sepsis and renal dysfunction[1-3]. The concept of preoperative biliary drainage to reduce the postoperative morbidity and mortality in patients with malignant obstructive jaundice has not proved its efficacy leading to a longstanding controversy on this issue. Studies assessing the impact of endoscopic or radiologic drainage procedures prior to surgery in jaundiced patients showed high rates of complications, highlighting the role of the factor “intervention” in general in this patient population[4]. Recently, a randomized controlled multicenter clinical trial was designed to seek evidence whether or not preoperative biliary drainage should be performed in patients with obstructive jaundice due to a periampullary tumor[4].
The reasons for the high morbidity and mortality encountered in the post operative period have been attributed to impaired immune function and the high incidence of systemic endotoxemia[5-8]. In obstructive jaundice, increased intestinal permeability has been postulated to be a key factor contributing to bacterial and endotoxin translocation to mesenteric lymph nodes, portal circulation and liver[9,10]. A suppressed clearance capacity of Kupffer cells, the main hepatic macrophage population, attributed to accumulation of bile acids into liver, permits the “spillover” of endotoxin from portal into systemic circulation, with consecutive release of proinflammatory cytokines, potentially leading to the development of the so called “gut derived sepsis”. Improved knowledge and understanding of the underlying pathophysiological mechanisms explaining the failure of the gut barrier in jaundiced patients may render us with better tools for prevention, treatment and patient selection.
Nowadays, it is accepted that the gastrointestinal tract is not only a passive organ of nutrient absorption, but it additionally displays important endocrine, immunologic, metabolic and barrier functions. The intestinal tract contains the body’s largest interface between a person and his or her external environment. The complexity of its function is obvious when thinking that at the same time the intestine has to serve two opposite functions; the selective permeability of needed nutrients from the intestinal lumen into the circulation and into the internal milieu in general, and, on the other hand, the prevention of the penetration of harmful entities including microorganisms, luminal antigens, and luminal proinflammatory factors. The latter function is known as barrier function. Gut barrier function is dependent on the immune barrier, composed of locally acting factors such as, the secretory IgA, intra-mucosal lymphocytes, Payer’s nodules, mesenteric lymph nodes and of the systemic host defense represented mainly by the reticuloendothelial system, the biological barrier, which is made up of normal intestinal flora responsible for colonization resistance, and the mechanical barrier, consisting of the closed-lining intestinal epithelial cells and by the capillary endothelial cells. All these components of gut barrier integrity can be affected by biliary obstruction and the absence of bile within the intestinal lumen.
The presence of bile and bile acids in the intestinal lumen is associated with a number of positive effects, contributing to a normal gut barrier function.
Experimental studies have shown that bile affects homing and distribution of T-lymphocytes in the gut-associated lymphatic tissue (GALT) and its absence results in decreased numbers of CD4+ and CD8+ T-lymphocytes and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) expressing cells in the lamina propria[11]. In addition, bile affects the size and number of B-lymphocytes in Peyer’s patches. In experimental animals, the ligation of the common bile duct induced efficient apoptosis in Peyer’s patch B-lymphocytes through a Toll-like receptors-2 dependent elevation of Fas expression and/or increase in sensitivity to Fas mediated apoptosis[12]. Bilirubin has been shown to impair bactericidal activity of neutrophils attenuating bacterial clearance mechanisms[13]. Bile also contains immunoglobulin A, which enhances mucosal defense either by maintaining mucosal integrity, or by binding to bacteria and viruses[14]. Circulating polymeric immunoglobulin A (IgA) binds to the secretory component (SC) on the surface of rat hepatocytes and is internalized and transported by vesicles to the canalicular membrane where the IgA-SC complex is secreted into bile. Secretion of IgA is sensitive to bile flow and the biliary secretory pathways for IgA and SC are dissociated after brief periods of cholestasis[15]. There is also evidence that specific or nonspecific antibodies contained in bile inhibit adhesion of enteric bacteria on the intestinal mucosa or inhibit bacterial endocytosis by enterocytes, thus preventing bacterial translocation[16].
Bile acids have been reported to inhibit the growth of certain bacteria such as Bacteroides, Clostridia, Lactobacillus and Streptococci[17-20]. Absence of bile salts results in a disturbed intestinal bacterial balance with overgrowth of gram negative bacteria[19,21]. Bile salts have a detergent-like activity, which can make bacterial membranes permeable and can eventually lead to membrane collapse and cell damage[22]. Alternatively, bile salts are thought to prevent intestinal endotoxin and bacterial translocation by binding directly intraluminal endotoxin and bacteria, and creating poorly absorbed detergent-like complexes[23].
