Published online Mar 7, 2010. doi: 10.3748/wjg.v16.i9.1155
Revised: January 15, 2010
Accepted: January 22, 2010
Published online: March 7, 2010
Splenic lymphangiomatosis is a very rare condition that, from 1990 to date, has been described only nine times. In the present report, we describe the first case of splenic lymphangiomatosis with rapid growth during lactation in a 35-year-old woman. We also underline the difficultly in making an accurate preoperative diagnosis, despite more modern imaging techniques. Total splenectomy was considered to be the treatment needed, both to make a definitive diagnosis and to exclude the presence of malignancy.
- Citation: Patti R, Iannitto E, Di Vita G. Splenic lymphangiomatosis showing rapid growth during lactation: A case report. World J Gastroenterol 2010; 16(9): 1155-1157
- URL: https://www.wjgnet.com/1007-9327/full/v16/i9/1155.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i9.1155
Splenic lymphangiomatosis is a very rare, benign entity that is characterized by the presence of multiple cystic lesions of different sizes that replace all the parenchyma. It is considered to result from developmental failure in the lymphatic system. In the majority of cases, this disease has an asymptomatic course and, despite the use of modern imaging techniques, often makes preoperative diagnosis difficult. Due to the nonspecific features of this disease, splenectomy is needed to make a definitive histological diagnosis and to exclude the possibility of malignant lesions.
In January 2006, a 35-year-old woman was admitted to the General Surgery Unit, Department of Surgical and Oncological Sciences, Palermo, Italy after a hematologist (IE) suggested that she undergo splenectomy.
The patient was referred about 5 wk after her second delivery. About 5 mo before, she had noted increasing pain in the left hypochondrium. Abdominal ultrasound showed an increased in spleen volume with a long axis of 10 cm, with no homogeneous ultrasound structure, which indicated the presence of multiple hypoechoic circular masses with a maximum diameter of 1 cm.
Further abdominal ultrasound performed up to 1 wk before delivery showed that the spleen had no additional changes. Immediately after delivery, the patient began to nurse the baby. In particular, this was the first time she had nursed her newborn, because she did not nurse her first child who was born 3 years before.
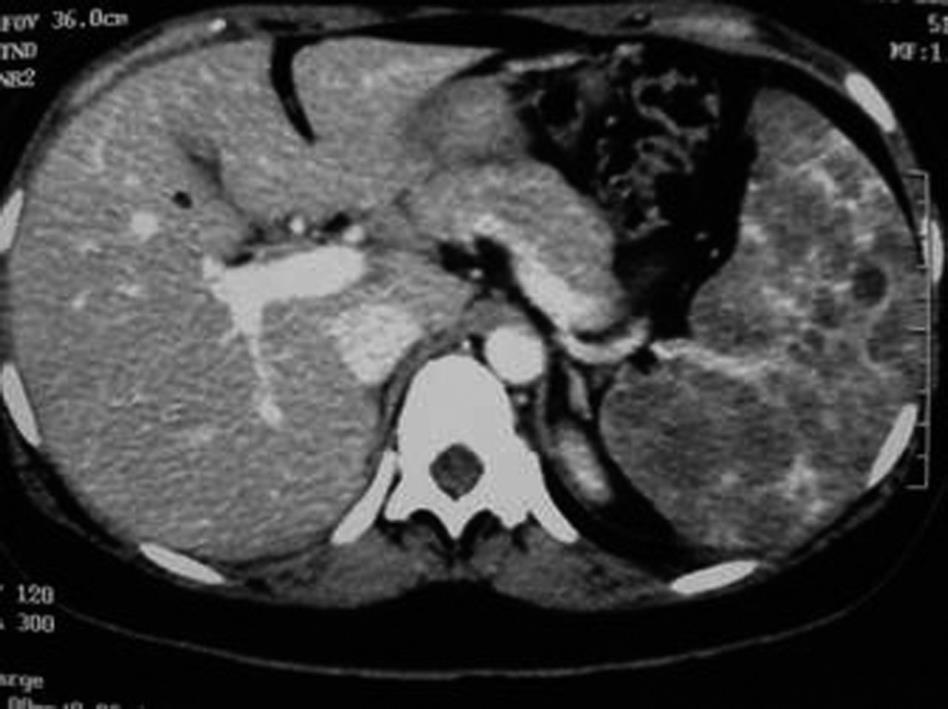
Due to recurrence of pain in the left hypochondrium, after about 5 wk from delivery, the patient underwent abdominal ultrasound that showed spleen enlargement, with a long axis that measured up to 18 cm, with a hypoechoic mass that had a diameter of 2.5 cm. Total body computed tomography showed only splenomegaly with multiple low-density lesions with a nodular appearance, which were considered by the radiologist to be features of lymphoma (Figure 1).
