Published online Nov 14, 2010. doi: 10.3748/wjg.v16.i42.5380
Revised: August 14, 2010
Accepted: August 21, 2010
Published online: November 14, 2010
AIM: To investigate the correlation between gastric cancer growth and signal transducer and activator of transcription-3 (STAT3) expression.
METHODS: We assessed the expressions of STAT3, phosphor-STAT3 (pSTAT3), suppressor of cytokine signaling-1 (SOCS-1), survivin and Bcl-2 in gastric cancer patients after gastrectomy by immunohistochemical method. In addition, in situ hybridization was used to further demonstrate the mRNA expression of STAT3 in gastric cancer.
RESULTS: With the univariate analysis, expressions of STAT3, pSTAT3, SOCS-1, survivin and Bcl-2, the size of primary tumor and the lymph node metastasis were found to be associated with the overall survival (OS) of gastric cancer patients. However, only pSTAT3 expression and the lymph node metastasis were identified as the independent factors of OS of gastric cancer with multivariate analysis. STAT3 expression was correlated with the lymph node metastasis. There were positive correlations between expressions of STAT3, survivin, Bcl-2 and pSTAT3 in gastric cancer, whereas there was negative correlation between STAT3 expression and SOCS-1 expression in gastric cancer.
CONCLUSION: STAT3 can transform into pSTAT3 to promote the survival and inhibit the apoptosis of gastric cancer cells. SOCS-1 might be the valid molecular antagonist to inhibit the STAT3 expression in gastric cancer.
- Citation: Deng JY, Sun D, Liu XY, Pan Y, Liang H. STAT-3 correlates with lymph node metastasis and cell survival in gastric cancer. World J Gastroenterol 2010; 16(42): 5380-5387
- URL: https://www.wjgnet.com/1007-9327/full/v16/i42/5380.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i42.5380
Gastric cancer is the fourth most common cancer worldwide with approximately 930 000 newly diagnosed cases annually, resulting in about 700 000 deaths per year[1,2]. Although the surgical skills and adjuvant chemotherapy have been improved, the survival rate of gastric cancer patients remains unsatisfactory. Most gastric cancer patients were identified at the advanced stage when they were initially diagnosed, which is deemed as the important factor for the increased recurrence rate and decreased overall survival (OS) after potentially curative resection[3]. Therefore, it is imperative to investigate the molecular mechanism so as to enhance the curative effects for gastric cancer.
Many researchers have demonstrated that the aggressive nature of gastric cancer is related to mutations of various oncogenes and tumor suppressor genes and abnormalities in some growth factors and their receptors[4-6]. Recently, the activation of signal transducers and activators of transcription (STAT) proteins has been shown to have a strong bearing on gastric cancer progression although the detailed mechanism for this relationship has not been available[7]. The STAT proteins, including seven members, are a family of transcription factors which regulate expression of genes involved in both normal and pathological cellular processes. The strong association was demonstrated between STAT proteins and progression of various epithelial cancers[8-11].
Of the STAT family members, STAT3 is the most commonly activated in human cancers[12]. STAT3 is being increasingly recognized as a molecular hub for diverse signaling pathways such as cell cycle progression, apoptosis, angiogenesis and immune evasion, thus being considered as a novel molecular marker for cancer treatment[13,14]. Although STAT3 is constitutively activated and contributes to oncogenesis through preventing apoptosis and enhancing cell proliferation in many kinds of human tumors, the duration of STAT3 activation is temporary under physiologic conditions[15]. Over-expression of the activated STAT3 has been found in various types of malignant tumors, including leukemia, breast cancer. pancreatic cancer, prostatic cancer and melanoma, and STAT3 has played a key role in carcinogenesis[16]. However, the role of STAT3 signaling in gastric cancer is still unclear. Many studies suggested that the phospho-STAT3 (pSTAT3) should be taken as an active form of STAT3 and an independent prognostic factor for disease free survival and poor survival after curative resection[14,15].
Theoretically, STAT3 has to be activated by phosphorylated tyrosine induced by Janus Kinase (JAK) before STAT3 binds to receptor successfully. Subsequently, self-phosphorylated STAT3 protein is released from pSTAT3, assumes the shape of “dimmers” and migrates into the nucleus to activate the transcription of target genes. Jackson et al[17] found that pSTAT3 was localized mainly in epithelial cells in both normal stomach and gastric cancer, but there was at least ten-fold more STAT3 activation in the latter, with substantial nuclear staining. Therefore, a higher level of pSTAT3 positive expression represents more nuclear translocations of STAT3, which can stimulate the target gene transcription upon tissue transformation in gastric cancer. Suppressor of cytokine signaling-1 (SOCS-1) seems to be one of the STATs activated genes, which contain the SH2 domains that interact with JAK and prevent activation of STATs[18,19]. Recent findings suggest that SOCS-1 can be significantly up-regulated by interleukin (IL)-6 and is involved in the down-regulation of the IL-6-induced activation of STAT3[20]. Bcl-2 and survivin are the potential downstream molecules to STAT3. These molecules regulate both cell cycle and apoptosis and are the known targets of the STAT3 signaling pathway[21].