In addition, bile exerts trophic effects on the intestinal mucosa, increasing villous density and inducing hypertrophy of the intestinal wall components[21,24]. In vitro experiments have shown that bile acids promote intestinal epithelial cell proliferation through a c-myc-dependent mechanism and protect against apoptotic cell death through activation of NF-κB[25,26]. These data support an important beneficial role of bile salts in regulation of mucosal growth and repair. Recent studies have also shown that bile is crucial for the maintenance of the integrity of enterocyte tight junctions, regulating the expression of the essential tight junction-associated proteins occludin and ZO-1, thus preserving the intestinal paracellular barrier[27,28].
Increased intestinal permeability has been postulated to be a key factor contributing to bacterial and endotoxin translocation and the pathogenesis of septic and renal complications in patients with extrahepatic biliary obstruction[29]. Beyond several experimental studies that have repeatedly demonstrated increased intestinal permeability in obstructive jaundice, this phenomenon has been confirmed in the clinical setting as well[5,29-31]. Increased intestinal permeability was evidenced in jaundiced patients either directly by the lactulose/mannitol permeability test[29,31], or indirectly by measurements of endotoxin concentrations in portal and systemic circulation[32], determination of anti-endotoxin core antibodies[31] and by multiple sampling during laparotomy in jaundiced patients, demonstrating growth of translocating bacteria of primarily enteric origin in extraintestinal sites[33]. Clinical data also demonstrate that surgical biliary decompression in obstructive jaundice exaggerates the pathophysiological disturbances and significantly increases intestinal permeability in the immediate post operative period as compared to non-surgically treated patients[5]. This probably reflects that the magnitude of an additional “trauma” in jaundiced patients is of importance and this should be considered in order not to further aggravate the patient’s condition and host defense and potentially increase morbidity and mortality.
Intestinal permeability is determined by interactions among several barrier components including the unstirred water layer, mucosal surface hydrophobicity, the surface mucous coat, epithelial factors (especially tight junctions) and endothelial factors[34]. Each of these components has different permeability properties. However, among these factors, the intestinal epithelium consisted of the epithelial cells which are linked close to the apical surface by the tight junctions seem to be the most important in determining intestinal permeability[35]. Up to now, the mechanism of increased intestinal permeability in obstructive jaundice remains an enigma, but in the last few years experimental studies have shed light on our knowledge in the field.
In several studies, obstructive jaundice does not seem to induce dramatic morphologic changes in the intestinal mucosa on routine light microscopy[10,36], while in others non-specific findings, such as subepithelial edema, lifting of the villus and sporadic mucosal denudation with exposure of lamina propria have been documented[9,19,37]. However, ultrastructural studies on intestinal mucosa revealed certain kinds of cell disruption, represented by alterations of cellular and mitochondrial membrane[37]. In general, most studies had demonstrated that obstructive jaundice increases intestinal permeability though epithelial continuity is retained and the mechanism for this was not evident.
The key event in the pathophysiology of obstructive jaundice-associated complications is gut derived endoto-xemia[7]. According to its size, this molecule as well as other bacterial byproducts, could have permeated the intestinal mucosa through the paracellular pathway[38]. Therefore, our research group investigated for the first time the expression of occludin, a bona fide integral component of the tight junction, in the intestinal epithelium of jaundiced rats. The results of this study showed that intestinal mucosal barrier dysfunction in obstructive jaundice is associated with regional loss of occludin expression in the intestinal epithelium, observed mainly at the upper part of the villi[28]. Our immunohistochemical observations were confirmed by immunoblotting by other investigators, who additionally showed that obstructive jaundice leads to decreased mucosal expression of the TJ-associated protein ZO-1 as well[27]. Those researchers applying in vitro experiments with enterocytic monolayers incubated in the presence or absence of graded concentrations of bile showed that the alterations of intestinal tight junctions were bile mediated, while this finding was also supported in vivo because gavaging mice with rat bile significantly ameliorated the deleterious effects of obstructive jaundice on intestinal permeability. Also, in a current study it was shown that intestinal electrophysiological parameters in jaundiced animals, which substantially depend on intestinal TJs’ integrity, were improved after oral supplementation with bile salts[39]. Further investigaton into the role of intestinal TJs alterations on gut barrier failure in obstructive jaundice demonstrated an up-regulation of claudin-4 expression in the upper part of the villi. Claudins are the only known variable elements in TJs and different expression, combination and mixing ratios of various members of the