Published online Jul 28, 2010. doi: 10.3748/wjg.v16.i28.3478
Revised: April 2, 2010
Accepted: April 9, 2010
Published online: July 28, 2010
Radiofrequency ablation (RFA) of pancreatic neoplasms is restricted to locally advanced, non-resectable but non-metastatic tumors. RFA of pancreatic tumors is nowadays an ultrasound-guided procedure performed during laparotomy in open surgery. Intraoperative ultrasound covers the mandatory role of staging, evaluation of feasibility, guidance and monitoring of the procedure. Different types of needle can be used. The first aim in the evaluation of RFA as a treatment for locally advanced pancreatic ductal adenocarcinoma, in order of evaluation but not of importance, is to determine the feasibility of the procedure. The second aim is to establish the effect of RFA on tumoral mass in terms of necrosis and cytoreduction. The most important aim, third in order of evaluation, is the potential improvement of quality of life and survival rate. Nowadays, only a few studies assess the feasibility of the procedure. The present paper is an overview of RFA for pancreatic adenocarcinoma.
- Citation: D’Onofrio M, Barbi E, Girelli R, Martone E, Gallotti A, Salvia R, Martini PT, Bassi C, Pederzoli P, Mucelli RP. Radiofrequency ablation of locally advanced pancreatic adenocarcinoma: An overview. World J Gastroenterol 2010; 16(28): 3478-3483
- URL: https://www.wjgnet.com/1007-9327/full/v16/i28/3478.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i28.3478
Ductal adenocarcinoma is the most common primary malignancy of the pancreas, and represents 80% of malignant pancreatic tumors[1-3]. Pain, anorexia and weight loss are common symptoms, even in early stages. Tumors of the pancreatic head frequently cause obstruction of the common bile duct and present with jaundice. Tumors of the body/tail of the pancreas may grow to a large size before obvious symptoms appear[4]. Macroscopically, pancreatic ductal adenocarcinoma is a white/yellow and firm mass with infiltrating epithelium that recapitulates ductal structures. Microscopically, it is composed of infiltrating glands surrounded by dense and reactive fibrous tissue[4]. Highly aggressive adenocarcinomas are characterized by the presence of intratumoral fibrosis and necrosis, typically with a reduction of the microvascular density and perfusion[5].
Diagnostic imaging aims to correctly detect and characterize the tumor, thus playing an important role in the management of pancreatic ductal adenocarcinoma[6]. Conventional ultrasonography (US) is often the initial noninvasive imaging modality chosen for the first evaluation of the pancreas, as it is inexpensive, easy to perform and widely available[7]. The detection of a solid hypoechoic mass should be considered to be a ductal adenocarcinoma until proven otherwise. Contrast-enhanced US can better characterize and stage pancreatic tumors already detected by US[8], thus differentiating between solid and cystic lesions and influencing the choice of further examinations. A solid hypovascular pancreatic mass has to be considered a ductal adenocarcinoma until proven otherwise and requires computed tomography (CT) confirmation and staging. more than 95% of cases, pancreatic ductal adenocarcinoma is diagnosed at an advanced stage[5], with locally advanced (presence of perineural and vascular invasion) or metastatic disease (commonly in the liver, lungs, peritoneum and adrenal glands)[2,3,6,7]. Prognosis and treatment approach depend on the resectability or non-resectablility of the lesion at presentation[6]. Thus, only 10%-20% of patients are candidates for surgery[4], whereas in most cases, there is worse survival[8,9] and only palliative therapies are feasible.
Palliative therapies for advanced pancreatic adenocarcinoma consist of chemotherapy to give both local and systemic effect and radiotherapy for local effect. Endo-prosthesis positioning, biliary and pancreatic anastomosis, or ethanol ablation of the celiac plexus for pain relief represent further palliative procedures.
