Published online Oct 28, 2010. doi: 10.3748/wjg.v16.i40.5122
Revised: July 15, 2010
Accepted: July 22, 2010
Published online: October 28, 2010
AIM: To investigate a therapeutic method for gastrointestinal stromal tumor (GIST) based on KIT RNA interference (RNAi) with AdMax adenovirus.
METHODS: KIT short hairpin RNA (shRNA), whose lateral sides were decorated with restriction endonuclease sequences, was designed. T4 DNA ligase catalyzed the joint of the KIT shRNA and the green fluorescent protein-containing PDC316-EGFP-U6 to form PDC316-EGFP-U6-KIT. Homologous recombination of AdEGFP-U6-KIT was performed with the AdMax system. Heterotopically transplanted GISTs were established in nude mice. AdEGFP-U6-KIT was intratumorally injected. The volume, inhibition ratio of tumor and CD117 expression of GIST graft tumor in nude mice were compared between test and control groups.
RESULTS: The length of KIT shRNA was determined to be about 50bp by agarose electrophoresis. Gene sequencing detected the designed KIT RNAi sequence in PDC316-EGFP-U6-KIT. After transfection with AdEGFP-U6-KIT, 293 cells displayed green fluorescence. The physical and infective titers of AdEGFP-U6-KIT were 5 × 1011 viral particles/mL and 5.67 × 107 plaque forming units/mL, respectively. The mean volume of the grafted tumor was significantly smaller in test mice than in control mice (75.3 ± 22.9 mm3vs 988.6 ± 30.5 mm3, t = -18.132, P < 0.05). The inhibition ratio of the tumors was 59.6% in the test group. CD117 positive expression was evident in two cases (20%) in the test group and 10 cases (100%) in the control group (χ2 = 10.2083, P < 0.005).
CONCLUSION: AdEGFP-U6-KIT is successfully constructed, and KIT RNAi mediated with Admax vector system can effectively inhibit the expression of the KIT gene and the growth of GIST in nude mice.
- Citation: Wang TB, Huang WS, Lin WH, Shi HP, Dong WG. Inhibition of KIT RNAi mediated with adenovirus in gastrointestinal stromal tumor xenograft. World J Gastroenterol 2010; 16(40): 5122-5129
- URL: https://www.wjgnet.com/1007-9327/full/v16/i40/5122.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i40.5122
Gastrointestinal stromal tumor (GIST) is a mesenchymal neoplasm and is the most prevalent gastrointestinal mesenchymal tumor. Its annual incidence is about 10-20 per million people[1-3]. However, it is difficult to give an exact incidence, since both the definition and classification of GIST are contentious[1]. GIST occurs in the stomach (50%-60%), small intestine (30%-40%), colon and rectum (5%-10%), and esophagus (5%)[1,2]. The gold standard therapy for GIST is complete resection, depending on the lesion size and location. It is unnecessary to dissect the lymph node since lymph node metastasis is rare. During surgery, tumor rupture must be avoided as it is the main factor resulting in post-operative recurrence. The 5-year survival rate for en bloc resection of GIST is 48%-65%[3]. In some cases, because of the anatomic site or the tumor size, only a partial resection can be performed. The two most important prognostic features of primary GIST are tumor size and mitotic index. GIST is an easily recurrent disease, which is found in the liver (65%), peritoneal surface (50%), or both (20%)[1,4]. Conventional chemotherapy is of little benefit for GIST because it is a non-epithelial neoplasm[1]. The use of Imatinib mesylate in advanced GIST produces a response in 50% of treated patients, and stabilizes the disease in 75%-85% of patients. The 2-year survival after Imatinib therapy is approximately 70%[5]. Imatinib therapy after 1 year is associated with a high risk of relapse[6]. Primary resistance to Imatinib affects about 15% of patients and 50% of patients become secondarily resistant by 2 years after Imatinib therapy[3,7]. Resistance to Imatinib is a major clinical problem, which has prompted the search for alternate drugs for Imatinib resistant cases.
The KIT proto-oncogene on chromosome 4 (4q11-q12) encodes the KIT protein, and appears to play an important role in the early stages of tumor formation as well as in late tumor progression. The sites of mutation are exon 11 (65%-70%), 9 (10%-20%), 13 (1%-2%) and 17 (< 1%)[8]. Inhibition of the KIT gene may block the formation and development of GIST. RNA interference (RNAi) is the most effective method to silence a target gene. RNAi can block KIT gene expression in GIST, while it is still uncertain if KIT RNAi can become an effective therapeutic method for GIST[9,10]. It was also reported that an adenovirus vector can effectively introduce RNAi to cancer gene therapy[11].
In this study, an adenovirus vector was successfully constructed to mediate KIT RNAi in GIST xenografts. The results demonstrated that the adenovirus system can silence efficiently KIT and inhibit the growth of grafted GIST. This approach may have therapeutic potential in GIST.