claudin family are essential in regulation of barrier properties of TJs[40]. There is evidence that the functional role of claudin-4 in the intestinal epithelium may be associated with loosening of intercellular junctions and opening of the paracellular route[41]; therefore, its overexpression is compatible with increased intestinal permeability. The key role of claudin-4 and occludin in obstructive jaundice-associated intestinal permeability alterations is further evidenced by improvement of gut mucosal barrier after restoration of their expression by regulatory peptides administration[28,42]. Apart from the role of bile deprivation, another explanation of altered intestinal occludin and claudin-4 expression in obstructive jaundice is through endotoxin-mediated mechanisms. The excessive presence of endotoxin in portal and systemic circulation stimulates a systemic inflammatory response, characterized by the release of cytokines and other proinflammatory mediators such as tumor necrosis factor-alpha (TNF-α), interleukin-1, interleukin-6, interferon-gamma (INF-γ), nitric oxide and oxygen free radicals[43]. These substances may produce injurious effects on TJs structure and function compromising intestinal epithelial barrier function[44-47]. Specifically, it has been demonstrated that TNF-α as well as INF-γ downregulate the human occludin promoter[48]. Given that increased levels of both TNF-α and INF-γ have been demonstrated in obstructive jaundice[43,49], it is tempting to speculate that these cytokines may account for occludin down-regulation. Furthermore, endotoxin reduces splachnic blood flow and disrupts intestinal microcirculation resulting in hypoxia of enterocytes and energy depletion[50]. Studies in epithelial cells monolayers have shown that adenosine triphosphate depletion induces the structural perturbation of the TJ leading to loss of the permeability barrier[51]. An additional contributory factor might be increased bacterial adherence to the enterocyte. Obstructive jaundice results in intestinal bacterial overgrowth, mainly represented by E. coli over growth[28]. Absence of bile deprives the gut from about 90% of secretory IgA, which normally prevents bacterial adherence to the intestinal mucosa[16]. Overgrowth of E. coli and lack of biliary IgA may lead to increased attachment of this bacterial strain to the intestinal mucosa. In vitro studies have shown that attachment of the enteropathogenic E. coli in intestinal epithelial cells monolayers dissociates occludin from the tight junctions, thus disrupting the paracellular barrier[52].
Absence of bile from the intestinal lumen is known to induce intestinal mucosal atrophy[21,53]. Epithelial homeostasis is highly dependent on the balance between cell proliferation and death, and knowledge of both factors is essential when elucidating how obstructive jaundice regulates intestinal cell turnover and mucosal cellularity. Experimental studies provided evidence of increased apoptosis of enterocytes in intestinal crypts in parallel with decreased mitotic activity[10,54]. These cellular events occurring in intestinal crypts, where the mucosal proliferation zone exists, may explain the induction of mucosal atrophy observed in cases of biliary obstruction[54].
The responsible mechanisms of increased intestinal apoptosis could reflect primary immunologic events following BDL (apoptosis has been shown to be induced by a variety of triggers, including proinflammatory cytokines such as TNF-α, IL-1 and IL-6, or by cytotoxic T lymphocytes that act through either granzyme B or Fas receptor pathways) or a direct action of bacterial toxins[43].
It is well known that bile salts exert a potent trophic effect on the intestinal epithelium. This action is based on their mitogenic effect on the enterocytes. In vitro studies have shown that intestinal cells exposed to physiological concentrations of the bile salt taurodeoxycholate, within 24 h are beginning to enter into S-phase of the cell cycle, while after 6 d of exposure to bile salts, cell growth is stimulated by almost 70% relative to cells grown in the absence of bile salts[26]. The proliferative effect of taurodeoxycholate is at least partly mediated by regulation of the transcription of the proto-oncogene c-myc, which has been shown to play an important regulatory role during intestinal epithelial proliferation[26].
The significant contribution of the imbalance between cell proliferation and apoptotic death in the phenomenon of gut barrier failure in obstructive jaundice is further evidenced by the improvement of gut barrier function and the reduction of gut derived endotoxemia when factors that restore intestinal homeostasis were administered. The enhancement of intestinal permeability by glutamine may be explained by its proliferative and antiapoptotic effect on the intestinal mucosa[55-57]. Administration of intestinal trefoil agents such as Growth hormone and Insulin-like growth factor I, which act on intestinal mucosal growth, development and metabolism, significantly improved intestinal barrier function and reduced portal and systemic endotoxemia in obstructive jaundice, exerting, beyond their trophic effect, a potent antiapoptotic action on the intestinal mucosa leading to preservation of mucosal homeostasis[10]. In addition, gut regulatory peptides Bombesin and Neurotensin which have been shown to prevent gut barrier dysfunction in obstructive jaundice, exerted also a combined mitogenic and antiapoptotic effect on the intestinal mucosa[54].
