Published online Sep 28, 2010. doi: 10.3748/wjg.v16.i36.4616
Revised: August 3, 2010
Accepted: August 10, 2010
Published online: September 28, 2010
AIM: To study the clinicopathological features of gastric glomus tumor and review the related Chinese literature published in 1990-2010.
METHODS: A case of gastric glomus tumor was reported. Clinicopathological findings in 56 cases of gastric glomus tumor were analyzed.
RESULTS: Gastric glomus tumor was far more common in women than in men with a female to male ratio of 1.6:1. The median age of the patients was 45 years (range 28-79 years). The patients often complained of epigastric pain and bloody stool. The tumor was located in antrum of the stomach. The greatest diameter of the tumor was 0.8-11cm. Histologically, the tumor was comprised of nests of glomus cells surrounding the capillaries. Glomus cells were small, uniform and round. Vimentin, smooth muscle actin and actin were expressed in the tumor. Other markers, including S-100 protein, CD34, CD117, desmin, CD56, synaptophysin, chromogranin A, neuron specific enolase and cytokeratin were all negative.
CONCLUSION: Gastric glomus tumor is a rare benign mesenchymal neoplasm. Its diagnosis depends on pathologic examination. Differential diagnosis includes gastrointestinal stromal tumor, paraganglioma and carcinoid tumor.
- Citation: Fang HQ, Yang J, Zhang FF, Cui Y, Han AJ. Clinicopathological features of gastric glomus tumor. World J Gastroenterol 2010; 16(36): 4616-4620
- URL: https://www.wjgnet.com/1007-9327/full/v16/i36/4616.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i36.4616