Plasmids pDC316-EGFP-U6 and pBHGlox-E1,3Cre, DH5α strain and HEK293 cells were purchased from Vector Gene Technology. Plasmid DNA extraction kit, RPMI1640, agarose, fetal bovine serum, and DMEM were purchased from Shanghai Sangon Biological Engineering Technology & Services. CD117 (mouse monoclonal IgG), T4 DNA ligase, HindIII, BamHI, and BgIII were purchased from New England Biolabs.
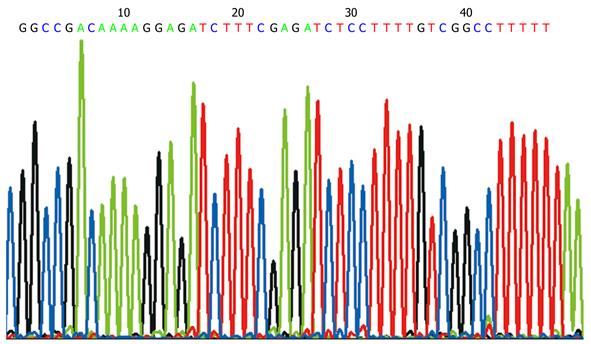
The sequence of KIT RNAi was previously reported as 5'-GGCCGACAAAAGGAGATCTTTCGAGATCTCCTTTTGTCGGCCTTTTT-3'[12]. These sense and antisense oligonucleotides were ligated to HindIII and BamHI and annealed to form double strains of DNA that designated KIT (B/H). Agarose gel electrophoresis was employed to identify the length of KIT (B/H).
Distilled deionized water (22 μL), 10 × K Buffer (5 μL), PDC316-EGFP-U6 (20 μL), BamHI (1.5 μL), and HindIII (1.5 μL) were added into 0.2 mL Eppendorf (EP) tube. The EP tube was incubated at 37°C overnight. One hundred microliters of solution BD was added to the tube, and then the solution was transferred to a DNA purification pillar. After 2 min, the pillar was centrifuged at 12 000 r/min at room temperature for 1 min. After removal of the filter liquor and the addition of 500 μL of solution PE, the pillar was centrifuged at 12 000 r/min at room temperature for 1 min. The step was repeated and the pillar was centrifuged at 12 000 r/min for 1 min. The pillar was put into a 1.5 mL EP tube, and 30 μL of 60°C sterile water was added into pillar. After centrifugation at 13 400 ×g for 1 min, PDC316-EGFP-U6 (B/H) solution was acquired and identified with agarose electrophoresis.
Distilled deionized water (2 μL), 10 × T4 DNA Ligation Buffer (1 μL), PDC316-EGFP-U6 (B/H) (3 μL), KIT (B/H) (3 μL), and T4 DNA Ligase (1 μL) were added to a 0.2 mL EP tube. The tube was incubated at 16°C for 2 h. The product was named PDC316-EGFP-U6-KIT.
Six microliters of PDC316-EGFP-U6-KIT was put into a 30 μL suspension of DH5α competent cells and rotated slightly for 30 min in an ice bath, prior to transfer to a 42°C water bath for 90 s and then to an ice bath for 2 min. Two hundred microliters of LB medium was added to the tube. The mixture was cultured with shaking (200 r/min) at 37°C for 1 h. The bacterial liquid was spread on a LB agar plate containing ampicillin (100 μg/mL). After absorption of liquid at room temperature, the agar was incubated overnight at 37°C.
Four colonies on the plate were collected and put separately into a tube containing 3 mL of LB, and cultivated in a rocking bed overnight at 37°C. The plasmid extraction kit was used to obtain plasmid DNA. Three microliters of bacterial liquid was put into a 1.5 mL EP tube, and centrifuged at 12 000 r/min for 1 min. After removal of the supernatant, 250 μL of solution I/Rnase A was added to suspend the bacteria. Then, 250 μL of solution II was inverted gently six times and placed at room temperature for 2 min. Two hundred and fifty microliters of solution III was then added to the tube and the contents were mixed by gentle inversion six times. After centrifugation at 12 000 r/min for 10 min, the supernatant was removed, added to a DNA purifying pillar, held for 2 min, centrifuged at 12 000 r/min for 1 min, and the filter liquor was removed. Five hundred microliters of PB solution was added to the pillar, the suspension was centrifuged at 12 000 r/min for 1 min, and the filter liquor was removed. Then, 500 μL of solution W was put into the pillar, centrifuged at 12 000 r/min for 1 min, and the filter liquor was removed. The step was repeated and the contents of the pillar were centrifuged at 12 000 r/min for 3 min. The pillar was put into a 1.5 mL EP tube and 50 μL of 60°C sterile water was added. After 2 min, the contents were centrifuged at 13 400 ×g for 1 min. The resulting solution contained PDC316-EGFP-U6-KIT. Distilled deionized water (4.6 μL), 10 × K Buffer (1 μL), PDC316-EGFP-U6-KIT (4 μL), and SalI (0.4 μL) were added to a 0.2 mL EP tube and incubated at 37°C for 2 h. Agarose gel electrophoresis was used to identify the recombinant plasmid.