Altered intestinal tight junction expression and increased intestinal apoptosis are accompanied by significant alterations of the intestinal oxidative state, which represent an additional important factor in promoting intestinal injury in obstructive jaundice[21,54,58]. Studies with experimental animals showed that obstructive jaundice induces intestinal oxidative stress evidenced, not only by increased lipid peroxidation and glutathione oxidation, but also by a general imbalance between protein or non protein thiols and protein or non protein disulfides (symmetric or mixed)[21,54,59]. Specifically, in the intestine we observed increased levels of the high oxidative stress markers of thiol redox state oxidized glutathione (GSSG), non-protein mixed disulfides (NPSSR) and protein symmetric disulfides (PSSP), accompanied by the decrease of the low oxidative stress markers glutathione (GSH), GSH:GSSG ratio and protein thiols (PSH). Our findings of increased intestinal oxidative stress in obstructive jaundice were also confirmed in the clinical setting[58]. The potential mechanisms of high intestinal oxidative stress in obstructive jaundice have been extensively reviewed previously[58,60]. Briefly, increased levels of bile acids, systemic endotoxemia and the subsequent inflammatory response[61], up-regulation of inducible nitric oxide synthase expression[62,63], increased neutrophil chemotaxis and superoxide anion generation[64] and decreased systemic levels of the antioxidant vitamin E[65], contribute to the promotion of the oxidative process in obstructive jaundice.
A question raised is whether intestinal cellular and oxidative alterations could be interrelated. It has been shown that reactive oxygen species may promote cell growth arrest, via a mitogen-activated protein kinases dependent pathway that alters the status of growth regulatory proteins, and apoptotic cell death, via a cytochrome c-mediated activation of the caspase family[66]. Thus, oxidative stress may promote intestinal apoptosis and inhibition of cell proliferation, leading to mucosal atrophy in obstructive jaundice. In addition, given that oxidative stress disrupts the TJ structural complex by modulating the assembly, localization, expression and function of their molecular components[46], this factor may underlie the altered intestinal TJ expression and increased permeability in obstructive jaundice. The interrelation of oxidative stress with intestinal cellular alterations in extrahepatic cholestasis is also supported by the fact that the oral administration of the antioxidant GSH preserves the intestinal mucosal redox state and prevents intestinal histological and electrophysiological changes[39]. In line with this observation, in a recent work[59], we demonstrated that administration of different antioxidant substances (N-acetyl-cysteine, allopurinol, α-tocopherol) in ten days’ cholestatic rats, induces a significant antioxidant action in the intestine, mediated by a certain influence profile on the thiol redox state by each substance, leading to improvement of intestinal barrier function and prevention of endotoxemia. In addition, administration of gut regulatory peptides bombesin and neurotensin in experimentally jaundiced rats, induces a potent antioxidant effect on the intestine, preventing all the mentioned intestinal cellular alterations (apoptosis, inhibition of cell proliferation, altered TJ expression) and improving intestinal mucosal barrier function[28,42,54]. Taken together all these data suggest that intestinal oxidative stress and the thiol redox state are important factors in the promotion of the deleterious effects of obstructive jaundice on the anatomical and functional integrity of the intestinal mucosa.
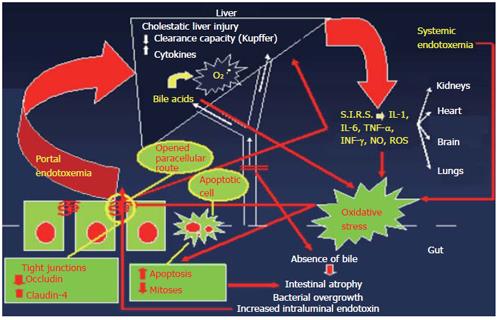
The pathophysiology of obstructive jaundice-induced gut barrier failure, endotoxemia and systemic complications, is schematically presented in Figure 1.