Radiofrequency ablation (RFA) of pancreatic ductal adenocarcinoma builds on its positive experiences in the liver. In fact, ablation therapy performed on the liver, usually minimally invasive through a percutaneous approach, provides extremely favorable results[10,11]. Thus, the first aim in the evaluation of RFA as a treatment for locally advanced pancreatic ductal adenocarcinoma, in order of evaluation but not of importance, is to determine the feasibility of the procedure. Nowadays, only few studies that are mainly concerned with the feasibility and complications of the procedure have been reported[12,13]. The second aim is to establish the effect of RFA on tumoral mass in terms of necrosis and cytoreduction. This is the same endpoint of radiotherapy, but with a possible better effect in terms of extent and type of tumor necrosis for RFA, even if performed during open surgery. The most important aim, third in order of evaluation, is the improvement of quality of life and survival rate.
RFA of pancreatic neoplasms is restricted to locally advanced, non-resectable but non-metastatic tumors. The resectability of a lesion represents an absolute exclusion criterion, because surgical resection is the treatment of choice. The presence of metastatic spread again represents an exclusion criterion. At preoperative imaging, the eligibility is related to the presence of a locally advanced, non-resectable pancreatic solid mass in the absence of any sign of metastatic spread, including ascites.
The anatomical complexity of the pancreatic and peri-pancreatic regions in which the pancreatic ductal adenocarcinoma grows makes the procedure of RFA different from that in other regions. In fact, independent from tumor size, the necrotic area must not overcome the lesion owing to the required safety margins in respect to the contiguous main vascular and digestive structures. Therefore, RFA has become a palliative treatment and could be included in a combined therapeutic plan.