Gastric glomus tumor is a rare benign mesenchymal neoplasm arising from the glomus body. The majority of glomus tumors occur in the distal extremities, particularly in the subungual region, hand, wrist, foot, bone and joints, skeletal muscle, soft tissue, mediastinum, trachea, kidney, lung, uterus and vagina. Since the first case of gastric glomus tumor was reported in 1951 by Kay et al[1], few cases have been reported[2-13]. The clinicopathological features of 56 cases of gastric glomus tumor were studied with a review of the related Chinese literature published in 1990-2010.
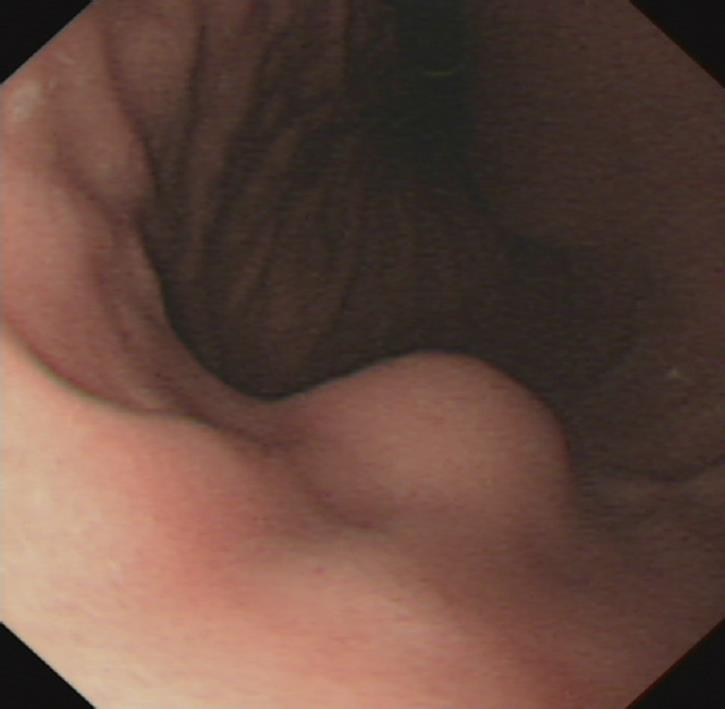
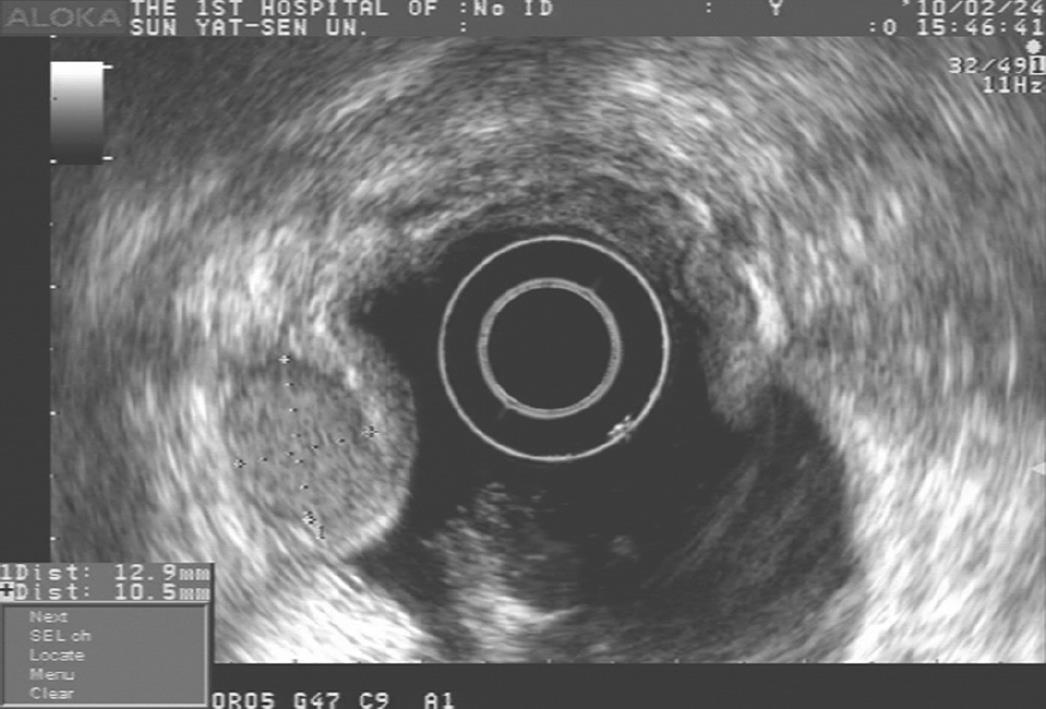
A 60-year-old woman was admitted to our institution due to a gastric mass found at upper gastrointestinal endoscopy. Endoscopy demonstrated a well-circumscribed elevated mass measuring 1.5 cm × 1.5 cm with normal overlying mucosa in the anterior wall of gastric corpus (Figure 1). Endoscopic ultrasonography showed a sharply demarcated homogeneous hypoechoic mass measuring 1.5 cm × 1.2 cm in the third and fourth sonographic layers of gastric wall with rich blood supply (Figure 2). The patient underwent wedge resection of the tumor.
Resected specimens were fixed in 4% formaldehyde and embedded in paraffin. Tissue block was cut into 4-micrometer thick sections which were stained with routine staining methods.
Immunohistochemical staining of the formalin-fixed and paraffin-embedded tissue was carried out using an EnVison kit (DAKO). Primary antibodies including vimentin, smooth muscle actin (SMA), actin, CD117, CD34, S-100 protein, desmin, CD56, synaptophysin, chromogranin A, neurone specific enolase and cytokeratin were purchased from the DAKO Corporation.