Clinicians handling patients with obstructive jaundice should not neglect protecting the intestinal barrier function before, during and after intervention for the relief of this condition, because failure of the intestinal barrier with consequent endotoxemia and the systemic inflammatory response may lead to serious and even life threatening complications. In this context, minimization of the additional surgical trauma, antibiotic prophylaxis, adequate fluid replacement to prevent visceral-microcirculatory disturbances, enteral nutrition to improve microcirculation, prevent mucosal atrophy and provide important nutrients for enterocytes and lactulose administration to reduce the incidence of endotoxemia are well demonstrated strategies. Growing research interest in this field has shed enough light in the pathophysiology of intestinal failure in obstructive jaundice demonstrating that the breakage of gut barrier is multi-factorial, involving disruption of the immunologic, biological and mechanical barrier. Altered intestinal tight junctions expression, oxidative stress and imbalance of cell proliferation and apoptosis may play a key role in gut permeability alterations in cases of biliary obstruction. Future studies focused on the pharmacological modulation of these factors may lead to a better control of intestinal permeability not only in obstructive jaundice, but also in diverse clinical states which may be complicated by gut-derived sepsis.
S- Editor Ma N L- Editor Rippe RA E- Editor Yin DH
| 1. | Greig JD, Krukowski ZH, Matheson NA. Surgical morbidity and mortality in one hundred and twenty-nine patients with obstructive jaundice. Br J Surg. 1988;75:216-219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 69] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Pain JA, Cahill CJ, Bailey ME. Perioperative complications in obstructive jaundice: therapeutic considerations. Br J Surg. 1985;72:942-945. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 104] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Sewnath ME, Karsten TM, Prins MH, Rauws EJ, Obertop H, Gouma DJ. A meta-analysis on the efficacy of preoperative biliary drainage for tumors causing obstructive jaundice. Ann Surg. 2002;236:17-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 314] [Cited by in F6Publishing: 341] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 4. | van der Gaag NA, de Castro SM, Rauws EA, Bruno MJ, van Eijck CH, Kuipers EJ, Gerritsen JJ, Rutten JP, Greve JW, Hesselink EJ. Preoperative biliary drainage for periampullary tumors causing obstructive jaundice; DRainage vs. (direct) OPeration (DROP-trial). BMC Surg. 2007;7:3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Parks RW, Halliday MI, McCrory DC, Erwin P, Smye M, Diamond T, Rowlands BJ. Host immune responses and intestinal permeability in patients with jaundice. Br J Surg. 2003;90:239-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Scott-Conner CE, Grogan JB. The pathophysiology of biliary obstruction and its effect on phagocytic and immune function. J Surg Res. 1994;57:316-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 118] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Clements WD, Parks R, Erwin P, Halliday MI, Barr J, Rowlands BJ. Role of the gut in the pathophysiology of extrahepatic biliary obstruction. Gut. 1996;39:587-593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 102] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Clements WD, Erwin P, McCaigue MD, Halliday I, Barclay GR, Rowlands BJ. Conclusive evidence of endotoxaemia in biliary obstruction. Gut. 1998;42:293-299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Deitch EA, Sittig K, Li M, Berg R, Specian RD. Obstructive jaundice promotes bacterial translocation from the gut. Am J Surg. 1990;159:79-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 207] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Scopa CD, Koureleas S, Tsamandas AC, Spiliopoulou I, Alexandrides T, Filos KS, Vagianos CE. Beneficial effects of growth hormone and insulin-like growth factor I on intestinal bacterial translocation, endotoxemia, and apoptosis in experimentally jaundiced rats. J Am Coll Surg. 2000;190:423-431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Sano T, Ajiki T, Takeyama Y, Kuroda Y. Internal biliary drainage improves decreased number of gut mucosal T lymphocytes and MAdCAM-1 expression in jaundiced rats. Surgery. 2004;136:693-699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Ogawa A, Tagawa T, Nishimura H, Yajima T, Abe T, Arai T, Taniguchi M, Takeda K, Akira S, Nimura Y. Toll-like receptors 2 and 4 are differentially involved in Fas dependent apoptosis in Peyer's patch and the liver at an early stage after bile duct ligation in mice. Gut. 2006;55:105-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Arai T, Yoshikai Y, Kamiya J, Nagino M, Uesaka K, Yuasa N, Oda K, Sano T, Nimura Y. Bilirubin impairs bactericidal activity of neutrophils through an antioxidant mechanism in vitro. J Surg Res. 2001;96:107-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Brown WR, Kloppel TM. The liver and IgA: immunological, cell biological and clinical implications. Hepatology. 1989;9:763-784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 130] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Kloppel TM, Hoops TC, Gaskin D, Le M. Uncoupling of the secretory pathways for IgA and secretory component by cholestasis. Am J Physiol. 1987;253:G232-G240. [PubMed] [Cited in This Article: ] |