PDC316-EGFP-U6-KIT sequencing was done by Life Technologies Corporation using ABI377DNA.
Transfection was performed according to the manufacturer’s instructions. Approximately 5 × 105 HEK293 cells were seeded in 60-cm plates 24 h before transfection, with a 80% confluency. Four micrograms of shuttle plasmid PDC316-EGFP-U6-KIT and 6 μg rescue plasmid pBHGlox(delta) E1, 3Cre were mixed well, then DMEM was added to a total volume of 300 mL and left at room temperature for 5 min. Three hundred microliters of DMEM and 10 μL Lipofectamine 2000 was added slowly to the tube with constant mixing, and the mixture was left at room temperature for 5 min. The mixed plasmids and diluted Lipofectamine 2000 were blended and kept at room temperature for 30 min. Afterwards, the mixture was added to a plate containing cultured HEK293 cells. The second day after transfection, the HEK293 cells were transferred to 75 cm2 cell culture bottles and cultured in DMEM containing 10% fetal calf serum. The bottles were monitored daily for the appearance of cytopathic effect (CPE), which was evident by a rounded and refractile appearance of the cells, and would begin to lift off the surface of the bottle. The CPE cells were observed under fluorescence microscope for green fluorescence. When > 90% of the cells showed CPE, the cells were harvested and subjected to three freeze (methanol/dry ice bath)/thaw(37°C water bath) cycles. After the cell debris was sedimented, the supernatant containing the adenovirus particles comprised the AdEGFP-U6-KIT stock. The stock was stored in small aliquots at -70°C after 10% glycerol was added.
When HEK293 cells had attained a 90% confluency in a 75 cm2 cell culture bottle, 2 mL of the stock unfrozen AdEGFP-U6-KIT supernatant was added. About 44 h after infection, the HEK293 cells presented total CPE and were harvested for three freeze/thaw cycles as described above. Supernatant was collected and added to four 75 cm2 cell culture bottles containing HEK293 cells and treated to recover supernatant as described above. Ten milliliters of AdEGFP-U6-KIT supernatant was added to 10 cell culture bottles, which were inoculated with 1.8 × 108 HEK293 cells that has attained a 90% confluency. About 70 h after infection, the cell suspension was centrifuged at 3000 r/min for 10 min. The precipitate of cells was suspended in Tris buffer and treated with three freeze/thaw cycles as described previously. After centrifugation at 6000 r/min for 10 min, the supernatant was collected, digested with 20 units Dnase, filtered through a 0.45 μm filter membrane, and purified with ion exchange chromatography. Further purification was achieved using molecular sieving. The purified AdEGFP-U6-KIT was stored in virus preservation fluid. After desalination and sterilization using a 0.22 μm sterile filter, the purified and sterile virus fluid was stored in small aliquots at -70°C after 10% glycerol was added.
According to the manufacturer’s instructions, 10 × virolysis solution was used to purify virus samples. The physical titer was calculated based on the equation of OD260 × 1.1 × 1012 (vp/mL), and 50% tissue culture infective dose [TCID50, plaque forming units (PFU)/mL] was also determined.
The study was approved by the Ethics Board of the First Affiliated Hospital of Sun Yat-sen University. Prior written informed consent was obtained from patients with gastric stromal tumors for use of samples. Balb/c-nu/nu mice were purchased from the Chinese Academy of Medical Sciences. All mice were maintained according to the ‘‘NIH Guide for the Care and Use of Laboratory Animals’’. Patient-derived GIST xenografts were established in Balb/c-nu/nu mice as described[13]. Briefly, primary GISTs were obtained and immediately placed in chilled RPMI 1640. The tumors were kept in an ice bath and quickly transferred to the laboratory. Thin slices of tumor were diced into 2-3 mm pieces and washed three times with RPMI 1640. These tumor pieces were minced into fine fragments that would pass through an 18-gauge needle and were then mixed 1:1 (v/v) with Matrigel to give a total volume of 0.1 mL/injection. The tissue mixture was subcutaneously injected into the flank of 9-10-wk-old Balb/c-nu/nu mice. In 6 primary gastric GISTs obtained at operation, only 1 was successfully grafted. Its size and frequency of mitosis was 20 cm × 12 cm × 14 cm and 14/50HPF, respectively. The tumor tissue showed CD117 positive staining. According to Miettinen et al’s report, it belonged to high risk GIST for recurrence and metastasis. For serial transplantation, tumor-bearing animals were anesthetized with diethyl ether and sacrificed by cervical dislocation. Tumors were minced under sterile conditions and injected into Balb/c-nu/nu mice as described above. Growth of established tumor xenografts was monitored at least twice a week by vernier caliper measurement of the length (a) and width (b) of tumor. Tumor volumes were calculated as (a × b2) /2[14]. Twenty-four days after graft, 20 mice harboring grafted tumors were randomly selected and divided into two groups. Fifty microliters of AdEGFP-U6-KIT (2.5 × 109 viral particles) was intratumorally injected into the test group of mice (n = 10), while blank AdEGFP-U6 was used in the control group of mice (n = 10). Forty-five days after injection, tumors were harvested, frozen in liquid nitrogen, fixed in buffer containing 10% formalin, and embedded in paraffin for histological study. The tumor inhibition rate referred to reduced degree of tumor [(tumor volume before injection - tumor volume at finish of test)/tumor volume before injection]. Positive value presented contraction of tumor, while a negative value indicated tumor growth.
