Published online Apr 21, 2011. doi: 10.3748/wjg.v17.i15.2007
Revised: December 14, 2010
Accepted: December 21, 2010
Published online: April 21, 2011
AIM: To investigate whether illness severity has an impact on gastric residual volume (GRV) in medical critically ill patients.
METHODS: Medical intensive care unit (ICU) patients requiring nasogastric feeding were enrolled. Sequential Organ Failure Assessment (SOFA) score was assessed immediately preceding the start of the study. Acute Physiology and Chronic Health Evaluation (APACHE) II scores were recorded on the first, fourth, seventh, and fourteenth day of the study period. GRV was measured every 4 h during enteral feeding. The relationship between mean daily GRV and SOFA scores and the correlation between mean daily GRV and mean APACHE II score of all patients were evaluated and compared.
RESULTS: Of the 61 patients, 43 patients were survivors and 18 patients were non-survivors. The mean daily GRV increased as SOFA scores increased (P < 0.001, analysis of variance). Mean APACHE II scores of all patients correlated with mean daily GRV (P = 0.011, Pearson correlation) during the study period. Patients with decreasing GRV in the first 2 d had better survival than patients without decreasing GRV (P = 0.017, log rank test).
CONCLUSION: GRV is higher in more severely ill medical ICU patients. Patients with decreasing GRV had lower ICU mortality than patients without decreasing GRV.
- Citation: Hsu CW, Sun SF, Lee DL, Lin SL, Wong KF, Huang HH, Li HJ. Impact of disease severity on gastric residual volume in critical patients. World J Gastroenterol 2011; 17(15): 2007-2012
- URL: https://www.wjgnet.com/1007-9327/full/v17/i15/2007.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i15.2007
Malnutrition is prevalent in intensive care unit (ICU) patients and is associated with increased morbidity and mortality[1]. Early administration of enteral nutrition to critically ill patients has been associated with a significantly lower incidence of infections and a reduced length of hospital stay[2,3]. However, intragastric enteral nutrition often is complicated by intolerance, as indicated by elevated volumes of aspirated gastric residuals[4]. Disordered upper gastrointestinal (GI) tract motility occurs frequently in ICU patients. Intolerance to nasogastric delivery of feeding is the most important consequence of the abnormal upper GI motility that occurs in critically ill patients[5]. Several factors related to critical illness have been reported to be associated with gastric dysmotility and feeding intolerance including age, admission diagnosis, hyperglycemia, the nature of the acute illness, mechanical ventilation, sedatives, cytokine release and splanchnic hypoperfusion due to shock and sepsis[6]. Two studies have shown that “upper digestive intolerance” and “enteral feeding intolerance” are linked to adverse outcomes, suggesting that decreased gastric emptying (GE) is related to clinical deterioration and worsening of patient outcomes[7,8]. Direct measurement of GE is usually inconvenient and impractical in routine clinical practice. Clinically, gastric residual volumes (GRVs) are easier to measure than GE, and GRV measurements are by far the most frequently recommended assessment for GE[9]. They are used as a surrogate marker to determine the success or failure of nutrition delivered via a nasogastric route. However, the relationship between GRV and disease severity is not clear. The aim of this study is to investigate whether disease severity has an impact on GRV and whether GRV is a predictor of ICU mortality.
This prospective, observational study was conducted during a 2-year period from January 2005 to December 2006 in a medical ICU of a tertiary medical center. Patients who required enteral feeding were enrolled. Criteria for exclusion included abdominal surgery, acute pancreatitis, GI bleeding, intestinal obstruction, and patients with subtotal or total gastrectomy. The protocol was approved by the Human Investigation and Research Committee of the hospital.
After informed consent was obtained, the following demographic data were collected: primary ICU admission diagnosis, age, gender, body mass index (BMI), use of mechanical ventilation, Sequential Organ Failure Assessment (SOFA) score[10], Acute Physiology and Chronic Health Evaluation (APACHE) II score[11], blood glucose level, number of ICU days, ventilator days, hospital days, and Glasgow Coma Scale score. A standard 12 French enteral feeding tube (Abbott, Chicago, IL, USA) for general patients was placed into the stomach. The correct position of the nasogastric tube was confirmed by injecting 50 mL of air with a syringe into the tube and auscultating the epigastric area, or by radiograph if necessary. We checked the tube position by measuring the exposed portion of the tube and compared the length with previous measurements. The patients were fed in a semi-recumbent position, and the patient’s position and tube length were kept the same in each measurement. As soon as the feeding tube was inserted, continuous tube feeding using enteral feeding pumps (Abbott) was started. Enteral feeding was initiated at 20 mL/h. The rate was increased by 20 mL/h every 4 h until the volume required to meet the patient’s optimum caloric support was achieved. The rate of continuous enteral feeding was controlled by the pumps. GRV was measured by aspirating with a 50-mL syringe every 4 h until the end of enteral feeding. Feeding was stopped for 30 min before GRV was measured. After measurement, the nurses stopped enteral tube feedings if residual volume was higher than 500 mL or residual volume was between 200 to 500 mL and patients had abdominal distension, absence of bowel sounds, or presence of nausea or vomiting[12]. Feeding re-started immediately at original rate if GRV < 200 mL and there was low risk of aspiration. Daily GRV was calculated by summation of each GRV measurement. Serum glucose was controlled by an intensive insulin control protocol in order to reach the target glucose level of 140 mg/dL.
