Published online Jul 14, 2010. doi: 10.3748/wjg.v16.i26.3330
Revised: March 9, 2010
Accepted: March 16, 2010
Published online: July 14, 2010
AIM: To investigated if paclitaxel can attenuate hepatic fibrosis in rat hepatic stellate cells (RHSCs).
METHODS: RHSCs were cultured in vitro and randomly assigned to four groups: normal control group (treated only with Dulbecco’s Modified Eagle’s Medium), Taxol group (200 nmol/L paclitaxel was added to the cell culture), transforming growth factor (TGF)-β group (5 ng/mL recombinant human TGF-β1 was added to the cell culture), and TGF-β + Taxol group. TGF-β signaling cascade and status of various extracellular matrix proteins were evaluated by real time reverse transcriptase polymerase chain reaction and Western blotting.
RESULTS: The paclitaxel treatment markedly suppressed Smad2/3 phosphorylation. This was associated with attenuated expression of collagen I and III and fibronectin in RHSCs.
CONCLUSION: These data indicate that 200 nmol/L paclitaxel ameliorates hepatic fibrosis via modulating TGF-β signaling, and that paclitaxel may have some therapeutic value in humans with hepatic fibrosis.
-
Citation: Zhou J, Zhong DW, Wang QW, Miao XY, Xu XD. Paclitaxel ameliorates fibrosis in hepatic stellate cells
via inhibition of TGF-β/Smad activity. World J Gastroenterol 2010; 16(26): 3330-3334 - URL: https://www.wjgnet.com/1007-9327/full/v16/i26/3330.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i26.3330
Hepatic fibrosis is the key stage in the pathological process from hepatic injury to cirrhosis or even to tumor. Transforming growth factor (TGF)-β1 is widely acknowledged as a key factor in acceleration of the hepatic fibrosis process. TGF-β1 mainly activates hepatic stellate cells (HSCs) through the TGF-β1/Smad signaling pathway, thus causing hepatic fibrosis[1-5]. TGF-β evokes diverse cellular responses by binding to and activating specific cell-surface receptors that have intrinsic serine/threonine kinase activity. The activated TGF-β receptors stimulate the phosphorylation of receptor-regulated Smad2 and Smad3 proteins (R-Smads), which in turn form complexes with Smad4. This complex translocates from the cytoplasm into the nucleus, where the Smads regulate the transcription of target genes. Inhibitory Smad7 acts in an opposing manner to the R-Smads, and downregulates TGF-β signaling[6].
Some studies have previously demonstrated that endogenous Smad-2, 3 and 4 bind to microtubules in several cell lines, and the binding provides a negative regulatory mechanism to modulate TGF-β activity. Disruption of the microtubule network by chemical agents, such as nocodazole and colchicine, leads to ligand-independent Smad nuclear accumulation and transcription of TGF-β-responsive genes, which in turn increases TGF-β-induced Smad activity[7].
The aim of this study was to assess if microtubule stabilization with low-dose paclitaxel (Taxol) could inhibit TGF-β/Smad signaling and ameliorate hepatic fibrosis in HSCs.
HSCs (supplied by the Institute of Liver Diseases, the Second Xiangya Hospital, Hunan, China ) were seeded in 24-hole plastic culture plates with a density of 1 × 106/mL. The cell viability was > 95% and purity was > 90%. After cell culture for 2 wk, nearly all HSCs were activated. These cells were first cultured for 3 d, followed by a cell cycle of synchronous culture for 48 h, then the HSCs were randomly divided into four groups: normal control group (treated only with Dulbecco’s Modified Eagle’s Medium), normal + Taxol group [200 nmol/L paclitaxel (Taxol; Sigma, St. Louis, MO, USA) was added to the cell culture], TGF-β group [5 ng/mL recombinant human TGF-β1 (R&D Systems, Minneapolis, MN, USA) was added to the cell culture], and TGF-β + Taxol group. The examinations were carried out after 48 h of culture. All the examinations were repeated three times for accuracy and consistency.