Immunohistochemistry was performed as described[15]. Briefly, each grafted tumor specimen was fixed in 10% formaldehyde and embedded in paraffin. Sections 4 μm thick were cut and mounted on glass slides. Immunohistochemical staining was performed using a standard avidin-biotin method. The formalin-fixed, paraffin-embedded 4 μm thick tissue sections were deparaffinized with xylene, dehydrated in ethanol, and incubated with 3% hydrogen peroxidase for 5 min. After being washed with phosphate buffered saline (PBS), tissue sections were incubated in 10% normal bovine serum for 20 min, followed by an overnight incubation with a 1:100 dilution of CD117 antibody. Biotinylated goat antimouse immunoglobulin was used as the secondary antibody. Peroxidase-conjugated avidin was at a 1:500 dilution. Finally, 0.2 g/L DAB and 10 mL/L hydrogen peroxide in PBS were used as the substrate. Specimens positive for CD117 served as the positive control, and those with the first antibody substituted by PBS as negative control. Brown granules in the cytoplasm of a tumor cell were considered indicative of a positive cell, and brown staining of more than 20% of the tumor cells was regarded as positive.
The χ2 or Fisher exact test was used to compare categorical variables, and Student’s t test was used to analyze continuous variables. Statistical analyses were performed using SPSS software version 11.5. Results were considered statistically significant at P < 0.05.
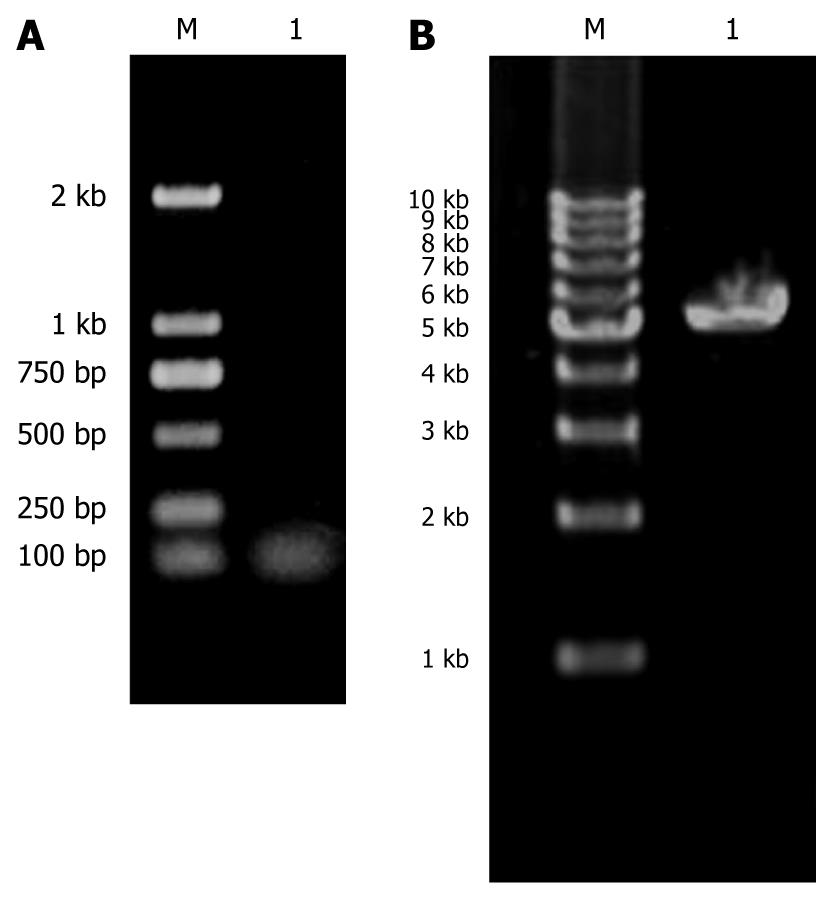
The length of KIT (B/H) band shown in agarose gel electrophoresis was 50 bp or so, which gave evidence that KIT (B/H) was synthesized correctly (Figure 1A).
In agarose gel electrophoresis, PDC316-EGFP-U6 (B/H) was about 5300 bp, consistent with the vector length (Figure 1B).