| 16. | Wells CL, Jechorek RP, Erlandsen SL. Inhibitory effect of bile on bacterial invasion of enterocytes: possible mechanism for increased translocation associated with obstructive jaundice. Crit Care Med. 1995;23:301-307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Binder HJ, Filburn B, Floch M. Bile acid inhibition of intestinal anaerobic organisms. Am J Clin Nutr. 1975;28:119-125. [PubMed] [Cited in This Article: ] |
| 18. | Floch MH, Gershengoren W, Elliott S, Spiro HM. Bile acid inhibition of the intestinal microflora--a function for simple bile acids. Gastroenterology. 1971;61:228-233. [PubMed] [Cited in This Article: ] |
| 19. | Ding JW, Andersson R, Soltesz V, Willén R, Bengmark S. The role of bile and bile acids in bacterial translocation in obstructive jaundice in rats. Eur Surg Res. 1993;25:11-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 93] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Ding JW, Andersson R, Soltesz VL, Pärsson H, Johansson K, Wang W, Bengmark S. Inhibition of bacterial translocation in obstructive jaundice by muramyl tripeptide phosphatidylethanolamine in the rat. J Hepatol. 1994;20:720-728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Assimakopoulos SF, Vagianos CE, Patsoukis N, Georgiou C, Nikolopoulou V, Scopa CD. Evidence for intestinal oxidative stress in obstructive jaundice-induced gut barrier dysfunction in rats. Acta Physiol Scand. 2004;180:177-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Bron PA, Marco M, Hoffer SM, Van Mullekom E, de Vos WM, Kleerebezem M. Genetic characterization of the bile salt response in Lactobacillus plantarum and analysis of responsive promoters in vitro and in situ in the gastrointestinal tract. J Bacteriol. 2004;186:7829-7835. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 103] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Bertók L. Physico-chemical defense of vertebrate organisms: the role of bile acids in defense against bacterial endotoxins. Perspect Biol Med. 1977;21:70-76. [PubMed] [Cited in This Article: ] |
| 24. | Parks RW, Stuart Cameron CH, Gannon CD, Pope C, Diamond T, Rowlands BJ. Changes in gastrointestinal morphology associated with obstructive jaundice. J Pathol. 2000;192:526-532. [PubMed] [Cited in This Article: ] |
| 25. | Toledo A, Yamaguchi J, Wang JY, Bass BL, Turner DJ, Strauch ED. Taurodeoxycholate stimulates intestinal cell proliferation and protects against apoptotic cell death through activation of NF-kappaB. Dig Dis Sci. 2004;49:1664-1671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Yamaguchi J, Toledo A, Bass BL, Celeste FA, Rao JN, Wang JY, Strauch ED. Taurodeoxycholate increases intestinal epithelial cell proliferation through c-myc expression. Surgery. 2004;135:215-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Yang R, Harada T, Li J, Uchiyama T, Han Y, Englert JA, Fink MP. Bile modulates intestinal epithelial barrier function via an extracellular signal related kinase 1/2 dependent mechanism. Intensive Care Med. 2005;31:709-717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Assimakopoulos SF, Scopa CD, Charonis A, Spiliopoulou I, Georgiou C, Nikolopoulou V, Vagianos CE. Experimental obstructive jaundice disrupts intestinal mucosal barrier by altering occludin expression: beneficial effect of bombesin and neurotensin. J Am Coll Surg. 2004;198:748-757. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Parks RW, Clements WD, Smye MG, Pope C, Rowlands BJ, Diamond T. Intestinal barrier dysfunction in clinical and experimental obstructive jaundice and its reversal by internal biliary drainage. Br J Surg. 1996;83:1345-1349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 98] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Kimmings AN, van Deventer SJ, Obertop H, Rauws EA, Huibregtse K, Gouma DJ. Endotoxin, cytokines, and endotoxin binding proteins in obstructive jaundice and after preoperative biliary drainage. Gut. 2000;46:725-731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 129] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 31. | Welsh FK, Ramsden CW, MacLennan K, Sheridan MB, Barclay GR, Guillou PJ, Reynolds JV. Increased intestinal permeability and altered mucosal immunity in cholestatic jaundice. Ann Surg. 1998;227:205-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 80] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Bailey ME. Endotoxin, bile salts and renal function in obstructive jaundice. Br J Surg. 1976;63:774-778. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 176] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 33. | Kuzu MA, Kale IT, Cöl C, Tekeli A, Tanik A, Köksoy C. Obstructive jaundice promotes bacterial translocation in humans. Hepatogastroenterology. 1999;46:2159-2164. [PubMed] [Cited in This Article: ] |