There was no fever, nausea or vomiting. Laboratory findings were all within the normal limits. Also, serological screening for hydatid disease was negative.
Due to the rapid increase in spleen volume, the patient suspended lactation and after 2 d, she underwent conventional open splenectomy. During the operation, careful examination of the abdominal cavity was performed to search for the presence of an accessory spleen, which in this case, was not found. The remaining abdominal viscera were normal.
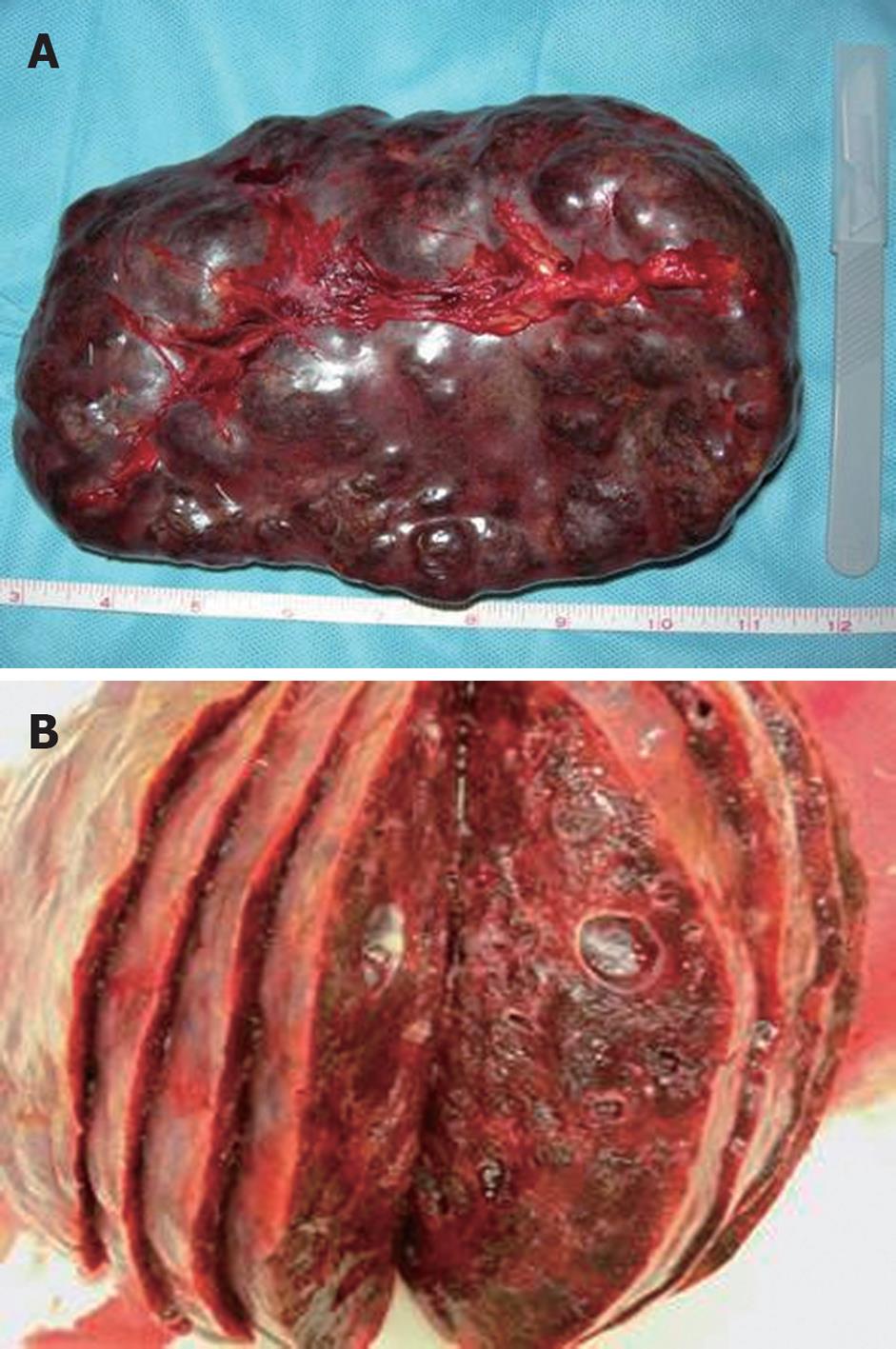
The spleen had an 18-cm long axis, and its macroscopic appearance was characterized by the presence of multiple cystic masses of clear appearance that replaced all the parenchyma, which altered the volume and profile of the spleen (Figure 2). Histological examination revealed various lymphatic space dilatations associated with slow stromal bleeding, which led to a diagnosis of splenic lymphangiomatosis.
Postoperative course was unremarkable and the patient was discharged 6 d after surgery. Additional clinical and instrumental controls did not reveal any further sites of lymphangiomatosis. After a 3-year follow-up, there was no sign of recurrence. The patient did not have a further pregnancy and felt in good health. Ultrasound controls performed on her parents and sons did not show signs of abdominal cystic lesions.