In the present study, we evaluated the expressions of STAT3, pSTAT3, SOCS-1, survivin, and Bcl-2 in both gastric cancer and normal gastric tissues after gastrectomy. We correlated these molecular variables, clinicopathologic features and prognoses of gastric cancer patients to study the potential mechanism of STAT3 signaling in carcinogenesis and progression of gastric cancer.
We used the human gastric cancer tissue and normal gastric tissue specimens preserved in the department of pathology and obtained patients’ data from Tianjin Medical University Cancer Hospital. Fifty-three gastric cancer specimens from the 53 patients with gastric cancer and 53 normal gastric tissue specimens from 53 patients without gastric cancer were included in this study. Primary gastric cancer in these patients was diagnosed and treated at Tianjin Medical University Cancer Hospital between January 2002 and December 2002. The gastric cancer patients had well-documented clinical histories and follow-up information. None of them underwent preoperative chemotherapy and/or radiation therapy. All of gastric cancer patients had undergone potentially radical gastrectomy with lymphadenectomy. Of them, 37 (69.8%) were male and 16 (30.2%) were female. Ages ranged from 31 to 78 years, with a median age of 55.0 years. The tumor location was as follows: lower third in 28 (52.8%) cases, middle third in 18 (34.0%) cases, and upper third in 7 (13.2%) cases. Thirty-seven (69.8%) patients had lymph node metastasis identified by pathologic examination after surgery, and 16 (30.2%) patients had no lymph node metastasis after surgery. At the end of June 2009, 24 (45.3%) gastric cancer patients were still alive, whereas 29 (54.7%) had died. No gastric cancer patient died during the initial hospital stay or one month after surgery. Follow-up ranged from 4 to 85 mo, and the median was 35 mo. Results of B ultrasonography, CT scans, chest X-ray and endoscopy were obtained.
For in situ hybridization, the biotinylated oligonucleotide probe complementary to STAT3 (CCTTGGATTGAGAGTCAAGATTGGGCATAT) mRNA was synthesized. All in situ hybridization was carried out using manual capillary action technology. Briefly, the slides were rapidly deparaffinized, cleared, and rehydrated. The tissues were then digested with pepsin (Boshide, China) at 2 mg/mL for 30 min at 37°C. The probe was applied to the slides, and the tissues were heated at 42°C for 2 h for denature and secondary mRNA structure. The hybridization of the probe and mRNA target was performed by exposing the slides in a calorstat at 42°C for 12 h. The biotinylated hybrids were detected with streptavidin-horseradish peroxidase (Jingmei, China) for 30 min at 37°C. After preincubation with 3,3’-diaminobenzidine solution for 3-5 min at 37°C, the tissues were washed with distilled water. Following the chromogen reaction, the tissues were counterstained with hematoxylin solution, washed with distilled water, air-dried, and cover-slipped with universal mount (Invitrogen US). In situ hybridization for negative control was performed with probe diluent.
Paraffin sections (4 μm thick) were deparaffinized and rehydrated. Antigen retrieval treatment was done at 95°C for 40 min in 0.01 mol/L sodium citrate buffer (pH 6.0), and endogenous peroxidases were blocked using 3% hydrogen peroxide for 30 min. The primary antibodies at 1:100 dilution were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA); STAT3 (SC-483, rabbit polyclonal IgG), SOCS-1 at 1:100 dilution (SC-9021, rabbit polyclonal IgG) from NeoMarkers Biotechnology, Inc. (Fremont, CA); survivin (RB-9245-P0, rabbit polyclonal IgG) at 1:50 dilution, and Bcl-2 (Ms-123-PABX, mouse monoclonal IgG) at 1:100 dilution from Jingmei Biotechnology (Zhongshan, China). All sections were incubated overnight with the primary antibody at 4°C. The sections were then treated with peroxidase by the labeled polymer method with Zhongshan peroxidase for 30 min. Antibody binding was visualized using the Avidin Biotin Complex Elite Kit and 3,3-diaminobenzine according to the manufacturer’s instructions (City Key Laboratory of Tianjin Cancer Center, China). Sections were then counterstained in hematoxylin. Breast cancer and colon cancer specimens confirmed to be immunoreactive for the relevant antigens in preliminary studies were used as positive controls for STAT3, pSTAT3, SOCS-1, survivin and Bcl-2. For general negative controls, the primary antibody was replaced with phosphate buffered saline.
All sections were scored blindly by two independent observers, and in cases of scoring disagreement, a third independent assessment was performed. Staining of STAT3, SOCS-1, survivin, and Bcl-2 was graded according to the intensity and extent of staining of the epithelium. The staining intensity was scored into 4 grades: 0, complete absence of the staining; 1, weak staining; 2, moderate; and 3, strong staining. The extent of the positively stained cells was also scored into 5 grades: 0, a complete negative staining; 1, < 5%; 2, 5%-25%; 3, 25%-75%; and 4, ≥ 75%. The final scores were derived from multiplication of the extent by the intensity. For statistical analysis, scores were further grouped into two categories: negative (final scores, < 3) and positive (final score, ≥ 3).