The indications depend on the different clinical presentations related to the site of the obstructive or non-obstructive tumors. Non-obstructive tumors are usually located in the body/tail of the pancreas. These neoplasms do not need immediate surgical treatment and neoadjuvant chemotherapy represents the first choice. The absence of distant metastases and a good local response to therapy are expected at the post-treatment restaging evaluation. Resectable masses are treated surgically, whereas RFA can be performed in cases of non-resectable tumor. Obstructive tumors are usually located in the pancreatic head and frequently cause obstructive jaundice that promptly requires endoprosthesis positioning or derivative treatment and subsequent neoadjuvant chemotherapy. The presence of a resectable mass leads to surgical treatment, whereas RFA can be performed in cases of inveterate non-resectable tumor.
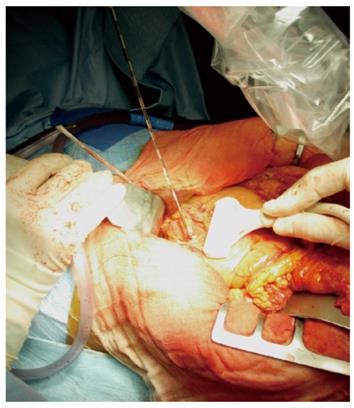
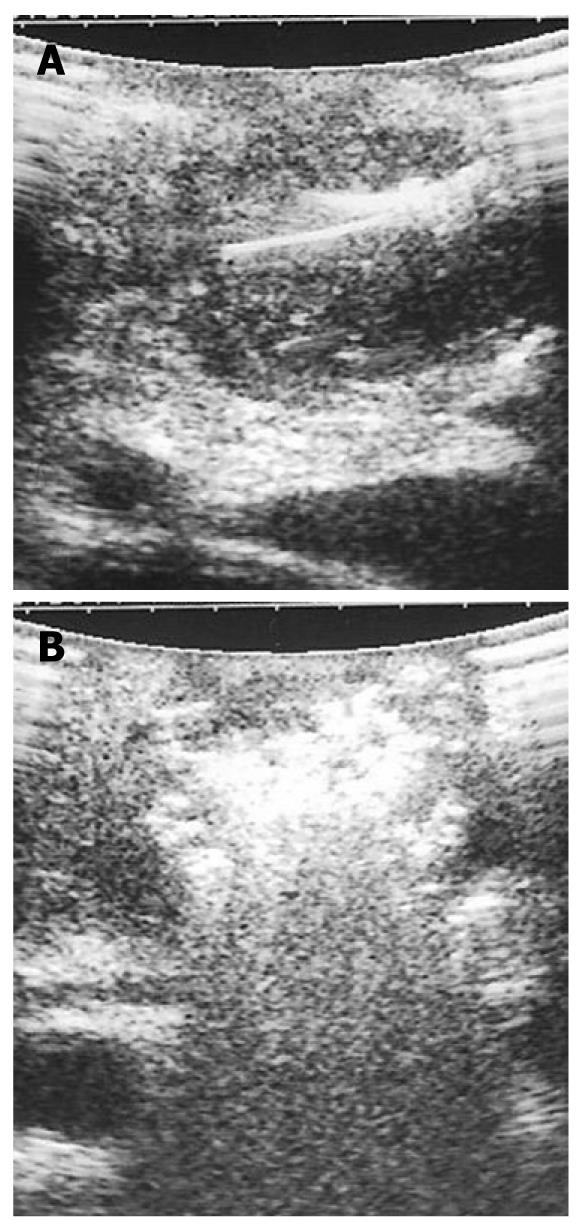
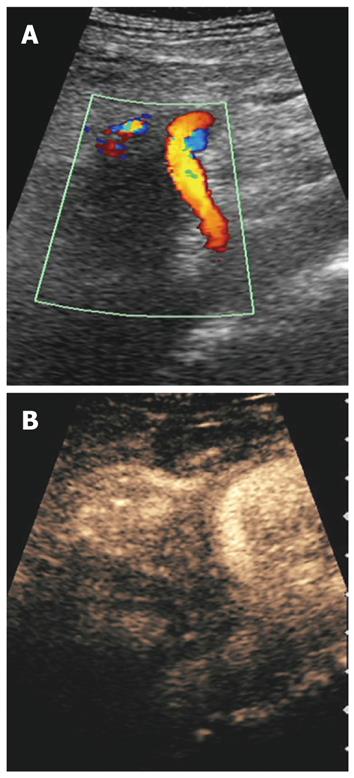
RFA of pancreatic tumors is nowadays an ultrasound-guided procedure that is performed during laparotomy in open surgery (Figure 1). Intraoperative ultrasound (IOUS) covers the mandatory role of staging, evaluation of feasibility, guidance and monitoring of the procedure (Figure 2).
The malignant nature of the lesion has to be confirmed pathologically before the procedure. Then, pre-surgical imaging staging needs confirmation. In particular, metastatic spread in the peritoneum (under inspection and extemporary histology of suspicious lesions, eventually present) and liver (under IOUS with extemporary cytology of doubtful lesions, eventually present) must be excluded. RFA will not be performed if the lesion is found resectable at IOUS, or if metastatic liver lesions are detected. The occurrence of very small lesions or those that envelop the main vessels without a true mass is a contraindication for the procedure. However, the possibility of RFA must be at first evaluated at preoperative imaging.
IOUS has to confirm the safety and feasibility of the RFA procedure without any risk of damage to the contiguous vascular and digestive structures, especially the duodenum. Tumor shape and diameters technically influence the procedure (approach, choice of needle and opening of the electrodes).