Clinical, imaging and pathological features of 56 patients with gastric glomus tumor were analyzed according to a review of related Chinese literature published in 1990-2010.
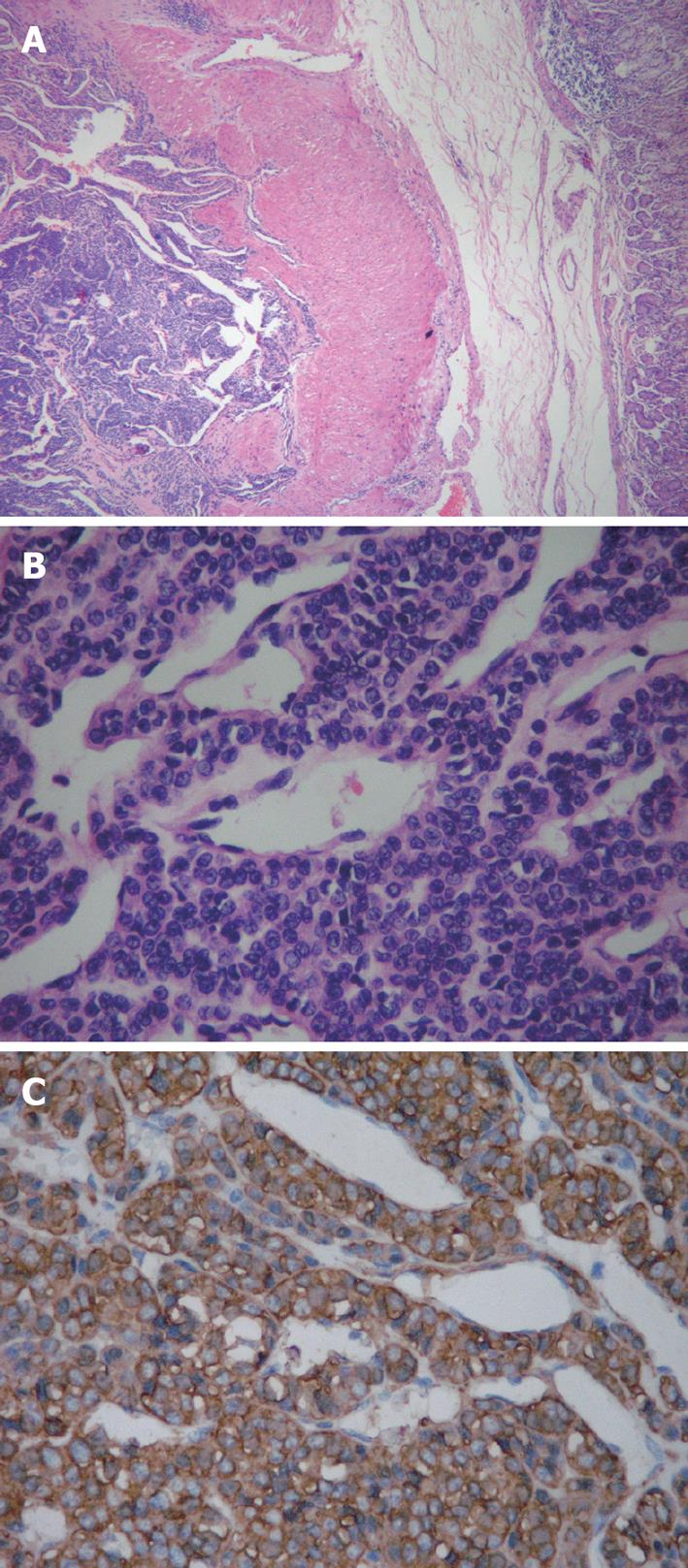
In our case, the cut surface of specimens demonstrated a grayish-white, circumscribed mass measuring 1 cm × 1 cm arising from the muscularis of stomach without involving the serosal surface. Histologically, the tumor was mainly located in the muscularis of stomach and comprised of small, uniform, rounded cells surrounding capillaries with diffuse sheet distributions but without nuclear pleomorphism and mitotic figures (Figure 3). Immunohistochemical staining was positive for vimentin, SMA and actin (Figure 3). Other markers, including S-100 protein, CD34, CD117, Desmin, CD56, synaptophysin, chromogranin A, neurone specific enolase and cytokeratin were all negative. A final diagnosis of gastric glomus tumor was made based on the typical histopathology and immunohistochemical staining.
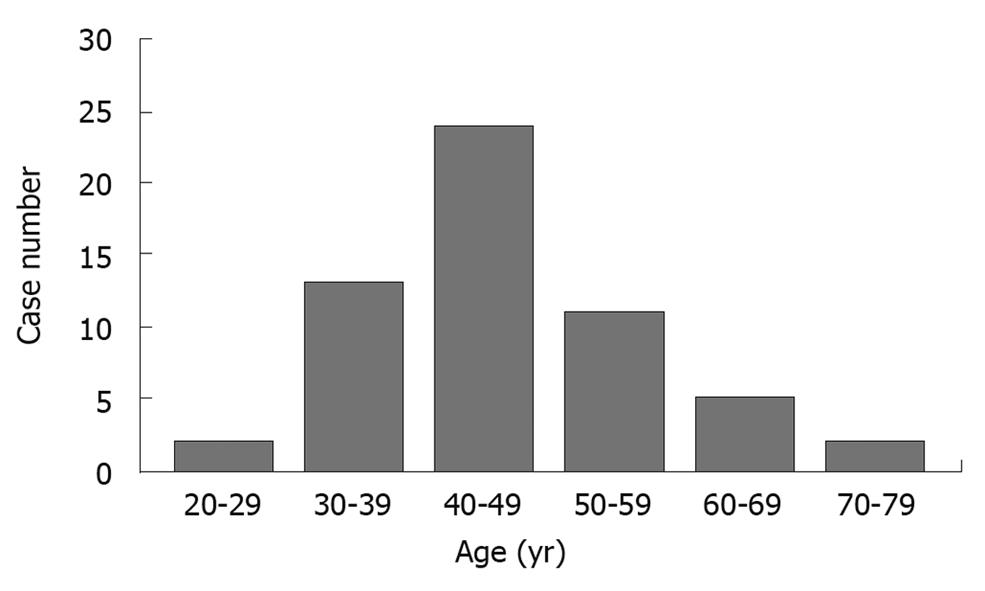
Of the 57 patients with gastric glomus tumor including our case, 35 were females (64.1%), 22 were males (35.9%) with a female to male ratio of 1.6:1. The median age of the patients was 45 years (range 28-79 years) (Figure 4). Of the 57 patients, 35 complained of upper abdominal pain or fullness (61.4%), 14 had bloody stools (24.6%) and 8 were asymptomatic (14%). The course of the disease ranged 1 wk-over 10 years. Gastric glomus tumor was found in gastric antrum of 53 patients, in gastric corpus of 3 patients, and in junction of gastric antrum-corpus of 1 patient, respectively (Table 1).