The staining of pSTAT3 was grouped as positive and negative. The positive staining of pSTAT3 was defined as ≥ 10% of the cells with the nucleus stained dark brown and the negative staining was defined as < 10% of the cells stained.
All statistical calculations were performed using SPSS version 13.0 (SPSS Inc., Chicago, IL). Differences in STAT3 expression, pSTAT3 expression, SOCS-1 expression, surviving expression, Bcl-2 expression and clinicopathologic variables of patients were estimated using the χ2 test for categorical data and independent-paired Student’s t test for continuous variables. Overall survival of gastric cancer patients was measured from date of surgery to the date of death by the Kaplan-Meier method. The log-rank test (χ2 comparison) was used to compare the overall survival based on expressions of STAT3, pSTAT3, SOCS-1, survivin and Bcl-2. Cox regression analysis was used to estimate the independent risk factors of overall survival of gastric cancer patients after radical surgery. In addition, linear regression was used to estimate the correlations among the variables which were significantly associated with the STAT3 immunohistochemical expression.
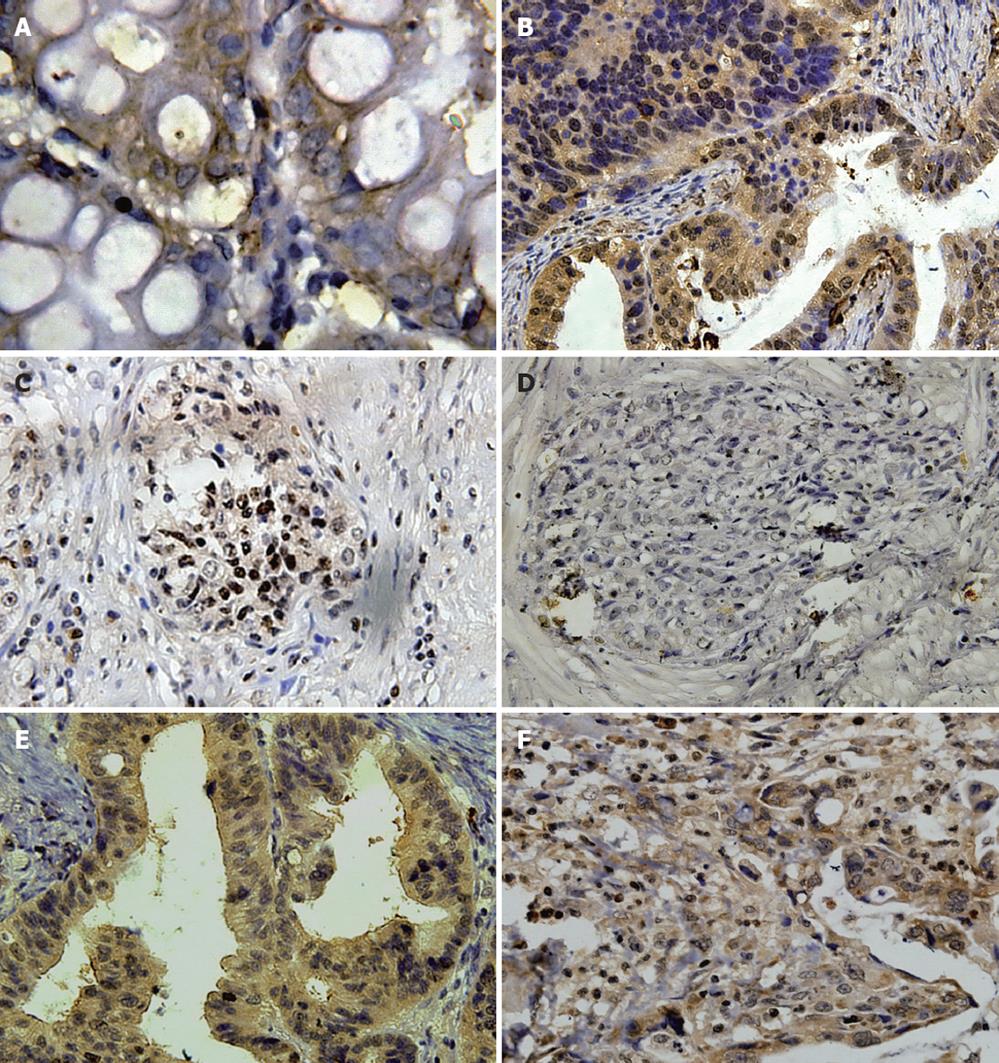
Almost all gastric cancer tissue specimens were positive for STAT3 mRNA expression, whereas only 8 of 53 normal gastric tissue specimens were positive for STAT3. STAT3 mRNA expression was strong in the cytoplasm of gastric cancer epithelium, while the epithelium of normal gastric tissues was weak or negative (Figure 1A).