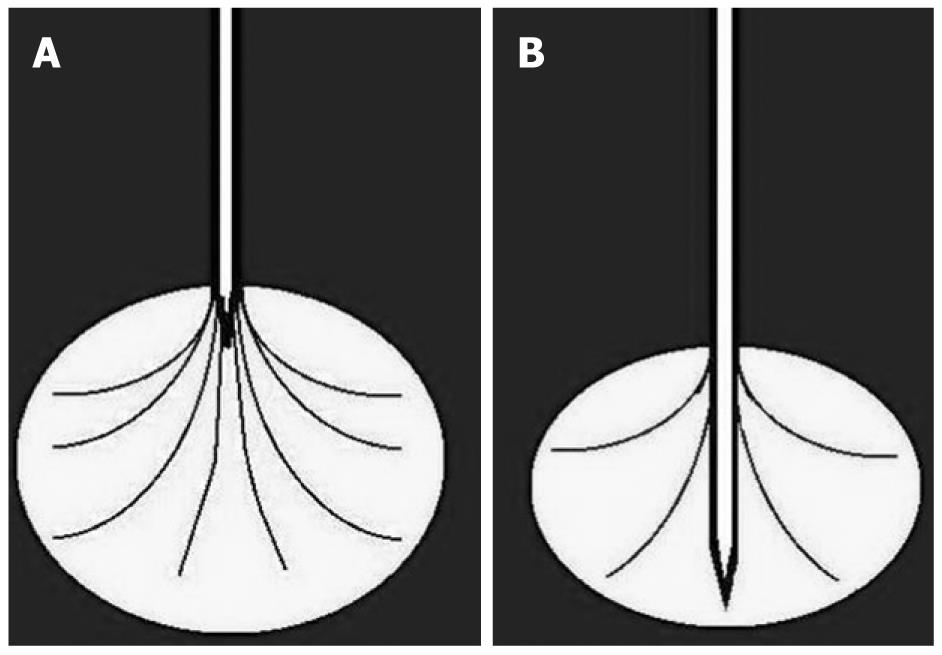
Two main types of needle are now available. In a needle with expandable electrodes, the electrodes can be opened from the top (Figure 3A) or the back (Figure 3B) of the needle. In the first case, the tip of the needle has to stop immediately prior to the lesion and electrodes (up to nine) open up and widen into the lesion. It is also possible to treat small tumors because the device can be opened at different degrees in relation to the size of the requested ablation zone. However, the flexibility of the electrode is not a negligible disadvantage in the treatment of very hard tumors. In the second case, by using a needle with electrodes coming from the back during the opening procedure, the electrodes (usually four) open up at about 2 cm behind the tip and move towards the outside. After complete opening, the electrodes arrive at the same level as the central needle tip. As a consequence, for correct positioning, the needle has to pass completely through the lesion, and the presence of the central needle for at least 2 cm within the mass assures the electrodes enter the neoplastic tissue. The central needle crossing throughout the mass and the stiffer electrodes guarantee excellent stability, even in the presence of hard tumor. On the other hand, the required introduction of the needle throughout the lesion for at least 2 cm can represent a disadvantage with respect to the dimensions and location of the tumor.
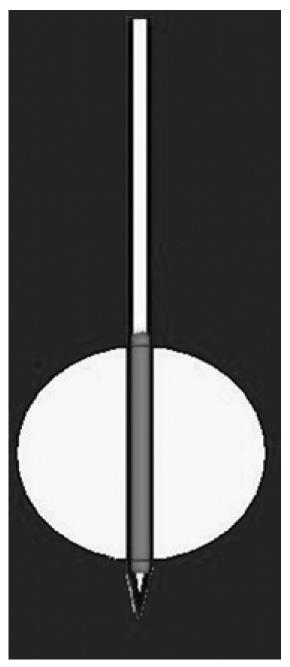
In a needle with a single electrode (Figure 4), the length of the area that has to be treated depends on the uncovered portion of the electrode. The width of the treated area depends on both the length of the uncovered portion and the time of ablation. The first type of needle produces a spherical/ovoid necrotic area, with a diameter ranging from 2 to 5-6 cm, depending on the needle and the electrode opening. The second type produces a cylindrical necrotic area ranging from 1 to 3 cm, depending on the extension of the uncovered metallic portion of the needle.