| n (%) | |
| Sex | |
| Male | 22 (38.6) |
| Female | 35 (61.4) |
| Symptoms | |
| Epigastric pain | 35 (61.4) |
| Bloody stool | 14 (24.6) |
| Asymptomatic | 8 (14.0) |
| Location | |
| Antrum | 53 (93.0) |
| Corpus | 3 (5.3) |
| Junction of antrum-corpus | 1 (1.7) |
| Histopathological subtype | |
| Solid glomus tumor | 54 (94.7) |
| Glomangioma | 3 (5.3) |
| Glomangiomyoma | 0 (0) |
Of the 57 patients with gastric glomus tumors, 8 had barium meal X-ray examination, which showed a circular or semi-circular filling defect with smooth surface and a broad base on the gastric angle. The abdominal sonogram revealed a sharply demarcated mass with a homogeneous hypoechoic pattern of gastric wall in 12 patients and a heterogeneous hypoechoic pattern with internal hyperechoic spots in a few patients. Endoscopic ultrasonography showed a hyperechoic mass in gastric antrum of 1 patient. However, endoscopic ultrasonography showed a sharply demarcated, homogeneous hypoechoic mass in the third and fourth sonographic layers of gastric wall with rich blood supply in our patient. Fifty patients underwent gastroscopy, showing round or oval elevated lesions with smooth surface on gastric mucosa of most patients and erosion or ulceration with blood clot attached in a few patients. Abdominal computed tomography scan revealed a demarcated soft tissue enhancing mass in the stomach of 16 patients, suggesting of a hypervascular benign gastric tumor.
Macroscopically, the greatest diameter of the tumor was 0.8-11 cm. On cut surface, the tumor was firm and solid or cystic, and gray or grayish-red or grayish-white, or dark brown in color.
Histologically, the tumor was well-circumscribed and located in gastric submucosa or muscularis and comprised of glomus cells surrounding capillaries. The glomus cells were small, uniform, and round without nuclear pleomorphism, mitotic figures or necrosis. The stroma showed hyalinization or myxoid change in some patients and sporadic mast cells could be seen in the stroma.
Immunohistochemical staining showed that gastric glomus tumor was strongly and diffusely positive for SMA (22/22), vimentin (18/18) and actin (10/10), calponin (5/7), type IV collagen (2/2) and laminin (1/1). Other markers, including desmin, CD31, CD34, CD99, CD117, cytokeratin, S-100 protein, synaptophysin, chromogranin A, and neuron specific enolase were negative.
According to the World Health Organization (WHO) Classification of Tumors of Soft Tissue and Bone (2002)[14], glomus tumour can be further divided into “solid glomus tumor”, “glomangioma”, and “glomangiomyoma” depending on the relative prominence of glomus cells, vascular structure and smooth muscle. According to the pathological features of 57 patients reported in Chinese literature including our patient, 54 patients had “solid glomus tumor” and 3 had “glomangioma”.
Of the 57 patients with gastric glomus tumor, 18 underwent subtotal gastrectomy, 36 gastric wedge resection of the tumor and 3 tumor excision. Follow-up information was available from 15 patients who were alive without recurrence or metastasis 1-7 years after surgery.
Gastric glomus tumor is a rare benign mesenchymal tumor arising from the glomus body. Its clinical features are nonspecific. Patients often complain of epigastric pain and bloody stool. We reviewed 57 cases including our case reported in Chinese literature in 1990-2010. Gastric glomus tumor is far more common in women than in men with a female to male ratio of 1.6:1. The median age of the patients was 45 years (range 28-79 years). Most of the tumors were located in gastric antrum. The greatest diameter of tumor was 0.8-11 cm, which is much larger than that in the distal extremities. The tumor is usually solitary but cases of multiple gastric glomus tumors have been reported[15-17].