STAT3 was detected mainly in the epithelium of gastric cancer tissues and less frequently in the normal gastric tissues. The strong granular patterns of STAT3 staining were observed mainly within the cytoplasm and partially within the nuclear of the epithelium of gastric cancer. Conversely, only weak granular patterns of STAT3 staining were observed within the cytoplasm of a few normal gastric tissue specimens (Figure 1B). STAT3 staining was quantitatively assessed and grouped into positive expression and negative expression. Positive STAT3 expression was detected in 31 of 53 gastric cancer tissue specimens and 6 of 53 normal gastric tissue specimens. The ratio of positive STAT3 expression in gastric cancer tissue specimens was significantly higher than that in normal gastric tissue specimens (58.5% vs 11.3%, χ2 = 25.950, P < 0.001).
pSTAT3 was detected mainly in the epithelium of gastric cancer tissues and less frequently in the normal gastric tissues. The strong granular patterns of pSTAT3 staining were observed mainly within the nuclear and partially within the cytoplasm of the epithelium of gastric cancer. Conversely, only weak granular patterns of pSTAT3 staining were observed within the nuclear of a few normal gastric tissue specimens (Figure 1C). pSTAT3 staining was quantitatively assessed and grouped into positive expression and negative expression. Positive pSTAT3 expression was detected in 26 of 53 gastric cancer tissue specimens and 2 of 53 normal gastric tissue specimens. The ratio of positive pSTAT3 expression in gastric cancer tissue specimens was significantly higher than that in normal gastric tissue specimens (49.1% vs 3.8%, χ2 = 27.956, P < 0.001).
SOCS-1 was detected mainly in the normal gastric tissues and less frequently in the epithelium of gastric cancer. The strong granular patterns of SOCS-1 staining were observed within the cytoplasm of the normal gastric tissue specimens. Conversely, only weak granular patterns of SOCS-1 staining were observed within the cytoplasm of a few gastric cancer tissue specimens (Figure 1D). SOCS-1 staining was quantitatively assessed and grouped into positive expression and negative expression. Positive SOCS-1 expression was detected in 6 of 53 gastric cancer tissue specimens and 23 of 53 normal gastric tissue specimens. The ratio of positive STAT3 expression in gastric cancer tissue specimens was significantly lower than that in normal gastric tissue specimens (11.3% vs 43.4%, χ2 = 13.719, P < 0.001).
Survivin was detected mainly in the epithelium of gastric cancer tissues and less frequently in the normal gastric tissues. The strong granular patterns of survivin staining were observed within the cytoplasm of the epithelium of gastric cancer tissue specimens. Conversely, only weak granular patterns of survivin staining were observed within the cytoplasm of a few normal gastric tissue specimens (Figure 1E). Survivin staining was quantitatively assessed and grouped into positive expression grade and negative expression. Positive survivin expression was detected in 39 of 53 gastric cancer tissue specimens and 4 of 53 normal gastric tissue specimens. The ratio of positive survivin expression in gastric cancer tissue specimens was significantly higher than that in normal gastric tissue specimens (73.6% vs 7.5%, χ2 = 47.933, P < 0.001).
Bcl-2 was detected mainly in the epithelium of gastric cancer tissues and less frequently in the normal gastric tissues. The strong granular patterns of Bcl-2 staining were observed within the cytoplasm of the malignant epithelium of gastric cancer tissue specimens. Conversely, only weak granular patterns of Bcl-2 staining were observed within the cytoplasm of a few normal gastric tissue specimens (Figure 1F). Bcl-2 staining was quantitatively assessed and grouped into positive expression and negative expression. Positive Bcl-2 expression was detected in 26 of 53 gastric cancer tissue specimens and in 0 of 53 normal gastric tissue specimens. The ratio of positive Bcl-2 expression in gastric cancer tissue specimens was significantly higher than that in normal gastric tissue specimens (49.1% vs 0%, χ2 = 34.450, P < 0.001).