As reported above, the shape and dimension of the tumor influence the choice of the needle. For example, in the case of rounded lesions, even if located in the head of the pancreas, the first type have to be chosen, whereas, to treat ovoid lesions, the second type of needle is preferred. The latter is also preferable for very small lesions with particularly difficult access and/or location; choosing the needle with a lower caliper (17 rather than 14 gauge) and taking advantage of the cool tip for safer placement.
After laparotomy, the gastrocolic ligament is divided to access the pancreas. For most tumors localized in the pancreatic head, after Kocher maneuver a cold wet gauze is placed over the inferior vena cava to protect it from heat, to reduce maximally the risk of complications. The duodenum is continuously perfused with cold saline solution through a nasogastric tube. Simultaneously, instillation of cool water to the areas around the tumor has to be performed during ablation.
IOUS guides the ablation procedure, mainly during the needle positioning and opening of the electrodes into the lesion (Figure 2A). During treatment, the tip of the needle and the electrodes must be kept at almost 5 mm from the sensitive structures such as the duodenum and peri-pancreatic vessels, as previously reported[13-15]. The correct needle positioning and electrode opening are followed by setting of the parameters for the procedure. These parameters differ depending on the system used. The time setting usually ranges from 5 to 10 min. The power supply affects the temperature directly, and the treated volume indirectly. Some systems also allow evaluation of impedance, which increases with the development of necrosis during the procedure. The temperature at the tip of each electrode can be monitored, thus assuring a more uniform distribution of the temperature in the mass. The temperature setting depends on the treatment aims. Since protein denaturation begins at 50-60°C, the higher temperatures used during the procedure achieve homogeneous necrosis. On the other hand, the use of too high temperatures (105°C) increases the risk of complications, without a favorable effect. Hence, during the ablation procedure of a pancreatic mass, middle-range temperatures are usually applied (90°C). During the procedure, monitored with ultrasound, the tumor gradually becomes hyperechoic owing to the gas produced inside the treated lesion (Figure 2B). This sign can be used to confirm the radiofrequency effect that monitors the integrity of the sensitive surrounding structures, as described previously[10]. At the end of the procedure, the electrodes have to be closed and the needle removed.
The post-treatment IOUS evaluation has to assess the volume of the resultant hyperechoic treated area. The presence of incomplete necrosis with a significant hypoechoic neoplastic remnant can be treated again. When technically possible, a biliary and gastric bypass is required for pancreatic head tumors[12]. In particular, Siriwardena et al[16] have recommended that no patient should undergo laparotomy simply for ablation, but the procedure should be used only in patients in whom palliative bypass is required or non-resectable disease is found at surgery. As a consequence, intraoperative RFA of tumor of the pancreatic body, which is non-resectable at imaging, seems unacceptable at this time, but it could be justifiable if positive survival results in large populations are found.
Moreover, in the future, endoscopic or percutaneous approaches for ablation of these tumors, under local anesthesia with sedation, are expected, as occurs in other regions.
Only a few studies have focused on the feasibility and complications of RFA, and none of these have reported major intraoperative complications[12,13,16,17]. Hadjicostas et al[17], based on results obtained in four patients, have concluded that RFA seems to be a feasible, potentially safe and promising option in patients with advanced and non-resectable pancreatic cancer. Girelli et al[12], based on results obtained in 50 patients, showed that RFA of locally advanced pancreatic cancer is feasible and relatively well tolerated, with a 24% complication rate. In a previously published series of 16 patients, however, a mortality rate of 25% was reported, with all deaths in patients treated for pancreatic head tumor and complicated with massive gastrointestinal hemorrhage[13]. However, the temperature applied exceeded 90°C and no protective/refrigeration practices were utilized. On the contrary, in the Girelli series in which the procedure was performed with protective/refrigeration practices, the reported mortality rate was 2%. Moreover, in the same series in the second part of the study, by using a temperature of 90°C, the complication rate decreased to 8%[12].