Total RNA was isolated using the High Pure RNA Isolation Kit according to the manufacturer’s instructions (Roche, Switzerland). Contaminated DNA was removed by treating the samples with RNase-free DNase I (Promega, Madison, WI, USA). Real-time polymerase chain reaction (PCR) was performed using Bio-Rad (Hercules, CA, USA) iQ SYBR Green supermix with Opticon (MJ Research Inc., Waltham, MA, USA) by following the vendor’s instructions. One hundred micrograms of total RNA was reverse-transcribed and subjected to PCR as follows: 94°C for 2 min followed by 40 cycles of: 94°C for 15 s, 58°C for 30 s and 72°C for 30 s, and a final extension at 72°C for 10 min. The primers used were as follows. Rat Smad2, forward: 5′-TCACAGCCATCATGAGCTCAAGG-3′, reverse: 5′-TGTGACGCATGGAAGGTCTCTC-3′; Smad3, forward: 5′-AGCACACAATAACTTGGACC-3′, reverse: 5′-TAAGACACACTGGAACAGCGGATG-3′; collagen I, forward: 5′-GAGCGGAGAGTACTGGATCG-3′, reverse: 5′-TACTCGAACGGGAATCCATC-3′; collagen III, forward: 5′-GTGCGGTTTGTGAAGCACCG-3′, reverse: 5′-GTTCTTCTCATGCACACTT-3′; fibronectin, forward: 5′-TGACTCGCTTTGACTTCACCAC-3′, reverse: 5′-TCTCCTTCCTCGCTCAGTTCGT-3′. All samples were subjected to reverse transcriptase (RT)-PCR along with the housekeeping gene GAPDH with the following primer sequences: forward: 5′-TGCTGAGTATGTCGTGGAGTCTA-3′, reverse: 5′-AGTGGGAGTTGCTGTTGAAATC-3′ as an internal standard. Reaction specificity was confirmed by gel electrophoresis of products after real-time PCR and melting curve analysis. Ratios for Smad2/GAPDH, Smad3/GAPDH, collagen III/GAPDH, fibronectin/GAPDH and collagen I/GAPDH mRNA were calculated for each sample and expressed as the mean ± SD.
Total protein was extracted from cells and analyzed with bicinchoninic acid protein concentration assay kit (Beijing Biosea Biotechnology Co. Ltd., China). Samples (20 μg) were fractionated by SDS-PAGE. After transfer onto nitrocellulose membrane (Amersham International, Bucks, UK), the blots were probed with a mouse monoclonal antibody to p-Smad3 (Cell Signaling Technology, Beverly, MA, USA 1:1000 dilution), a goat polyclonal antibody to p-Smad2 (Upstate