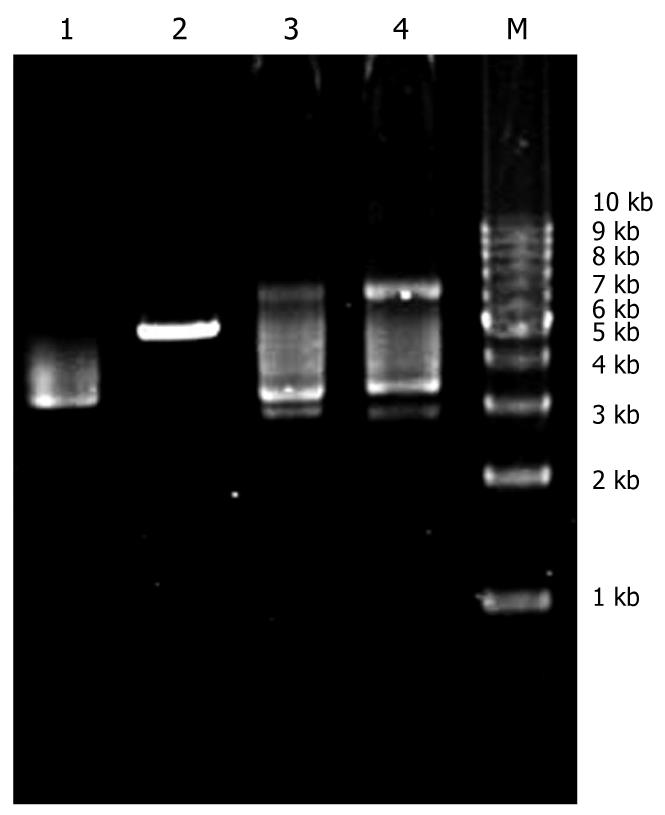
There was incision enzyme site SalI between HindIII and BamHI in blank PDC316-EGFP-U6 plasmid. After recombination, restrictive endonuclease site SalI was eliminated. The blank plasmid could be linearized by SalI digestion, while the recombinant one could not. Figure 2 shows that the plasmids on lanes 3 and 4 were the recombinant PDC316-EGFP-U6-KIT.
The sequencing graph showed that the KIT RNAi sequence in PDC316-EGFP-U6-KIT plasmid was correct (Figure 3).
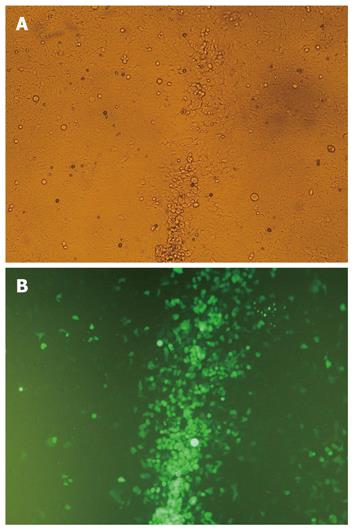
The CPE and green fluorescence in HEK293 cells were observed 8 d after recombination of PDC316-EGFP-U6-KIT and pBHGlox(delta)E1,3Cre (Figure 4A and B). The physical titer and TCID50 was 5 × 1011 (viral particles/mL) and 1.26 × 1010/mL, respectively.
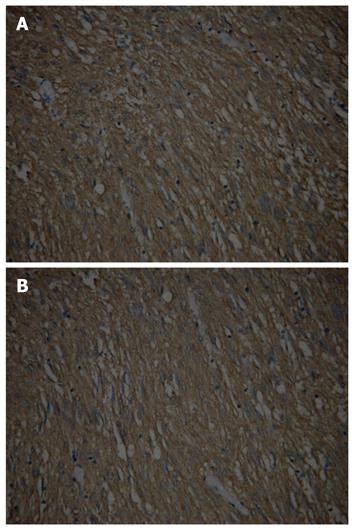
The GIST xenograft was incubated for 9-15 d. There was no histological difference between primary gastric GIST and xenografts, and both presented CD117 positive expressions (Figure 5A and B). An expansive, mobile and hard mass was observed in all animals. The mean tumor volume was similar between the test and control groups before injection of AdEGFP-U6-KIT (186.3 ± 33.6 mm3vs 176.8 ± 30.9 mm3, t = 0.3642, P > 0.10). Twenty-one days after the intervention with recombinant virus, the mean volume of graft tumor was smaller in the test animals than in the control animals (75.3 ± 22.9 mm3vs 988.6 ± 30.5 mm3, t = -18.132, P < 0.05). The tumor inhibition rate in the test and control mice was +59.6% and -459.2%, respectively. There were two cases in the test group and 10 cases in the control group who presented positive CD117 expression (20% vs 100%, χ2 = 10.2083, P < 0.005).