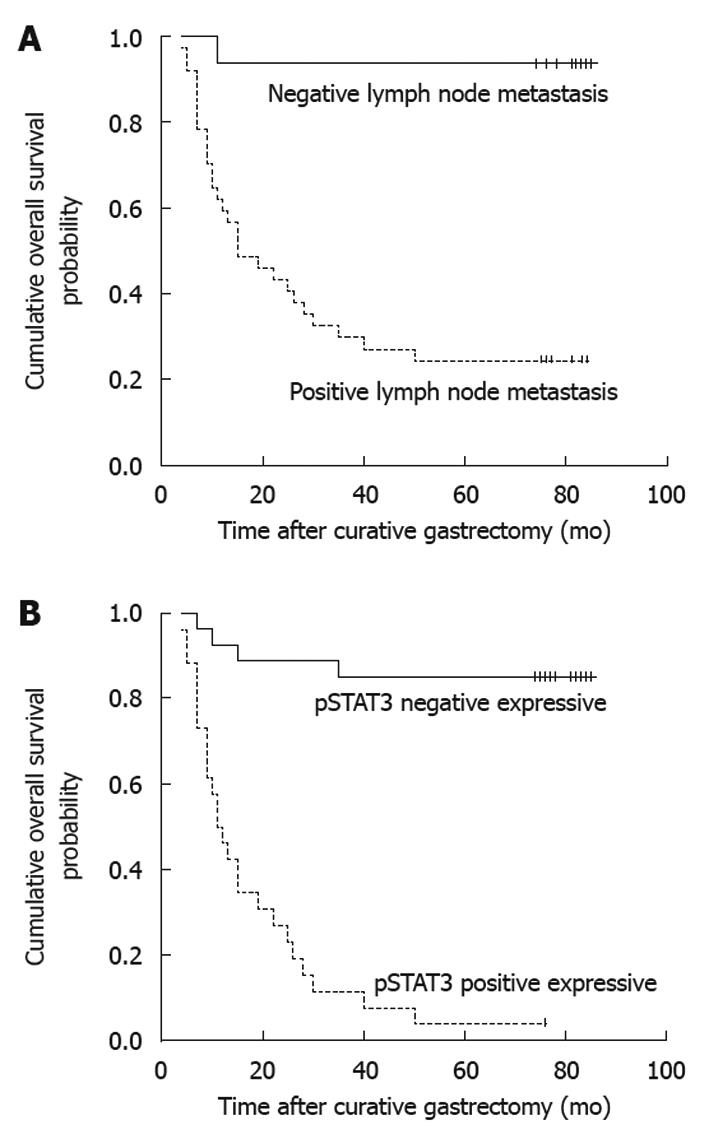
The following clinicopathologic variables were analyzed: (1) age at surgery (< 65 years or ≥ 65 years); (2) gender (male or female); (3) tumor location (lower third, middle third, or upper third); (4) tumor size (< 4 cm or ≥ 4 cm); (5) depth of primary tumor invasion (UICC) (T1+T2, or T3+T4); (6) Lauren’s classification of primary tumor (intestinal or diffuse); and (7) metastatic status of lymph nodes (positive or negative). In addition, the following antibodies were analyzed with OS of gastric cancer patients: STAT3, pSTAT3, SOCS-1, survivin and Bcl-2. With the univariate analysis, seven factors were found to have statistically significant associations with OS of gastric cancer patients after curative surgery (Table 1), including tumor size, metastatic status of lymph nodes (Figure 2A), and STAT3, pSTAT3 (Figure 2B), SOCS-1, survivin and Bcl-2 expressions.
| Factor | n | 5-yr survival rate (%) | χ2 | P value |
| Gender | 0.217 | 0.641 | ||
| Male | 37 | 45.9 | ||
| Female | 16 | 43.8 | ||
| Age at surgery (yr) | 0.260 | 0.610 | ||
| < 65 | 40 | 42.5 | ||
| ≥ 65 | 13 | 53.8 | ||
| Tumor size (cm) | 5.711 | 0.017 | ||
| < 4 | 13 | 76.9 | ||
| ≥ 4 | 40 | 35.0 | ||
| Tumor location | 2.619 | 0.270 | ||
| Lower stomach | 28 | 57.1 | ||
| Middle stomach | 18 | 33.3 | ||
| Upper stomach | 7 | 28.6 | ||
| Depth of primary tumor invasion | 3.089 | 0.079 | ||
| T1 + T2 | 6 | 83.3 | ||
| T3 + T4 | 47 | 40.4 | ||
| Status of lymph node metastasis | 17.950 | < 0.001 | ||
| Negative | 16 | 93.8 | ||
| Positive | 37 | 24.3 | ||
| Lauren’s classification | 3.280 | 0.070 | ||
| Intestinal | 25 | 60.0 | ||
| Diffuse | 28 | 32.1 | ||
| STAT3 | 19.432 | < 0.001 | ||
| Negative | 22 | 81.8 | ||
| Positive | 31 | 19.4 | ||
| pSTAT3 | 40.444 | < 0.001 | ||
| Negative | 27 | 85.2 | ||
| Positive | 26 | 3.8 | ||
| SOCS-1 | 5.712 | 0.017 | ||
| Negative | 47 | 38.3 | ||
| Positive | 6 | 100.0 | ||
| Survivin | 4.295 | 0.038 | ||
| Negative | 14 | 71.4 | ||
| Positive | 39 | 35.9 | ||
| Bcl-2 | 13.866 | < 0.001 | ||
| Negative | 27 | 70.4 | ||
| Positive | 26 | 19.2 | ||
All above seven variables were included in a multivariate Cox proportional hazards model (forward stepwise procedure) to adjust for the effects of covariates (Table 2). In this model, metastatic status of lymph nodes (HR = 8.591, P = 0.040), and pSTAT3 (HR = 9.605, P < 0.001) showed significant correlations with OS of gastric cancer patients after curative surgery.
| Factors | P value | Relative risk | 95% CI |
| Status of lymph node metastasis | 0.040 | 8.591 | 1.104-66.858 |
| Tumor size | 0.701 | ||
| STAT3 | 0.225 | ||
| pSTAT3 | < 0.001 | 9.605 | 3.107-29.694 |
| SOCS-1 | 0.429 | ||
| Survivin | 0.749 | ||
| Bcl-2 | 0.213 |
We found that the immunohistochemical expression level of STAT3 was positively associated with that of pSTAT3 (β = 0.451, P = 0.001), survivin (β = 0.504, P = 0.001), and Bcl-2 (β = 0.588, P < 0.001). The immunohistochemical expression level of STAT3 was negatively associated with that of SOCS-1 (β = -0.660, P = 0.002). In addition, the STAT3 immunohistochemical expression was positively associated with the status of lymph node metastasis (β = 0.480, P = 0.001).