Lymphangioma is a cystic, benign, slow-growing tumor and is a very rare condition, usually seen in children, where it is discovered incidentally[1]. These tumors occur more frequently in females, and 80%-90% are detected before the end of the second year of life. They are generally considered to be a developmental malformation in which obstruction or agenesis of lymphatic tissue results in lymphangiectasia, which is caused by a lack of normal communication of the lymphatic system.
The neck (75%) and axillary regions (20%) are the most common locations of lymphangioma. However, cases have been reported to originate from any part of the gastrointestinal tract. Lymphangioma can be isolated or diffuse and involve one or more organs. The lymphatic alteration, which involves different organs, is termed systemic lymphangiomatosis. The spleen can be involved alone or it can be a part of multivisceral involvement.
Isolated splenic lymphangioma can arise with different manifestations[2]: focal lesions of small size, subcapsular rather than intraparenchymal, with no pathological significance; large cystic lesions that may attain sufficient size to cause significant splenomegaly and left-upper quadrant symptoms; and lymphangiomatosis in which the spleen is replaced diffusely by expanding lymphangioma, thus leaving very little splenic parenchyma. Isolated splenic lymphangiomatosis is a rarer form; from 1990 to date, only nine cases have been described in the literature[1-9]. In this form, the cysts can have different sizes that vary from a few millimeters to two or three centimeters. The spleen, when it is involved, rarely has a normal size, and its volume is frequently greatly increased.