GIST is the most common mesenchymal tumor of the digestive tract. Metastasis of tumor cells to lymph nodes in GIST is rare, however, recurrence and liver metastasis of GIST often occured[15]. Heinrich et al[10] reported that GIST is generally distinguished from other abdominal sarcomas by the expression of KIT receptor tyrosine kinase. This kinase is important not only as a diagnostic marker for GIST, but as a primary oncogene in approximately 80% of these tumors, as evidenced by activating mutations of the KIT gene. About 90% of GIST had gain-of-function mutations of the KIT, and half of GIST without KIT mutation presented gain-of-function mutations in the PDGFRA (platelet-derived growth factor receptor-α) that encodes another receptor tyrosine kinase[16]. Other studies also showed that approximately 85% of GISTs gain activating mutations in KIT or the homologous RTK gene, with the PDGFRA. KIT activation was associated with proliferation, apoptosis, adhesion and chemotaxis[17,18]. With GIST’s resistance to conventional chemotherapy, tyrosine kinase inhibitors are an emerging class of anticancer therapies that have shown a pivotal role in clinical practice. Imatinib, which inhibits the enzymatic activity of KIT, can present a satisfactory response, while about 15% of patients show initial resistance, and many patients who respond positively at first show secondary resistance later[5-7,19]. In clinical studies, 75%-90% of patients with advanced GISTs treated with Imatinib experienced a clinical benefit[10]. The Imatinib induced responses correlated with tumor kinase mutational status. Patients with KIT exon 11-mutant GIST have a higher response rate and a significantly longer median survival compared with patients with exon 9-mutant GISTs, and those whose GISTs lack KIT or PDGFRA mutations[20]. The duration and dose of Imatinib in the neoadjuvant setting are yet undecided, however, less than 5% patients have complete clinical response to Imatinib[1]. Due to the unfavorable status of GIST therapy, a new method is necessary, especially one based on management to inactivate KIT expression.
RNAi is a process in which double-stranded RNA is used to generate degradation of cognate mRNA[11]. Synthetic 21-23 nucleotide (siRNA) has been demonstrated to induce transient and efficient RNAi[21]. Plasmid vector designed to produce siRNA presents transient siRNA expression and low transfection efficiency. For the high titer and level of recombinant adenoviruses in transgene expression, they currently are widely used in gene interventions, including RNAi[11,22]. In the present study, plasmids pDC316-EGFP-U6 and pBHGlox_E1,3Cre were employed to generate recombinant adenovirus. Shuttle plasmid pDC316-EGFP-U6 was reconstructed based on pDC316, in which the U6-promotor was designed to drive the goal gene and enhanced green fluorescent protein was employed to observe transfection. The multiple cloning site (MCS) for shRNA in pDC316-EGFP-U6 was U6 promoter-BamHI-SalI-HindIII. KIT (B/H) was correctly inserted into the MCS between BamHI and HindIII, and DNA sequencing and agarose gel electrophoresis could provide a strong evidence. The physical titer and TCID50 of recombinant virus AdEGFP-U6-KIT was 5 × 1011 viral particles/mL and 1.26 × 1010/mL, respectively. Wang et al[23] reported that a recombinant adenovirus vector designed for expression of a fusion gene was constructed successfully using the AdMax Adenovirus Vector Creation System, and its titer was 8 × 1010 PFU/mL. Other studies also demonstrated that AdMax system has a high recombinant efficiency and is comparatively simple[24-26]. It is reasonable to draw a conclusion that the Admax system could effectively recombine adenovirus in molecular organism research.
Based on the pivotal role of KIT in some diseases, including mast cell leukemia and GIST[1,27], KIT RNAi might be an alternative to control disease development. Ruano et al[27] discovered that retroviral transduction of HMC1.1 and HMC1.2 cell lines with vectors carrying DNA to be transcribed for RNAi against the wild type or mutant KIT messengers lowered KIT protein levels considerably, decreased cell proliferation, and raised the apoptotic levels. Furthermore, the same study suggested that the highly specific effect of RNAi in reducing KIT mRNA could be used for the treatment of other cancers resistant to Imatinib mesylate, such as GIST[27]. A study reported that KIT protein was detected in spermatogonial cells and knocked down to undetectable levels at 24 h after transfection with KIT siRNA[28]. Catalano et al[12] showed that exposure of a malignant mesothelioma cell line to KIT siRNA was associated with down-regulation of KIT expression and an increase in apoptosis. In the present study, KIT RNAi whose addition was mediated with Admax adenovirus suppressed dramatically KIT protein (CD117) expression, providing evidence that the KIT mRNA was knocked down by RNAi. Furthermore, the GIST xenograft was reduced markedly in the test group of animals and the tumor inhibition rate was 59.6%, while in the control mice, the xenograft grew rapidly and the mean volume increased by 5.6 times at the end of the experiment compared with that observed before intervention with KIT RNAi. The sequence of KIT RNAi in our study was not located in the mutant regions, therefore, the recombinant adenovirus could be used in most GISTs[1,8,10]. Zhu et al[9] evaluated interactions with the KIT oncoproteins and determined signaling pathways that are dependent on KIT oncogenic activation in GIST. Tyrosine-phosphorylated KIT oncoproteins interacted with PDGFRA, PDGFRB, phosphatidylinositol 3-kinase and PKCtheta in GIST cells, and these interactions were abolished by KIT inhibition with Imatinib or KIT RNAi. Another study used a KIT lentiviral shRNA to infect GIST882, shRNA knockdown of total KIT expression in Imatinib sensitive GIST882 cell line resulted in parallel decreases in phosphorylated-KIT. KIT knockdown in the cell lines also provided flow cytometric evidence for G1 block, decreased S phase, and markedly increased apoptosis[10]. Yang et al[29] reported that, excepting mutations of KIT or PDGFRA gene, there were cytogenetic aberrations and molecular genetic aberrations. A new paradigm of classification integrating the standard clinical and pathological criteria with molecular aberrations may permit personalized prognosis and treatment.