The immunohistochemical expression level of pSTAT3 was positively associated with that of survivin (β = 0.473, P = 0.002) and Bcl-2 (β = 0.623, P < 0.001). The immunohistochemical expression level of pSTAT3 was negatively associated with that of SOCS-1 (β = -0.553, P = 0.010). In addition, the level of pSTAT3 immunohistochemical expression was positively associated with the status of lymph node metastasis (β = 0.524, P < 0.001).
Recently, the molecular markers are being studied, as it may improve the prognostic prediction for gastric cancer patients after curative resection. Although many investigators have suggested that several molecular markers should be considered as key prognostic indicators in gastric cancer, such as epidermal growth factor receptor, vascular endothelial growth factor, hypoxia inducible factor 1-α and p53, none of them have been defined as a unified standard in clinical practice[22-24]. The STAT proteins are a family of transcription factors which regulate expression of multiple genes involved in both physiological and pathological conditions, which seems to be one of the most promising molecular markers for predicting the prognosis of patients with various cancers.
Of the STAT family members, STAT3 is the most commonly activated in human cancers[12]. Several previous reports showed increased or activated STAT3 expression in gastric cancer[15,16,25]. Recently, Kim et al[7] found that 39% of 100 patients with gastric cancer had positive STAT3 expression, whereas only 14% of 71 patients with normal gastric tissues had positive STAT3 expression. They also demonstrated that positive STAT3 expression was significantly associated with regional lymph node invasion (P = 0.008). Jackson et al[17] indicated that inappropriate STAT3 activation was an early event in the initiation and progression of gastric cancer. They found that gastric cancer cells showed increased total STAT3 staining in both the cytoplasm and the nucleus, and a high degree of predominantly nuclear pSTAT3 staining as compared with normal gastric cells. At present study, the ratio of positive STAT3 expression in gastric cancer tissue specimens was 58.5%, which was significantly higher than that in normal gastric tissue specimens (11.3%) (P < 0.001). Similarly, our results also showed that positive STAT3 expression was significantly associated with the status of lymph node metastasis (P = 0.001). Our positive STAT3 expression was higher than that reported by Kim et al[7], and lower than that (73%) reported by Gong et al[16]. We think these differences might be induced due to the different stages of patients, subjective interpretation by pathologists, and production of antibodies. To avoid the limitation of STAT3 expression detected by the immunohistochemical method, we also adopted the in situ hybridization for a more precise detection of STAT3 mRNA expression. All gastric cancer tissue specimens were positive for STAT3 mRNA expression, whereas only 8 of 53 normal gastric tissue specimens were positive for STAT3. With the survival analysis, we found that patients with STAT3 negative expression in primary tumors were more likely to have a longer median OS compared with those with STAT3 positive expression (P < 0.001). This result was similar to the above-mentioned studies. Therefore, we thought that the STAT3 positive expression was significantly associated with the progression and prognosis of gastric cancer patients.
Choi et al[26] analyzed 137 cases of gastric cancer tissues obtained from radical gastrectomy and found that pSTAT3 positive expression occurred more frequently than in non-neoplastic gastric tissues and pSTAT3 positive expression was significantly associated with low pathological grade and lymph node metastasis. Lee et al[14] assessed the STAT3 activation in 311 cases of surgically resected gastric cancer tissues and found that pSTAT3 was an independent prognostic factor for poor survival following curative resection. We also found that pSTAT3 positive expression in gastric cancer tissues occurred significantly more often than that in normal gastric tissues and pSTAT3 was significantly associated with the status of lymph node metastasis. The survival analysis showed that pSTAT3 was an independent factor of OS after curative resection in gastric cancer patients instead of STAT3. Therefore, pSTAT3 might be a functional transformant which could preferably reflect the biological effects of STAT3.
Many studies demonstrated that the inactivated SOCS-1 was one of the targets in cancer development and a tumor suppressor in the JAK/STAT pathway[27-29]. Ni et al[30] found that there was down-regulation of SOCS-1 gene in gastric cancer cell line AGS due to gene promoter hypermethylation, which provided evidence that JAK/STAT pathway was activated by aberrant SOCS-1 methylation in gastric cancer. In our study, the SOCS-1 positive expression in gastric cancer was much lower than that in normal gastric tissues (P < 0.001). Besides, the SOCS-1 expression was negatively correlated with the expression of STAT3 (P = 0.002) or pSTAT3 (P = 0.010). We considered that SOCS-1 was an inhibitor for the expression, activation and phosphorylation of STAT3 in gastric cancer.