| 34. | Farhadi A, Banan A, Fields J, Keshavarzian A. Intestinal barrier: an interface between health and disease. J Gastroenterol Hepatol. 2003;18:479-497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 338] [Cited by in F6Publishing: 351] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 35. | Arrieta MC, Bistritz L, Meddings JB. Alterations in intestinal permeability. Gut. 2006;55:1512-1520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 419] [Cited by in F6Publishing: 433] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 36. | Reynolds JV, Murchan P, Leonard N, Clarke P, Keane FB, Tanner WA. Gut barrier failure in experimental obstructive jaundice. J Surg Res. 1996;62:11-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Sileri P, Morini S, Sica GS, Schena S, Rastellini C, Gaspari AL, Benedetti E, Cicalese L. Bacterial translocation and intestinal morphological findings in jaundiced rats. Dig Dis Sci. 2002;47:929-934. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 38. | Hollander D. Intestinal permeability, leaky gut, and intestinal disorders. Curr Gastroenterol Rep. 1999;1:410-416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 175] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 39. | Portincasa P, Grattagliano I, Testini M, Caruso ML, Wang DQ, Moschetta A, Calamita G, Vacca M, Valentini AM, Renna G. Parallel intestinal and liver injury during early cholestasis in the rat: modulation by bile salts and antioxidants. Free Radic Biol Med. 2007;42:1381-1391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 40. | Heiskala M, Peterson PA, Yang Y. The roles of claudin superfamily proteins in paracellular transport. Traffic. 2001;2:93-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 176] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 41. | Tamagawa H, Takahashi I, Furuse M, Yoshitake-Kitano Y, Tsukita S, Ito T, Matsuda H, Kiyono H. Characteristics of claudin expression in follicle-associated epithelium of Peyer's patches: preferential localization of claudin-4 at the apex of the dome region. Lab Invest. 2003;83:1045-1053. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 42. | Assimakopoulos SF, Vagianos CE, Charonis AS, Alexandris IH, Spiliopoulou I, Thomopoulos KC, Nikolopoulou VN, Scopa CD. Experimental obstructive jaundice alters claudin-4 expression in intestinal mucosa: effect of bombesin and neurotensin. World J Gastroenterol. 2006;12:3410-3415. [PubMed] [Cited in This Article: ] |
| 43. | Bemelmans MH, Gouma DJ, Greve JW, Buurman WA. Cytokines tumor necrosis factor and interleukin-6 in experimental biliary obstruction in mice. Hepatology. 1992;15:1132-1136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 148] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 44. | Madara JL. Warner-Lambert/Parke-Davis Award lecture. Pathobiology of the intestinal epithelial barrier. Am J Pathol. 1990;137:1273-1281. [PubMed] [Cited in This Article: ] |
| 45. | Schmitz H, Fromm M, Bentzel CJ, Scholz P, Detjen K, Mankertz J, Bode H, Epple HJ, Riecken EO, Schulzke JD. Tumor necrosis factor-alpha (TNFalpha) regulates the epithelial barrier in the human intestinal cell line HT-29/B6. J Cell Sci. 1999;112:137-146. [PubMed] [Cited in This Article: ] |
| 46. | Rao RK, Basuroy S, Rao VU, Karnaky Jr KJ, Gupta A. Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem J. 2002;368:471-481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 303] [Cited by in F6Publishing: 318] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 47. | Blikslager AT, Roberts MC. Nitric oxide and the intestinal epithelial barrier: does it protect or damage the gut. J Pediatr Gastroenterol Nutr. 1997;25:439-440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 48. | Mankertz J, Tavalali S, Schmitz H, Mankertz A, Riecken EO, Fromm M, Schulzke JD. Expression from the human occludin promoter is affected by tumor necrosis factor alpha and interferon gamma. J Cell Sci. 2000;113:2085-2090. [PubMed] [Cited in This Article: ] |