Histologically, lymphangioma is classified as simple, cavernous and cystic, depending on the size of the dilated lymphatics. At gross examination, these cysts have a thick fibrous wall with an internal morphology that is characterized by fibrous trabeculae. Hyalinization and calcification of the fibrous connective tissue may be present. Also, lymphangioma has an endothelial lining, foam cells and a wall that contains lymphatic spaces, lymphoid tissue and smooth muscle. Histochemical staining of the endothelium demonstrates reactivity with CD31, CD34, factor VIII-related antigen and keratin to varying degrees[10]. Sexual hormones can influence the growth of lymphangioma. Quack Loetscher et al[11] have described a case of axillary cavernous lymphangioma that rapidly increased in size during pregnancy. They also hypothesized that the possible pathological mechanism for growth of lymphangioma could be related to the overproduction of cytokines such as vascular endothelial growth factor (VEGF)[11]. Animal studies have shown that prolactin, produced during lactation, stimulates production of VEGF and induces overexpression of its receptor[12]. In the present case, growth of lymphangiomatosis was observed during the period of lactation.
In the adult population, lymphangioma has an asymptomatic course, and often is found as an occasional feature during instrumental investigations performed for other reasons. When symptoms are present, they are related to the size of the spleen and pain is the most common complaint. Less commonly, patients can present with a palpable mass located close to the hypochondrium.
Due to the rarity of presentation and the asymptomatic course of this disease, it is often difficult to make a correct diagnosis. Imaging studies are useful, both to characterize the splenic malformations and to exclude eventual involvement of other organs. Diagnostic studies of lymphangioma usually begin with ultrasonography, which shows the entire spleen as appearing to be replaced by cystic lesions with an anechoic or hypoechoic pattern[13]. Color-Doppler integration can demonstrate the vasculature of the mass, including the intrasplenic arteries and veins along the cyst wall, which helps to determine the organ of origin[13]. Angiography typically reveals an avascular mass. The parenchymogram generally shows multiple focal lucencies of various sizes, the so-called “Swiss-cheese” appearance. Computed tomography demonstrates multiple low-attenuation masses with sharp margins that are typically subcapsular and do not enhance[14]. Upon magnetic resonance, generally lymphatic malformations appear as hyperdense on T2 imaging, whereas T1 imaging is only slightly increased[15]. Fine needle aspiration biopsy in splenic lymphangiomatosis is contraindicated because of the bleeding risk and limited amount of tissue for accurate diagnosis[16]. The differential diagnoses includes malignant (i.e. angiosarcoma or lymphoma) and benign (i.e. hemangioma, littoral cell angioma, and peliosis) splenic vascular proliferation. The instrumental investigations alone are not always useful to make a correct differential diagnosis. This is caused by the rarity of vascular tumors of the spleen and the absence of pathognomonic signs. For these reasons, usually, the correct and definitive diagnosis can be obtained only with histological confirmation; histochemical investigations are needed to make a differential diagnosis among different vascular tumors.
Lymphangiomatosis involves all the splenic parenchyma, therefore, a total splenectomy is the treatment needed. Laparoscopic splenectomy is considered the procedure of choice for normal or moderately enlarged spleens, whereas in the case of severe splenomegaly, open splenectomy is preferred[17]. During surgery, both open and laparoscopic, the search for accessory spleens is an important step. These must be removed even if they appear macroscopically normal, because they could be involved in the pathological process[7,10].
Although the development of lymphangioma in pregnancy has been described in the literature[11,18], the main interest of this case was the rapid growth of splenic lymphangiomatosis, with an increase from 10 to 18 cm in 5 wk during lactation. The rapid growth of the spleen and the suspicion of malignancy led us to perform an open splenectomy.