STAT3 has been considered as a molecular hub for several crucial signaling pathways in different cancer types. It was suggested that the activation of cell proliferation and inhibition of apoptosis were induced by STAT3[30]. Several researchers showed the similar conclusion that oncogenesis of the STAT3 was closely associated with the cell survival and the cell apoptosis[15,25,31]. In our study, the ratio of positive Bcl-2 expression in gastric cancer tissues was significantly higher than that in normal gastric tissues and the Bcl-2 expression was significantly associated with STAT3 (or pSTAT3) expression. Similar results were found in survivin expression. Therefore, we considered that STAT3 could facilitate the progression of gastric cancer by supporting cell survival and inhibiting cell apoptosis.
In summary, the STAT3 expression was significantly associated with the lymph node status, poor prognosis, and the expressions of pSTAT3, survivin and Bcl-2. The SOCS-1 expression was inversely associated with the STAT3 expression. STAT3 could promote tumor cell survival and inhibit cell apoptosis in gastric cancer.
Signal transducer and activator of transcription-3 (STAT3) was identified as the key factor which was associated with cancers by many investigators. However, the correlation between gastric cancer and STAT-3 is not unclear.
The authors detected the expressions of STAT3, phosphor-STAT3 (pSTAT3), SOCS-1, survivin and Bcl-2 proteins in gastric cancer and analyzed the clinicopathological variables associated with the prognosis of gastric cancer. Multivariate analysis showed that pSTAT3 expression and lymph node metastasis were independent factors of overall survival of gastric cancer patients. The expression of STAT3 protein was associated with the status of lymph node metastasis of gastric cancer.
STAT3 could transform into pSTAT3 to promote the survival and inhibit the apoptosis of gastric cancer cells. suppressor of cytokine signaling-1 (SOCS-1) might be the valid molecular antagonist to inhibit the STAT3 expression in gastric cancer.
The molecular contributions of STAT3 to gastric cancer have been elucidated in this study.
STAT3 is significantly associated with the lymph node metastasis, cell survival and inhibition of cell apoptosis in gastric cancer.
Deng et al studied the expressions of STAT3, pSTAT3, SOCS-1, survivin and Bcl-2 proteins in human gastric cancer. The study is of interest and potential clinical importance.
Peer reviewer: Hitoshi Tsuda, MD, PhD, Diagnostic Pathology Section, Clinical Laboratory Division, National Cancer Center Hospital, 5-1-1 Tsukiji, Chuo-ku, Tokyo 104-0045, Japan
S- Editor Tian L L- Editor Ma JY E- Editor Lin YP
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [Cited in This Article: ] |
| 2. | Pisani P, Parkin DM, Bray F, Ferlay J. Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer. 1999;83:18-29. [Cited in This Article: ] |
| 3. | Rohatgi PR, Yao JC, Hess K, Schnirer I, Rashid A, Mansfield PF, Pisters PW, Ajani JA. Outcome of gastric cancer patients after successful gastrectomy: influence of the type of recurrence and histology on survival. Cancer. 2006;107:2576-2580. [Cited in This Article: ] |
| 4. | Chan AO, Luk JM, Hui WM, Lam SK. Molecular biology of gastric carcinoma: from laboratory to bedside. J Gastroenterol Hepatol. 1999;14:1150-1160. [Cited in This Article: ] |
| 5. | Xie K, Huang S. Regulation of cancer metastasis by stress pathways. Clin Exp Metastasis. 2003;20:31-43. [Cited in This Article: ] |
| 6. | Siewert JR, Böttcher K, Stein HJ, Roder JD. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg. 1998;228:449-461. [Cited in This Article: ] |
| 7. | Kim DY, Cha ST, Ahn DH, Kang HY, Kwon CI, Ko KH, Hwang SG, Park PW, Rim KS, Hong SP. STAT3 expression in gastric cancer indicates a poor prognosis. J Gastroenterol Hepatol. 2009;24:646-651. [Cited in This Article: ] |
| 8. | Scholz A, Heinze S, Detjen KM, Peters M, Welzel M, Hauff P, Schirner M, Wiedenmann B, Rosewicz S. Activated signal transducer and activator of transcription 3 (STAT3) supports the malignant phenotype of human pancreatic cancer. Gastroenterology. 2003;125:891-905. [Cited in This Article: ] |
| 9. | Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474-2488. [Cited in This Article: ] |
| 10. | Huang M, Page C, Reynolds RK, Lin J. Constitutive activation of stat 3 oncogene product in human ovarian carcinoma cells. Gynecol Oncol. 2000;79:67-73. [Cited in This Article: ] |
| 11. | Song JI, Grandis JR. STAT signaling in head and neck cancer. Oncogene. 2000;19:2489-2495. [Cited in This Article: ] |