Biotechnology, Billerica, MA, USA 1:1000 dilution) and rabbit polyclonal antibodies to collagen I (Santa Cruz Biotechnology, Santa Cruz, CA, USA, 1:2000 dilution), collagen III (Abcam, Cambridge, MA, USA 1:2000 dilution), and fibronectin (Santa Cruz Biotechnology, 1:1000 dilution). The second antibodies were peroxidase-conjugated goat anti-mouse IgG (1:20 000 dilution)and the swine anti-rabbit IgG or rabbit anti-goat IgG , which be diluited in PBS that contained 1% normal goat serum or 1% fetal calf serum. β-actin was used as an internal control.
Data were calculated as the mean ± SD and the groups were compared using one-way ANOVA. Statistical significance was set at P < 0.05.
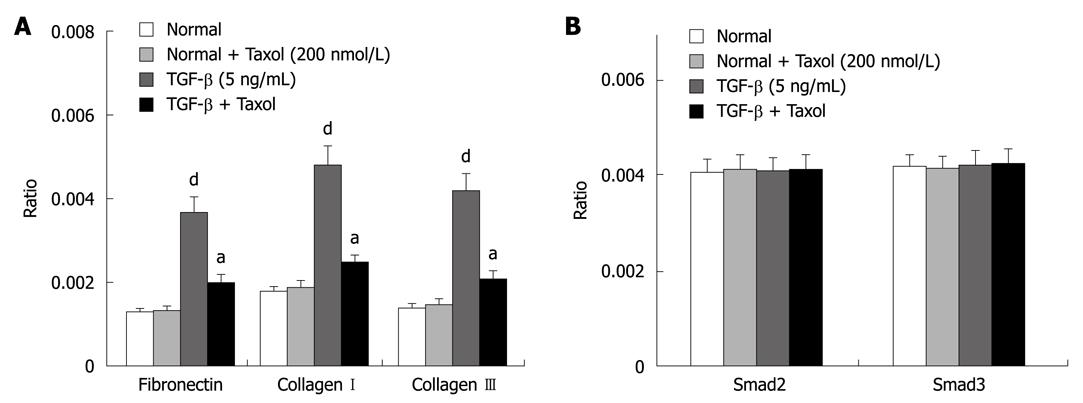
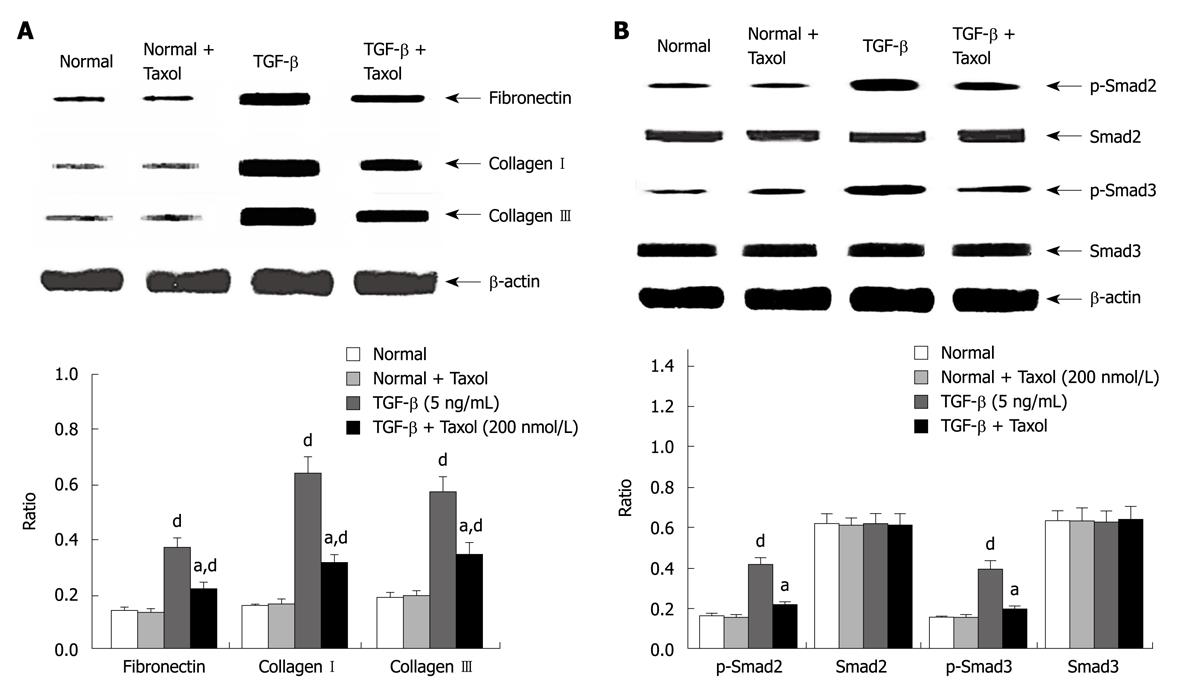
The suppressive effects of paclitaxel on mRNA and protein expression of fibronectin and collagen I and III were assessed by real-time RT-PCR and Western blotting. Figure 1A shows RT-PCR analysis that indicated that paclitaxel had no effect on basal mRNA expression for fibronectin and collagen I and III in rat HSCs. The expression of integrin-linked kinase, α-smooth muscle actin and collagen I was reduced in cells treated with paclitaxel and TGF-β at 48 h (P < 0.01). Western blotting analyses revealed similar trends in mRNA expression (P < 0.05, n = 6) (Figure 2A).
The effects of paclitaxel on expression of Smad2 and Smad3 mRNA were assessed by real-time RT-PCR. The analysis showed that paclitaxel had no effect on Smad2 and Smad3 mRNA expression in the normal, Taxol and TGF-β + Taxol groups at 48 h (Figure 1B). Western blotting analysis showed that phosphorylated Smad2/3 but not total Smad2/3 expression was markedly reduced in HSCs treated with paclitaxel and TGF-β at 48 h (P < 0.01). In contrast, paclitaxel had no effect on expression and phosphorylation of Smad2/3 in the Taxol group (Figure 2B).
In the present study, we observed that fibrosis in HSCs was substantially decreased by paclitaxel, a microtubule-stabilizing agent, thus suggesting that low-dose paclitaxel has therapeutic benefits in the amelioration of hepatic fibrosis.