Peer reviewer: Dr. Ronan A Cahill, Department of General Surgery, Waterford Regional Hospital, Waterford, Cork, Ireland
S- Editor Tian L L- Editor Kerr C E- Editor Lin YP
| 1. | Wadsworth DT, Newman B, Abramson SJ, Carpenter BL, Lorenzo RL. Splenic lymphangiomatosis in children. Radiology. 1997;202:173-176. [Cited in This Article: ] |
| 2. | Morgenstern L, Bello JM, Fisher BL, Verham RP. The clinical spectrum of lymphangiomas and lymphangiomatosis of the spleen. Am Surg. 1992;58:599-604. [Cited in This Article: ] |
| 3. | Talarico C, Cerasoli V, Mancini B, Mulieri G, Cancellario D'Alena F, Montemurro L, Verna F. [Lymphangiomatosis of the spleen. Report of a clinical case]. Ann Ital Chir. 2000;71:599-602. [Cited in This Article: ] |
| 4. | Spapen HD, Reynaert H, Debeuckelaere S, Achten E, Somers G. An unusual case of cystic lymphangiomatosis of the spleen. Neth J Med. 1990;37:24-26. [Cited in This Article: ] |
| 5. | Gómez A, Toscano R, Sánchez E, Vara C. [Splenic lymphangiomatosis]. J Chir (Paris). 1992;129:35-37. [Cited in This Article: ] |
| 6. | Panich V. Splenic cystic lymphangiomatosis: an unusual cause of massive splenomegaly: report of a case. J Med Assoc Thai. 1994;77:165-168. [Cited in This Article: ] |
| 7. | Barrier A, Lacaine F, Callard P, Huguier M. Lymphangiomatosis of the spleen and 2 accessory spleens. Surgery. 2002;131:114-116. [Cited in This Article: ] |
| 8. | Bader TR, Ranner G, Klimpfinger M. Case report: CT appearance of capillary and cavernous lymphangiomatosis of the spleen in an adult. Clin Radiol. 1998;53:379-381. [Cited in This Article: ] |
| 9. | Kwon AH, Inui H, Tsuji K, Takai S, Imamura A, Kamiyama Y. Laparoscopic splenectomy for a lymphangioma of the spleen: report of a case. Surg Today. 2001;31:258-261. [Cited in This Article: ] |
| 10. | Qutub W, Lewis K, Gonzalez R, Quaife R, Russ P, McCarter M. Lymphangiomatosis masquerading as metastatic melanoma. Am Surg. 2006;72:367-370. [Cited in This Article: ] |
| 11. | Quack Loetscher KC, Jandali AR, Garzoli E, Pok J, Beinder E. Axillary cavernous lymphangioma in pregnancy and puerperium. Gynecol Obstet Invest. 2005;60:108-111. [Cited in This Article: ] |
| 12. | Goldhar AS, Vonderhaar BK, Trott JF, Hovey RC. Prolactin-induced expression of vascular endothelial growth factor via Egr-1. Mol Cell Endocrinol. 2005;232:9-19. [Cited in This Article: ] |
| 13. | Komatsuda T, Ishida H, Konno K, Hamashima Y, Naganuma H, Sato M, Ishida J, Masamune O. Splenic lymphangioma: US and CT diagnosis and clinical manifestations. Abdom Imaging. 1999;24:414-417. [Cited in This Article: ] |
| 14. | Abbott RM, Levy AD, Aguilera NS, Gorospe L, Thompson WM. From the archives of the AFIP: primary vascular neoplasms of the spleen: radiologic-pathologic correlation. Radiographics. 2004;24:1137-1163. [Cited in This Article: ] |
| 15. | Solomou EG, Patriarheas GV, Mpadra FA, Karamouzis MV, Dimopoulos I. Asymptomatic adult cystic lymphangioma of the spleen: case report and review of the literature. Magn Reson Imaging. 2003;21:81-84. [Cited in This Article: ] |
| 16. | Alkofer B, Lepennec V, Chiche L. [Splenic cysts and tumors: diagnosis and management]. J Chir (Paris). 2005;142:6-13. [Cited in This Article: ] |
| 17. | Maurus CF, Schäfer M, Müller MK, Clavien PA, Weber M. Laparoscopic versus open splenectomy for nontraumatic diseases. World J Surg. 2008;32:2444-2449. [Cited in This Article: ] |
| 18. | Torashima Y, Yamaguchi J, Taniguchi K, Fujioka H, Shimokawa I, Izawa K, Kanematsu T. Surgery for ileal mesenteric lymphangioma during pregnancy: case report and review of the literature. J Gastrointest Surg. 2004;8:616-620. [Cited in This Article: ] |