In summary, GIST is prevalent and serious, and efficacious therapy is still required. The recombinant adenovirus AdEGFP-U6-KIT was correctly constructed and potently inhibited KIT expression and growth of GIST xenografts. AdMax adenovirus vector can effectively introduced RNAi into cancer gene therapy. KIT RNAi mediated with adenovirus might become a method for GIST treatment.
About 90% of gastrointestinal stromal tumor (GIST) had gain-of-function mutations of KIT. The gold standard therapy for GIST is complete resection, depending on the lesion size and location. GIST is an easily recurrent disease, which is frequently found in the liver (65%), peritoneal surface (50%), or both (20%). Conventional chemotherapy is of little benefit for GIST because it is a non-epithelial neoplasm. The use of Imatinib mesylate in advanced GIST produces a response in 50% patients. Primary resistance to Imatinib affects about 15% of patients, and 50% of patients become secondarily resistant by 2 years after Imatinib therapy. Clearly, a new strategy is required.
The KIT proto-oncogene on chromosome 4 (4q11-q12), which encodes for the KIT protein, appears to play an important role in early stages of tumor formation as well as in late tumor progression. Inhibition of KIT gene may block the formation and development of GIST. RNAi is the most effective method to silence a special gene. Two documents gave evidences that RNAi could block KIT gene expression in GIST, but all of them are not involved in the growth of GIST. Up to now, it is still uncertain if KIT RNAi could become an effective therapeutic method for GIST.
In this study, the authors successfully constructed an adenovirus vector to mediate KIT RNAi in GIST xenografts. The results demonstrate that an adenovirus system that induces KIT RNAi can silence efficiently KIT, inhibit the growth of GIST xenografts, therefore it may be a promising method for GIST treatment. This may be the first investigation about KIT RNAi mediated with adenovirus for the treatment of GIST xenograft.
The results of the present study show that it is possible that KIT RNAi mediated by adenovirus might become a treatment method for diseases related to KIT, including GIST, and it is also a useful method to study the KIT function in cytogenic and molecular research.
The authors described that an adenovirus vector was successfully constructed to mediate KIT RNAi in GIST xenografts. The results demonstrated that the adenovirus system can silence efficiently KIT and inhibit the growth of grafted GIST. This approach may have therapeutic potential in GIST. Their results are very attractive. However, additional data should be required.
Peer reviewers: Tamara Vorobjova, Senior Researcher in Immunology, Department of Immunology, Institute of General and Molecular Pathology, University of Tartu, Ravila, 19, Tartu, 51014, Estonia; Mitsunori Yamakawa, Professor, Department of Pathological Diagnostics, Yamagata University, Faculty of Medicine, 2-2-2 Iida-Nishi, Yamagata 990-9585, Japan
S- Editor Tian L L- Editor Ma JY E- Editor Zheng XM
| 1. | Stamatakos M, Douzinas E, Stefanaki C, Safioleas P, Polyzou E, Levidou G, Safioleas M. Gastrointestinal stromal tumor. World J Surg Oncol. 2009;7:61. [Cited in This Article: ] |
| 2. | Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1-12. [Cited in This Article: ] |
| 3. | Parfitt JR, Streutker CJ, Riddell RH, Driman DK. Gastrointestinal stromal tumors: a contemporary review. Pathol Res Pract. 2006;202:837-847. [Cited in This Article: ] |
| 4. | Blay JY, Bonvalot S, Casali P, Choi H, Debiec-Richter M, Dei Tos AP, Emile JF, Gronchi A, Hogendoorn PC, Joensuu H. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20-21 March 2004, under the auspices of ESMO. Ann Oncol. 2005;16:566-578. [Cited in This Article: ] |
| 5. | Joensuu H. Gastrointestinal stromal tumor (GIST). Ann Oncol. 2006;17 Suppl 10:x280-x286. [Cited in This Article: ] |
| 6. | Blay JY, Le Cesne A, Ray-Coquard I, Bui B, Duffaud F, Delbaldo C, Adenis A, Viens P, Rios M, Bompas E. Prospective multicentric randomized phase III study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: the French Sarcoma Group. J Clin Oncol. 2007;25:1107-1113. [Cited in This Article: ] |
| 7. | Pauwels P, Debiec-Rychter M, Stul M, De Wever I, Van Oosterom AT, Sciot R. Changing phenotype of gastrointestinal stromal tumours under imatinib mesylate treatment: a potential diagnostic pitfall. Histopathology. 2005;47:41-47. [Cited in This Article: ] |