The TGF-β/Smad signaling pathway plays an important role in hepatic fibrosis. In chronic hepatic injury, after HSCs are transformed into myofibroblasts, Smad2 and Smad3 are continuously phosphorylated and the inhibitory expression of Smad7 is at a low level. As a result, TGF-β1 signal transduction cannot be effectively inhibited. This might be one of the mechanisms for progression from chronic hepatic injury to hepatic fibrosis[8,9]. The worse the hepatic fibrosis and the higher the expression of TGF-β1, the higher is the Smad3 protein expression. Therefore, whatever is transferred by Smad3 might be the signal that can induce hepatic fibrosis[10-12]. Currently, TGF-β1 is the most effective fibrosis-promoting known, and it can promote activation of HSCs, and increase synthesis of extracellular matrix (ECM)[4,5]. Furthermore, it has been previously shown in several different cell lines that microtubules serve as a negative regulator for TGF-β/Smad signaling by forming a complex with endogenous Smad2, 3 and 4, thus sequestering the R-Smads away from the TGF-β receptor[7]. Therefore, it is conceivable that stabilization of microtubules by low-dose paclitaxel can dampen the exacerbated TGF-β signaling, as reported in TGF-β-induced inhibition of myogenesis in C2C12 myoblasts[13]. Similarly, in an earlier study, Liu et al[14] also have found that paclitaxel can significantly suppress TGF-β/Smad activity in SCID mice. In the present study, we provided evidence that low-dose paclitaxel suppressed phosphorylation of Smad2 and Smad3, two homologous Smad proteins that transduce signals from TGF-β and activin, in rat HSCs. These data support the notion that TGF-β/Smad signaling is regulated by the dynamic stability/instability of microtubules that are sensitive to low-dose microtubule-stabilizing agents, like paclitaxel.
Paclitaxel is an anticancer agent[15], which by stabilizing polymerized microtubules and enhancing microtubule assembly, arrests the cell cycle in the G0/G1 and G2/M phases, thus leading to cell death[16,17]. Prolonged treatment with paclitaxel has been associated with scleroderma-like changes or pulmonary fibrosis, albeit in only a small fraction of patients. It is noteworthy that inhibition of tumor cell proliferation can be achieved by much higher doses of paclitaxel. The inhibition of TGF-β/Smad signaling, however, can be attained with very low doses of paclitaxel. However, some of the recent studies have indicated that low-dose paclitaxel has minimal, if any, detectable effects on cell proliferation and other cellular activities, including fibrosis. Intriguingly, low-dose paclitaxel has been shown to inhibit collagen-induced arthritis and fibrosis associated with systemic sclerosis in SCID mice[14,18,19]. Type I collagen, the major ECM component of fibrotic tissue, is a heterotrimer composed of two α1 chains and one α2 chain. Increased production of type I collagen is a common hallmark of fibrotic diseases in various organs including the liver. Once stimulated by fibrogenic stimuli, HSCs are the only cells that respond by expressing increased amounts of all three different isoforms of TGF-β. As activated HSCs are the principal cells to produce type I collagen in fibrotic liver, they contribute to the development of liver fibrosis through autocrine and paracrine loops of TGF-β-stimulated collagen production[4]. In the present study, low-dose paclitaxel treatment effectively reduced expression of type I and III collagen and fibronectin in rat HSCs.
In conclusion, we demonstrated that low-dose paclitaxel significantly suppressed the exacerbated TGF-β/Smad signaling and decreased interstitial fibrosis in rat HSCs. It is hoped that the current results will give an impetus to future investigations to explore the therapeutic potential of paclitaxel in the amelioration of hepatic fibrosis.
Hepatic fibrosis is the key stage in the pathological progress from hepatic injury to cirrhosis. Transforming growth factor (TGF)-β is widely acknowledged as a key factor in accelerating hepatic fibrosis. Smad proteins have been identified to play an important role in regulating the expression of extracellular matrix (ECM) proteins via the TGF-β signaling pathway. Aberrant TGF-β/Smad signaling can be modulated by stabilization of microtubules with paclitaxel.
In this study, the authors’ research group for the first time reported that 200 nmol/L paclitaxel ameliorated fibrosis in rat hepatic stellate cells (HSCs) via inhibition of TGF-β/Smad activity.
In future experiments, the authors will use the hepatic fibrosis model to observe the treatment effect of paclitaxel in vivo.
Paclitaxel is an anticancer agent. It is noteworthy that the inhibition of tumor cell proliferation can be achieved by much higher doses of paclitaxel. The inhibition of TGF-β/Smad signaling, however, can be attained with very low doses of paclitaxel.
The findings that low-dose paclitaxel significantly suppressed the exacerbated TGF-β/Smad signaling and decreased interstitial fibrosis in rat HSCs are interesting and important.