| 12. | Jackson CB, Giraud AS. STAT3 as a prognostic marker in human gastric cancer. J Gastroenterol Hepatol. 2009;24:505-507. [Cited in This Article: ] |
| 13. | Gritsko T, Williams A, Turkson J, Kaneko S, Bowman T, Huang M, Nam S, Eweis I, Diaz N, Sullivan D. Persistent activation of stat3 signaling induces survivin gene expression and confers resistance to apoptosis in human breast cancer cells. Clin Cancer Res. 2006;12:11-19. [Cited in This Article: ] |
| 14. | Lee J, Kang WK, Park JO, Park SH, Park YS, Lim HY, Kim J, Kong J, Choi MG, Sohn TS. Expression of activated signal transducer and activator of transcription 3 predicts poor clinical outcome in gastric adenocarcinoma. APMIS. 2009;117:598-606. [Cited in This Article: ] |
| 15. | Kanda N, Seno H, Konda Y, Marusawa H, Kanai M, Nakajima T, Kawashima T, Nanakin A, Sawabu T, Uenoyama Y. STAT3 is constitutively activated and supports cell survival in association with survivin expression in gastric cancer cells. Oncogene. 2004;23:4921-4929. [Cited in This Article: ] |
| 16. | Gong W, Wang L, Yao JC, Ajani JA, Wei D, Aldape KD, Xie K, Sawaya R, Huang S. Expression of activated signal transducer and activator of transcription 3 predicts expression of vascular endothelial growth factor in and angiogenic phenotype of human gastric cancer. Clin Cancer Res. 2005;11:1386-1393. [Cited in This Article: ] |
| 17. | Jackson CB, Judd LM, Menheniott TR, Kronborg I, Dow C, Yeomans ND, Boussioutas A, Robb L, Giraud AS. Augmented gp130-mediated cytokine signalling accompanies human gastric cancer progression. J Pathol. 2007;213:140-151. [Cited in This Article: ] |
| 18. | Endo TA, Masuhara M, Yokouchi M, Suzuki R, Sakamoto H, Mitsui K, Matsumoto A, Tanimura S, Ohtsubo M, Misawa H. A new protein containing an SH2 domain that inhibits JAK kinases. Nature. 1997;387:921-924. [Cited in This Article: ] |
| 19. | Starr R, Willson TA, Viney EM, Murray LJ, Rayner JR, Jenkins BJ, Gonda TJ, Alexander WS, Metcalf D, Nicola NA. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917-921. [Cited in This Article: ] |
| 20. | Nicholson SE, Willson TA, Farley A, Starr R, Zhang JG, Baca M, Alexander WS, Metcalf D, Hilton DJ, Nicola NA. Mutational analyses of the SOCS proteins suggest a dual domain requirement but distinct mechanisms for inhibition of LIF and IL-6 signal transduction. EMBO J. 1999;18:375-385. [Cited in This Article: ] |
| 21. | Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003;3:46-54. [Cited in This Article: ] |
| 22. | Galizia G, Lieto E, Orditura M, Castellano P, Mura AL, Imperatore V, Pinto M, Zamboli A, De Vita F, Ferraraccio F. Epidermal growth factor receptor (EGFR) expression is associated with a worse prognosis in gastric cancer patients undergoing curative surgery. World J Surg. 2007;31:1458-1468. [Cited in This Article: ] |
| 23. | Sumiyoshi Y, Kakeji Y, Egashira A, Mizokami K, Orita H, Maehara Y. Overexpression of hypoxia-inducible factor 1alpha and p53 is a marker for an unfavorable prognosis in gastric cancer. Clin Cancer Res. 2006;12:5112-5117. [Cited in This Article: ] |
| 24. | Vidal O, Soriano-Izquierdo A, Pera M, Elizalde JI, Palacín A, Castells A, Piqué JM, Volant A, Metges JP. Positive VEGF immunostaining independently predicts poor prognosis in curatively resected gastric cancer patients: results of a study assessing a panel of angiogenic markers. J Gastrointest Surg. 2008;12:1005-1014. [Cited in This Article: ] |
| 25. | Sekikawa A, Fukui H, Fujii S, Ichikawa K, Tomita S, Imura J, Chiba T, Fujimori T. REG Ialpha protein mediates an anti-apoptotic effect of STAT3 signaling in gastric cancer cells. Carcinogenesis. 2008;29:76-83. [Cited in This Article: ] |
| 26. | Choi JH, Ahn MJ, Park CK, Han HX, Kwon SJ, Lee YY, Kim IS. Phospho-Stat3 expression and correlation with VEGF, p53, and Bcl-2 in gastric carcinoma using tissue microarray. APMIS. 2006;114:619-625. [Cited in This Article: ] |
| 27. | Fukushima N, Sato N, Sahin F, Su GH, Hruban RH, Goggins M. Aberrant methylation of suppressor of cytokine signalling-1 (SOCS-1) gene in pancreatic ductal neoplasms. Br J Cancer. 2003;89:338-343. [Cited in This Article: ] |
| 28. | Galm O, Yoshikawa H, Esteller M, Osieka R, Herman JG. SOCS-1, a negative regulator of cytokine signaling, is frequently silenced by methylation in multiple myeloma. Blood. 2003;101:2784-2788. [Cited in This Article: ] |
| 29. | Rottapel R, Ilangumaran S, Neale C, La Rose J, Ho JM, Nguyen MH, Barber D, Dubreuil P, de Sepulveda P. The tumor suppressor activity of SOCS-1. Oncogene. 2002;21:4351-4362. [Cited in This Article: ] |