Postoperative observation (clinical surveillance, laboratory tests and imaging studies) is mandatory because of the potential for major or minor, early or later complications. The most frequent complications encountered in the earlier postoperative period (within 1 wk) are fluid collection, pancreatic fistula, duodenal perforation and vascular damage. At later times, digestive or abdominal bleeding, infections or abscesses are more common. Severe acute pancreatitis is a rare complication[17]: in Girelli’s study, there was only one case, and none was reported in Wu’s study[12,13]. Major complications frequently are present with RFA of pancreatic head tumors, mainly owing to the closeness of the duodenum. These lesions are more difficult to treat, as reported previously[13].
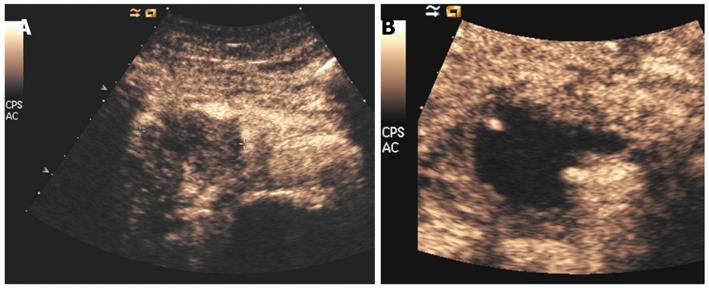
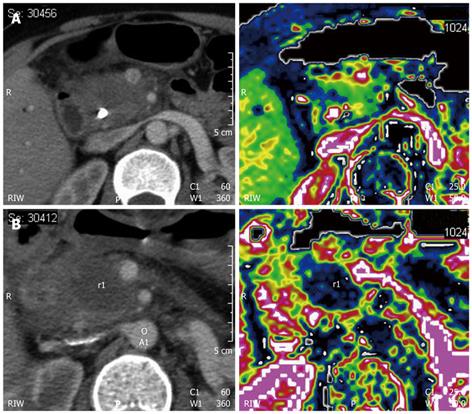
The necrotic area obtained by RFA is complete. In comparison with radiotherapy (Figure 5), the RFA-treated area (Figure 6) is completely avascular at perfusion imaging. Immediate ultrasound evaluation is useful for identification of possible fluid collections. In the presence of clinical suspicion of major complications, CT is mandatory. Dynamic studies after the administration of contrast agents, usually starting 1 mo after the procedure, are performed to detect the intratumoral necrotic area produced by RFA.
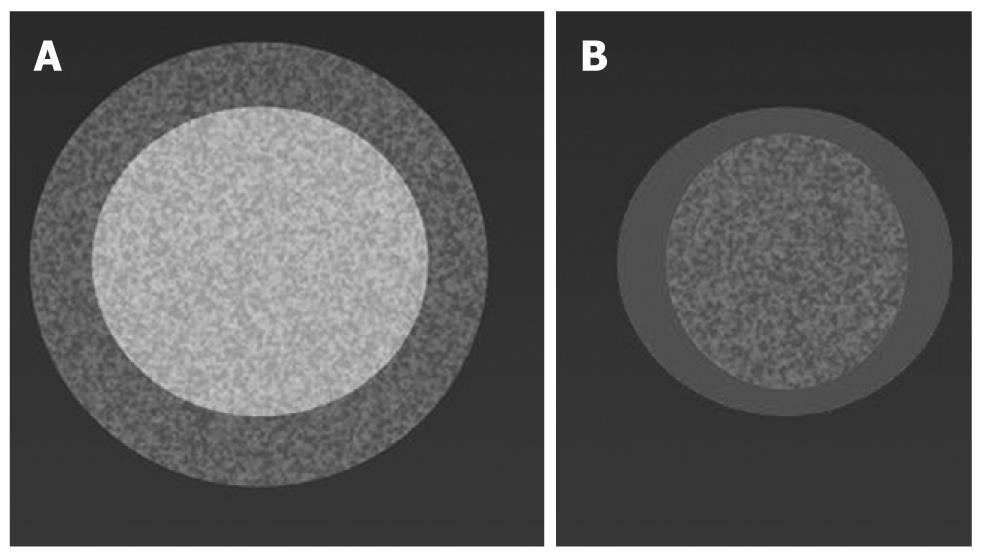
Postoperative imaging of RFA of the pancreas differs from that of the liver. With hepatocellular carcinoma, detection of the ablated area after treatment is immediate because of the hypervascular nature of the tumor. On the contrary, ductal adenocarcinoma is markedly hypovascular, such that after the procedure, the identification of the necrotic area with respect to residual viable tumor tissue can be difficult (Figure 7). It is more important to assess the type and extent of the post-ablation necrotic area, rather than the presence of remnant tumor. In treating pancreatic tumors, the presence of residual viable tumor at the periphery of the treated area is an intrinsic aspect of the procedure. This is the other important difference in comparison with RFA of the liver, in which the lesion must be covered by the necrotic area. On the contrary, in RFA of the pancreas, the necrotic area must be included in the tumor (Figure 8).