The tumor can be further divided into “solid glomus tumor”, “glomangioma”, and “glomangiomyoma” depending on the relative prominence of glomus cells, vascular structure and smooth muscle[14]. According to the review of the pathological features of 57 patients including our case, 54 patients (94.7%) had “solid glomus tumor” and 3 (5.3%) had “glomangioma”. Although gastric glomus tumor is usually benign, malignant behavior cannot be excluded. According to the WHO Classification of Tumors of Soft Tissue and Bone[14], “glomus tumor” should be defined as a malignant tumor when its size is > 2 cm and located at subfascia or viscera, with atypical mitotic figures or marked nuclear atypia and mitotic activity. However, no criteria are available for the diagnosis of gastric malignant glomus tumor. Several cases of malignant or metastatic gastric glomus tumor have been reported[11,18-20]. It seems that the biological behavior, especially metastasis, is more valuable for the diagnosis of malignant gastric glomus tumor.
Of the 57 patients with gastric glomus tumor, 18 underwent subtotal gastrectomy, 36 wedge resection of the tumor, and 3 tumor excision depending on the location and size of the tumor. Wedge resection with negative margins was the major treatment of choice. Follow-up information was available from 15 patients who were alive without recurrence or metastasis 1-7 years after surgery.
Gastric glomus tumor should be differentiated from other lesions, such as gastrointestinal stromal tumor (GIST), paraganglioma and carcinoid tumor, etc. GIST is the most common mesenchymal tumor in the stomach. Tumor often shows a variety of histological patterns, including the epithelioid pattern which may be confused with glomus tumor. However, GIST usually lacks of dilated capillaries and tumor cells are positive for CD117 and CD34, which is different from glomus tumor[21]. Paraganglioma cells are arranged in a characteristic alveolar or Zellballen pattern with rich thin-walled blood vessels in stroma which can easily be confused with glomus tumor. However, paraganglioma is comprised of large cells with nuclear enlargement, hyperchromatism and cytoplasm varying from pink to clear in color, or amphophilic and positive for S-100 protein, synaptophysin, chromogranin A, and neurone specific enolase. Carcinoid tumor is a neuroendocrine carcinoma and comprised of oval or spindle tumor cells arranged in cords or nests with thin-walled blood vessels. Carcinoid tumor cells are positive for cytokeratin, S-100 protein, synaptophysin, chromogranin A, and neurone specific enolase, but negative for SMA.
In conclusion, Gastric glomus tumor is rare benign mesenchymal neoplasm. Since patients have no specific clinical and imaging findings, it is difficult to diagnose before operation. Histopathological features of small, uniform and round tumor cells surrounding capillaries and strongly positive for SMA, are helpful to make its diagnosis. The differential diagnosis includes gastrointestinal stromal tumor, paraganglioma and carcinoid tumor.
Gastric glomus tumor is an extremely rare mesenchymal tumor. Few cases have been reported in the literature. Its clinicopathological features are unclear.
In this study, the authors reported 1 case of gastric glomus tumor and reviewed 56 cases reported in Chinese literature in 1990-2010.
This is the first study to analyze the clinicopathological findings of gastric glomus tumor in large series.
This study provided important information for the diagnosis and treatment of gastric glomus tumor.
Gastric glomus tumor is a rare benign mesenchymal neoplasm. Histopathological features of small, uniform and rounded tumor cells surrounding capillaries and strongly positive for smooth muscle actin, are helpful to make its diagnosis. Differential diagnosis includes gastrointestinal stromal tumor, paraganglioma and carcinoid tumor.
This is an interesting paper and provides important information for the diagnosis and treatment of gastric glomus tumor.