| 49. | Sewnath ME, Van Der Poll T, Van Noorden CJ, Ten Kate FJ, Gouma DJ. Endogenous interferon gamma protects against cholestatic liver injury in mice. Hepatology. 2002;36:1466-1477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 50. | Nakajima Y, Baudry N, Duranteau J, Vicaut E. Microcirculation in intestinal villi: a comparison between hemorrhagic and endotoxin shock. Am J Respir Crit Care Med. 2001;164:1526-1530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 87] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 51. | Tsukamoto T, Nigam SK. Role of tyrosine phosphorylation in the reassembly of occludin and other tight junction proteins. Am J Physiol. 1999;276:F737-F750. [PubMed] [Cited in This Article: ] |
| 52. | Simonovic I, Rosenberg J, Koutsouris A, Hecht G. Enteropathogenic Escherichia coli dephosphorylates and dissociates occludin from intestinal epithelial tight junctions. Cell Microbiol. 2000;2:305-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 214] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 53. | Ogata Y, Nishi M, Nakayama H, Kuwahara T, Ohnishi Y, Tashiro S. Role of bile in intestinal barrier function and its inhibitory effect on bacterial translocation in obstructive jaundice in rats. J Surg Res. 2003;115:18-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 54. | Assimakopoulos SF, Scopa CD, Zervoudakis G, Mylonas PG, Georgiou C, Nikolopoulou V, Vagianos CE. Bombesin and neurotensin reduce endotoxemia, intestinal oxidative stress, and apoptosis in experimental obstructive jaundice. Ann Surg. 2005;241:159-167. [PubMed] [Cited in This Article: ] |
| 55. | Margaritis VG, Filos KS, Michalaki MA, Scopa CD, Spiliopoulou I, Nikolopoulou VN, Vagianos CE. Effect of oral glutamine administration on bacterial tanslocation, endotoxemia, liver and ileal morphology, and apoptosis in rats with obstructive jaundice. World J Surg. 2005;29:1329-1334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 56. | Sheen-Chen SM, Ho HT, Chen WJ, Eng HL. Obstructive jaundice alters proliferating cell nuclear antigen expression in rat small intestine. World J Surg. 2003;27:1161-1164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 57. | Assimakopoulos SF, Nikolopoulou VN, Scopa CD, Vagianos CE. Beneficial effects of glutamine on intestinal barrier function in obstructive jaundice. World J Surg. 2005;29:935-936. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 58. | Assimakopoulos SF, Thomopoulos KC, Patsoukis N, Georgiou CD, Scopa CD, Nikolopoulou VN, Vagianos CE. Evidence for intestinal oxidative stress in patients with obstructive jaundice. Eur J Clin Invest. 2006;36:181-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 59. | Assimakopoulos SF, Maroulis I, Patsoukis N, Vagenas K, Scopa CD, Georgiou CD, Vagianos CE. Effect of antioxidant treatments on the gut-liver axis oxidative status and function in bile duct-ligated rats. World J Surg. 2007;31:2023-2032. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 60. | Assimakopoulos SF, Vagianos CE, Zervoudakis G, Filos KS, Georgiou C, Nikolopoulou V, Scopa CD. Gut regulatory peptides bombesin and neurotensin reduce hepatic oxidative stress and histological alterations in bile duct ligated rats. Regul Pept. 2004;120:185-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 61. | Sakaguchi S, Furusawa S, Yokota K, Sasaki K, Takayanagi M, Takayanagi Y. The enhancing effect of tumour necrosis factor-alpha on oxidative stress in endotoxemia. Pharmacol Toxicol. 1996;79:259-265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 62. | Pata C, Cağlikülekçi M, Cinel L, Dirlik M, Colak T, Aydin S. The effects of antithrombin-III on inducible nitric oxide synthesis in experimental obstructive jaundice. An immunohistochemical study. Pharmacol Res. 2002;46:325-331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 63. | Unno N, Wang H, Menconi MJ, Tytgat SH, Larkin V, Smith M, Morin MJ, Chavez A, Hodin RA, Fink MP. Inhibition of inducible nitric oxide synthase ameliorates endotoxin-induced gut mucosal barrier dysfunction in rats. Gastroenterology. 1997;113:1246-1257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 237] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 64. | Tsuji K, Kubota Y, Yamamoto S, Yanagitani K, Amoh Y, Takaoka M, Ogura M, Kin H, Inoue K. Increased neutrophil chemotaxis in obstructive jaundice: an in vitro experiment in rats. J Gastroenterol Hepatol. 1999;14:457-463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 65. | Tsai LY, Lee KT, Lu FJ. Biochemical events associated with ligation of the common bile duct in Wistar rats. J Formos Med Assoc. 1997;96:17-22. [PubMed] [Cited in This Article: ] |
| 66. | Kamata H, Hirata H. Redox regulation of cellular signalling. Cell Signal. 1999;11:1-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 846] [Cited by in F6Publishing: 815] [Article Influence: 32.6] [Reference Citation Analysis (0)] |