All these particular features of pancreatic RFA have to be considered during postoperative imaging. Precise and accurate dynamic imaging evaluation, such as contrast-enhanced US or perfusion CT (Figures 6 and 7), is necessary.
RFA of locally advanced pancreatic neoplasms is currently performed under ultrasound guidance in open surgery during laparotomy, with the palliative aim of tumor reduction in a combined therapeutic plan. Minimally invasive laparoscopic or percutaneous approaches, as in other regions, are expected.
The primary endpoint, represented by improvement in quality of life, has been achieved, given that the treatments obtain excellent pain relief[12,13,17]. In particular, in the series of Wu et al[13], pain relief was reported in 50% of cases, and in 68% in the series of Girelli[12]. Pain relief seems not to be related to ablation necrotic volume. Girelli et al[12] have reported a decreased of CA 19-9 concentration 7 d after surgery.
Regarding the more ambitious clinical endpoint, significant results about improved survival are still missing. At this time, only one study has investigated survival in a group of 25 consecutive patients, which showed a significant difference in survival between RFA and control groups in patients with stage III disease[18]. Further studies are needed to validate the reported preliminary results, to evaluate a possible correlation with tumor markers eventually expressed during the preoperative period, and particularly, to validate the technique.
RFA of locally advanced, non-resectable but non-metastatic, pancreatic tumors is a feasible palliative treatment that leads to tumor reduction and improved quality of life. Further studies are needed to validate therapeutic strategies and associations for the best possible results on survival.
Peer reviewers: Toru Ishikawa, MD, Department of Gastroenterology, Saiseikai Niigata Second Hospital, Teraji 280-7, Niigata, Niigata 950-1104, Japan; Patrick Veit-Haibach, MD, Department of Diagnostic and Interventional Radiology and Neuroradiology, University Hospital Essen, Hufelandstrasse 55, Essen 45122, Germany
S- Editor Tian L L- Editor Kerr C E- Editor Lin YP
| 1. | Schima W, Ba-Ssalamah A, Kölblinger C, Kulinna-Cosentini C, Puespoek A, Götzinger P. Pancreatic adenocarcinoma. Eur Radiol. 2007;17:638-649. [Cited in This Article: ] |
| 2. | Cubilla AL, Fitzgerald PJ. Tumors of the exocrine pancreas. 2nd series, fascicle 19. Washington, DC: Armed Forces Institute of Pathology 1984; 98-108. [Cited in This Article: ] |
| 3. | O'Connor TP, Wade TP, Sunwoo YC, Reimers HJ, Palmer DC, Silverberg AB, Johnson FE. Small cell undifferentiated carcinoma of the pancreas. Report of a patient with tumor marker studies. Cancer. 1992;70:1514-1519. [Cited in This Article: ] |
| 4. | Cameron JL. American Cancer Society Atlas of Clinical Oncology: Pancreatic Cancer. Hamilton, London: BC Decker 2001; . [Cited in This Article: ] |