Peer reviewer: Jai Dev Wig, MS, FRCS, Former Professor and Head, Department of General Surgery, Postgraduate Institute of Medical Education and Research, Chandigarh 160012, India
S- Editor Wang YR L- Editor Wang XL E- Editor Lin YP
| 1. | Kay S, Callahan WP Jr, Murray MR, Randall HT, Stout AP. Glomus tumors of the stomach. Cancer. 1951;4:726-736. [Cited in This Article: ] |
| 2. | Vassiliou I, Tympa A, Theodosopoulos T, Dafnios N, Fragulidis G, Koureas A, Kairi E. Gastric glomus tumor: a case report. World J Surg Oncol. 2010;8:19. [Cited in This Article: ] |
| 3. | Patel TH, Horton KM, Hruban RH, Fishman EK. Glomus Tumor of the Stomach: Depiction by Multidetector CT and Three-Dimensional Volume Rendering Imaging. Case Report Med. 2010;2010:126095. [Cited in This Article: ] |
| 4. | Chou KC, Yang CW, Yen HH. Rare gastric glomus tumor causing upper gastrointestinal bleeding, with review of the endoscopic ultrasound features. Endoscopy. 2010;42 Suppl 2:E58-E59. [Cited in This Article: ] |
| 5. | Chou HP, Tiu CM, Chen JD, Chou YH. Glomus tumor in the stomach. Abdom Imaging. 2010;35:390-392. [Cited in This Article: ] |
| 6. | Batra RB, Mehta A, Rama Mohan PV, Singh KJ. Glomus tumor of the stomach. Indian J Pathol Microbiol. 2009;52:77-79. [Cited in This Article: ] |
| 7. | Zissis D, Zizi-Serbetzoglou A, Glava C, Grammatoglou X, Katsamagkou E, Nikolaidou ME, Vasilakaki T. Glomus tumor of the stomach: a case report. J BUON. 2008;13:581-584. [Cited in This Article: ] |
| 8. | Yan SL, Yeh YH, Chen CH, Yang CC, Kuo CL, Wu HS. Gastric glomus tumor: a hypervascular submucosal tumor on power Doppler endosonography. J Clin Ultrasound. 2007;35:164-168. [Cited in This Article: ] |
| 9. | Lee HW, Lee JJ, Yang DH, Lee BH. A clinicopathologic study of glomus tumor of the stomach. J Clin Gastroenterol. 2006;40:717-720. [Cited in This Article: ] |
| 10. | Kapur U, Hobbs CM, McDermott E, Mooney EE. Gastric glomus tumor. Ann Diagn Pathol. 2004;8:32-35. [Cited in This Article: ] |
| 11. | Miettinen M, Paal E, Lasota J, Sobin LH. Gastrointestinal glomus tumors: a clinicopathologic, immunohistochemical, and molecular genetic study of 32 cases. Am J Surg Pathol. 2002;26:301-311. [Cited in This Article: ] |
| 12. | Gu M, Nguyen PT, Cao S, Lin F. Diagnosis of gastric glomus tumor by endoscopic ultrasound-guided fine needle aspiration biopsy. A case report with cytologic, histologic and immunohistochemical studies. Acta Cytol. 2002;46:560-566. [Cited in This Article: ] |
| 13. | Almagro UA, Schulte WJ, Norback DH, Turcotte JK. Glomus tumor of the stomach. Histologic and ultrastructural features. Am J Clin Pathol. 1981;75:415-419. [Cited in This Article: ] |
| 14. | Folpe AL. Glomus tumours. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon: IARC Press 2002; 136-137. [Cited in This Article: ] |
| 15. | Alempijevic T, Knezevic S, Knezevic D, Ostojic S, Stojakov D, Micev M, Tomic D, Krstic M. Gastric multicentric glomangioma: a case report of this rare cause of abdominal pain. Med Sci Monit. 2008;14:CS5-CS8. [Cited in This Article: ] |
| 16. | Haque S, Modlin IM, West AB. Multiple glomus tumors of the stomach with intravascular spread. Am J Surg Pathol. 1992;16:291-299. [Cited in This Article: ] |
| 17. | Nadkarni KM, Deshpande RB, Patel VC, Mody BB, Kinare SG, Bhalerao RA. Multicentric glomus tumour of the stomach (a case report). J Postgrad Med. 1982;28:118-120B. [Cited in This Article: ] |
| 18. | Bray AP, Wong NA, Narayan S. Cutaneous metastasis from gastric glomus tumour. Clin Exp Dermatol. 2009;34:e719-e721. [Cited in This Article: ] |
| 19. | Britvin AA, Zeĭnalov FA. [Malignant glomus tumor of the stomach (case report)]. Vopr Onkol. 1986;32:106-107. [Cited in This Article: ] |
| 20. | Rozenberg EE. [Malignant glomus tumor of the stomach (1 case)]. Vopr Onkol. 1986;32:100-102. [Cited in This Article: ] |
| 21. | Liegl-Atzwanger B, Fletcher JA, Fletcher CD. Gastrointestinal stromal tumors. Virchows Arch. 2010;456:111-127. [Cited in This Article: ] |