| 8. | Din OS, Woll PJ. Treatment of gastrointestinal stromal tumor: focus on imatinib mesylate. Ther Clin Risk Manag. 2008;4:149-162. [Cited in This Article: ] |
| 9. | Zhu MJ, Ou WB, Fletcher CD, Cohen PS, Demetri GD, Fletcher JA. KIT oncoprotein interactions in gastrointestinal stromal tumors: therapeutic relevance. Oncogene. 2007;26:6386-6395. [Cited in This Article: ] |
| 10. | Heinrich MC, Corless CL, Blanke CD, Demetri GD, Joensuu H, Roberts PJ, Eisenberg BL, von Mehren M, Fletcher CD, Sandau K. Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. J Clin Oncol. 2006;24:4764-4774. [Cited in This Article: ] |
| 11. | Chen LM, Le HY, Qin RY, Kumar M, Du ZY, Xia RJ, Deng J. Reversal of the phenotype by K-rasval12 silencing mediated by adenovirus-delivered siRNA in human pancreatic cancer cell line Panc-1. World J Gastroenterol. 2005;11:831-838. [Cited in This Article: ] |
| 12. | Catalano A, Rodilossi S, Rippo MR, Caprari P, Procopio A. Induction of stem cell factor/c-Kit/slug signal transduction in multidrug-resistant malignant mesothelioma cells. J Biol Chem. 2004;279:46706-46714. [Cited in This Article: ] |
| 13. | Huynh H, Soo KC, Chow PK, Panasci L, Tran E. Xenografts of human hepatocellular carcinoma: a useful model for testing drugs. Clin Cancer Res. 2006;12:4306-4314. [Cited in This Article: ] |
| 14. | Huynh H, Lee JW, Chow PK, Ngo VC, Lew GB, Lam IW, Ong HS, Chung A, Soo KC. Sorafenib induces growth suppression in mouse models of gastrointestinal stromal tumor. Mol Cancer Ther. 2009;8:152-159. [Cited in This Article: ] |
| 15. | Wang TB, Qiu WS, Wei B, Deng MH, Wei HB, Dong WG. Serum vascular endothelial growth factor and angiogenesis are related to the prognosis of patients with gastrointestinal stromal tumors. Ir J Med Sci. 2009;178:315-320. [Cited in This Article: ] |
| 16. | Shinomura Y, Kinoshita K, Tsutsui S, Hirota S. Pathophysiology, diagnosis, and treatment of gastrointestinal stromal tumors. J Gastroenterol. 2005;40:775-780. [Cited in This Article: ] |
| 17. | Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol. 2004;22:3813-3825. [Cited in This Article: ] |
| 18. | Liegl-Atzwanger B, Fletcher JA, Fletcher CD. Gastrointestinal stromal tumors. Virchows Arch. 2010;456:111-127. [Cited in This Article: ] |
| 19. | Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472-480. [Cited in This Article: ] |
| 20. | Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, McGreevey LS, Chen CJ, Van den Abbeele AD, Druker BJ. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342-4349. [Cited in This Article: ] |
| 21. | Paddison PJ, Caudy AA, Bernstein E, Hannon GJ, Conklin DS. Short hairpin RNAs (shRNAs) induce sequence-specific silencing in mammalian cells. Genes Dev. 2002;16:948-958. [Cited in This Article: ] |
| 22. | Xia H, Mao Q, Paulson HL, Davidson BL. siRNA-mediated gene silencing in vitro and in vivo. Nat Biotechnol. 2002;20:1006-1010. [Cited in This Article: ] |
| 23. | Wang XD, Liu H, Cao S, Li H, Ren XB, Hao XS. [Construction of recombinant adenovirus vector expressing extracellular domain of TbetaR-II-RANTES fusion gene and its anti-tumor effects]. Zhonghua Zhongliu Zazhi. 2007;29:405-410. [Cited in This Article: ] |
| 24. | Qi H. [Construction of a recombinant adenovirus expression vector for human renal tumor- associated antigen G250 gene with AdMax system]. Nanfang Yike Daxue Xuebao. 2008;28:1617-1620, 1625. [Cited in This Article: ] |
| 25. | Gong WD, Zhao Y, Yi J, Ding J, Liu J, Xue CF. Anti-HBV activity of TRL mediated by recombinant adenovirus. World J Gastroenterol. 2005;11:2574-2578. [Cited in This Article: ] |
| 26. | Nilsson M, Ljungberg J, Richter J, Kiefer T, Magnusson M, Lieber A, Widegren B, Karlsson S, Fan X. Development of an adenoviral vector system with adenovirus serotype 35 tropism; efficient transient gene transfer into primary malignant hematopoietic cells. J Gene Med. 2004;6:631-641. [Cited in This Article: ] |
| 27. | Ruano I, Izquierdo M. Selective RNAi-mediated inhibition of mutated c-kit. J RNAi Gene Silencing. 2009;5:339-344. [Cited in This Article: ] |
| 28. | Sikarwar AP, Rambabu MK, Reddy KV. Differential regulation of gene expression in mouse spermatogonial cells after blocking c-kit-SCF interaction with RNAi. J RNAi Gene Silencing. 2008;4:302-311. [Cited in This Article: ] |