Peer reviewer: Dariusz M Lebensztejn, Associate Professor, IIIrd Department of Pediatrics, Medical University of Bialystok, 17 Waszyngtona Str., Bialystok, 15-247, Poland
S- Editor Wang YR L- Editor Kerr C E- Editor Lin YP
| 1. | Li YR, Wei L. [Mechanism of transforming growth factor beta/Smad signaling and the relationship between its transduction and liver fibrosis]. Zhonghua Ganzangbing Zazhi. 2005;13:476-478. [Cited in This Article: ] |
| 2. | Wu XL, Zeng WZ, Jiang MD, Qin JP, Xu H. Effect of Oxymatrine on the TGFbeta-Smad signaling pathway in rats with CCl4-induced hepatic fibrosis. World J Gastroenterol. 2008;14:2100-2105. [Cited in This Article: ] |
| 3. | Parsons CJ, Takashima M, Rippe RA. Molecular mechanisms of hepatic fibrogenesis. J Gastroenterol Hepatol. 2007;22 Suppl 1:S79-S84. [Cited in This Article: ] |
| 4. | Inagaki Y, Okazaki I. Emerging insights into Transforming growth factor beta Smad signal in hepatic fibrogenesis. Gut. 2007;56:284-292. [Cited in This Article: ] |
| 5. | Gressner AM, Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets. J Cell Mol Med. 2006;10:76-99. [Cited in This Article: ] |
| 6. | Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577-584. [Cited in This Article: ] |
| 7. | Dong C, Li Z, Alvarez R Jr, Feng XH, Goldschmidt-Clermont PJ. Microtubule binding to Smads may regulate TGF beta activity. Mol Cell. 2000;5:27-34. [Cited in This Article: ] |
| 8. | Breitkopf K, Godoy P, Ciuclan L, Singer MV, Dooley S. TGF-beta/Smad signaling in the injured liver. Z Gastroenterol. 2006;44:57-66. [Cited in This Article: ] |
| 9. | Gressner AM, Weiskirchen R, Breitkopf K, Dooley S. Roles of TGF-beta in hepatic fibrosis. Front Biosci. 2002;7:d793-d807. [Cited in This Article: ] |
| 10. | Uemura M, Swenson ES, Gaça MD, Giordano FJ, Reiss M, Wells RG. Smad2 and Smad3 play different roles in rat hepatic stellate cell function and alpha-smooth muscle actin organization. Mol Biol Cell. 2005;16:4214-4224. [Cited in This Article: ] |
| 11. | Tao YY, Cui HY, Liu CH. [Dynamic characteristics of SARA during liver fibrogenesis in rats]. Zhonghua Ganzangbing Zazhi. 2006;14:909-913. [Cited in This Article: ] |
| 12. | Tahashi Y, Matsuzaki K, Date M, Yoshida K, Furukawa F, Sugano Y, Matsushita M, Himeno Y, Inagaki Y, Inoue K. Differential regulation of TGF-beta signal in hepatic stellate cells between acute and chronic rat liver injury. Hepatology. 2002;35:49-61. [Cited in This Article: ] |
| 13. | Zhu S, Goldschmidt-Clermont PJ, Dong C. Transforming growth factor-beta-induced inhibition of myogenesis is mediated through Smad pathway and is modulated by microtubule dynamic stability. Circ Res. 2004;94:617-625. [Cited in This Article: ] |
| 14. | Liu X, Zhu S, Wang T, Hummers L, Wigley FM, Goldschmidt-Clermont PJ, Dong C. Paclitaxel modulates TGFbeta signaling in scleroderma skin grafts in immunodeficient mice. PLoS Med. 2005;2:e354. [Cited in This Article: ] |
| 15. | Gelmon K. The taxoids: paclitaxel and docetaxel. Lancet. 1994;344:1267-1272. [Cited in This Article: ] |
| 16. | Donaldson KL, Goolsby GL, Kiener PA, Wahl AF. Activation of p34cdc2 coincident with taxol-induced apoptosis. Cell Growth Differ. 1994;5:1041-1050. [Cited in This Article: ] |
| 17. | Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277:665-667. [Cited in This Article: ] |
| 18. | Brahn E, Tang C, Banquerigo ML. Regression of collagen-induced arthritis with taxol, a microtubule stabilizer. Arthritis Rheum. 1994;37:839-845. [Cited in This Article: ] |
| 19. | Cao L, Sun D, Cruz T, Moscarello MA, Ludwin SK, Whitaker JN. Inhibition of experimental allergic encephalomyelitis in the Lewis rat by paclitaxel. J Neuroimmunol. 2000;108:103-111. [Cited in This Article: ] |