| 5. | D'Onofrio M, Zamboni GA, Malagò R, Mantovani W, Principe F, Gallotti A, Faccioli N, Falconi M, Capelli P, Mucelli RP. Resectable pancreatic adenocarcinoma: is the enhancement pattern at contrast-enhanced ultrasonography a pre-operative prognostic factor? Ultrasound Med Biol. 2009;35:1929-1937. [Cited in This Article: ] |
| 6. | Hermanek P. Staging of exocrine pancreatic carcinoma. Eur J Surg Oncol. 1991;17:167-172. [Cited in This Article: ] |
| 7. | Sahani DV, Shah ZK, Catalano OA, Boland GW, Brugge WR. Radiology of pancreatic adenocarcinoma: current status of imaging. J Gastroenterol Hepatol. 2008;23:23-33. [Cited in This Article: ] |
| 8. | Nagakawa T, Mori K, Nakano T, Kadoya M, Kobayashi H, Akiyama T, Kayahara M, Ohta T, Ueno K, Higashino Y. Perineural invasion of carcinoma of the pancreas and biliary tract. Br J Surg. 1993;80:619-621. [Cited in This Article: ] |
| 9. | Drapiewski JF. Carcinoma of the pancreas: a study of neoplastic invasion of nerves and its possible clinical significance. Am J Clin Pathol. 1944;14:549-556. [Cited in This Article: ] |
| 10. | Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD 3rd, Dupuy DE, Gervais DA, Gillams AR, Kane RA, Lee FT Jr. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009;20:S377-S390. [Cited in This Article: ] |
| 11. | Livraghi T. Single HCC smaller than 2 cm: surgery or ablation: Interventional oncologist's perspective. J Hepatobiliary Pancreat Surg. 2009;Epub ahead of print. [Cited in This Article: ] |
| 12. | Girelli R, Frigerio I, Salvia R, Barbi E, Tinazzi Martini P, Bassi C. Feasibility and safety of radiofrequency ablation for locally advanced pancreatic cancer. Br J Surg. 2010;97:220-225. [Cited in This Article: ] |
| 13. | Wu Y, Tang Z, Fang H, Gao S, Chen J, Wang Y, Yan H. High operative risk of cool-tip radiofrequency ablation for unresectable pancreatic head cancer. J Surg Oncol. 2006;94:392-395. [Cited in This Article: ] |
| 14. | Ng KK, Lam CM, Poon RT, Fan ST. Portal vein thrombosis after radiofrequency ablation for recurrent hepatocellular carcinoma. Asian J Surg. 2003;26:50-53; discussion 54. [Cited in This Article: ] |
| 15. | Ng KK, Lam CM, Poon RT, Shek TW, Fan ST, Wong J. Delayed portal vein thrombosis after experimental radiofrequency ablation near the main portal vein. Br J Surg. 2004;91:632-639. [Cited in This Article: ] |
| 16. | Siriwardena AK. Radiofrequency ablation for locally advanced cancer of the pancreas. JOP. 2006;7:1-4. [Cited in This Article: ] |
| 17. | Hadjicostas P, Malakounides N, Varianos C, Kitiris E, Lerni F, Symeonides P. Radiofrequency ablation in pancreatic cancer. HPB (Oxford). 2006;8:61-64. [Cited in This Article: ] |
| 18. | Spiliotis JD, Datsis AC, Michalopoulos NV, Kekelos SP, Vaxevanidou A, Rogdakis AG, Christopoulou AN. Radiofrequency ablation combined with palliative surgery may prolong survival of patients with advanced cancer of the pancreas. Langenbecks Arch Surg. 2007;392:55-60. [Cited in This Article: ] |