APACHE II scores[11] were recorded on the first, fourth, seventh, and fourteenth day of the study period. Study observations continued from start of enteral feeding until one of the following events occurred: the enteral tube was removed, the patient was discharged from the ICU, or he/she expired. SOFA scores[10] were assessed within a 24-h period preceding the start of study as the presence or absence of prospectively defined cardiovascular, respiratory, renal, hepatic and hematologic dysfunction, as well as level of consciousness. Dysfunction of cardiovascular, respiratory, renal, hepatic, hematologic and central nervous systems was determined based on laboratory data, vasopressor dosage, Glasgow Coma Scale score, and PaO2/FiO2.
Patients were classified as diabetic on the basis of their medical history. Survivors were defined as patients who were alive when discharged from the ICU or transferred to a general medical ward; this was determined at time of ICU discharge.
All the statistical analyses were done with the SPSS (Inc., Chicago, IL, USA) version 12.0. mean ± SE were recorded for all continuous variables. For discrete variables, the frequencies were reported. One way analysis of variance (ANOVA) was used to compare differences among more than 2 groups. Pearson correlation was used to compare correlation between daily GRV and APACHE II score. Kaplan-Meier curves were used to estimate the probability of survival. Log-rank test was used to compare the difference between the patients with decreasing daily GRV and patients without decreasing daily GRV in the first 2 d. Cox model was used to construct the relative risk among the percentage change of daily GRV in the first 2 d. All P values were two-tailed. A P value < 0.05 was considered as significant.
Sixty-one patients were enrolled in this study. Patient characteristics are summarized in Table 1. The mean patient age was 67.9 ± 2.0 years, and 70.1% of the patients were male. The mean BMI was 23.1 ± 0.5 kg/m2. Thirty-six percent of patients had diabetes and all patients were mechanically ventilated. The mean number of study days was 11.7 ± 0.8 d, mean number of ICU days was 19.1 ± 1.6 d, mean number of ventilator days was 23.8 ± 2.3 d, mean number of hospital days was 31.7 ± 2.7 d, mean SOFA score was 7.5 ± 0.6, mean APACHE II score was 20.2 ± 0.9, and mean blood glucose was 184.7 ± 8.6 mg/dL. The ICU mortality rate was 29.5%. The 4 most common admission diagnoses were pneumonia (n = 19), sepsis (n = 18), congestive heart failure (n = 6) and acute respiratory distress syndrome (n = 6).
| Average age (yr) | 67.9 ± 2.0 |
| Gender (male) | 43/61 (70.1) |
| Body mass index (kg/m2) | 23.1 ± 0.5 |
| Diabetic patients | 22/61 (36.1) |
| Mechanically ventilated patients | 61/61 (100) |
| Study days | 11.7 ± 0.8 |
| ICU days | 19.1 ± 1.6 |
| Ventilator days | 23.8 ± 2.3 |
| Hospital days | 31.7 ± 2.7 |
| SOFA score | 7.5 ± 0.6 |
| APACHE II score | 20.2 ± 0.9 |
| Blood glucose level (mg/dL) | 184.7 ± 8.6 |
| ICU mortality rate | 18/61 (29.5) |
| Admission diagnosis | |
| Pneumonia | 19/61 (31.1) |
| Sepsis | 18/61 (29.5) |
| CHF | 6/61 (9.8) |
| ARDS | 6/61 (9.8) |
| Stroke | 5/61 (8.2) |
| COPD or asthma | 5/61 (8.2) |
| Myocardial infarction | 1/61 (1.6) |
| Toxic overdose | 1/61 (1.6) |
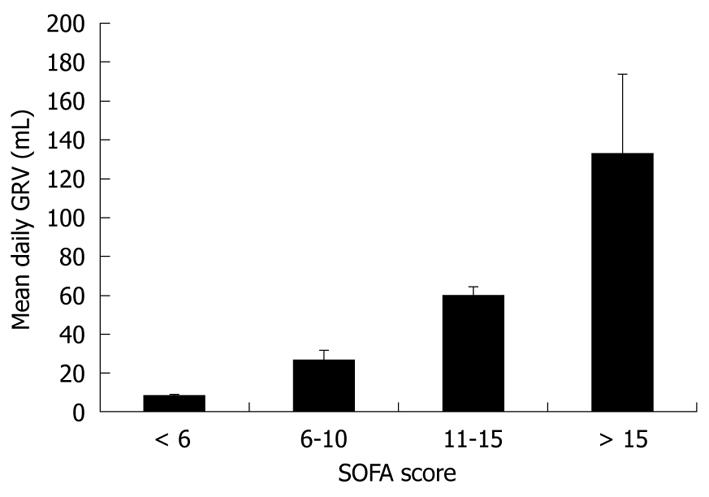
We stratified study patients into 4 groups by SOFA score. There were 27 patients with SOFA scores below 6; 18 patients with scores in the range of 6-10; 10 patients with scores in the range of 11-15; and 6 patients with scores above 15. Figure 1 demonstrates that the mean daily GRV was 7.8 ± 1.3 mL in the patients with a SOFA score below 6, 26.6 ± 4.8 mL in the patients with SOFA scores in the range of 6-10, 59.8 ± 4.5 mL in the patients with SOFA scores in the range of 11-15, and 133.2 ± 40.0 mL in the patients with a SOFA score above 15. Patient with higher SOFA scores had significantly higher daily GRV (P < 0.001, ANOVA).
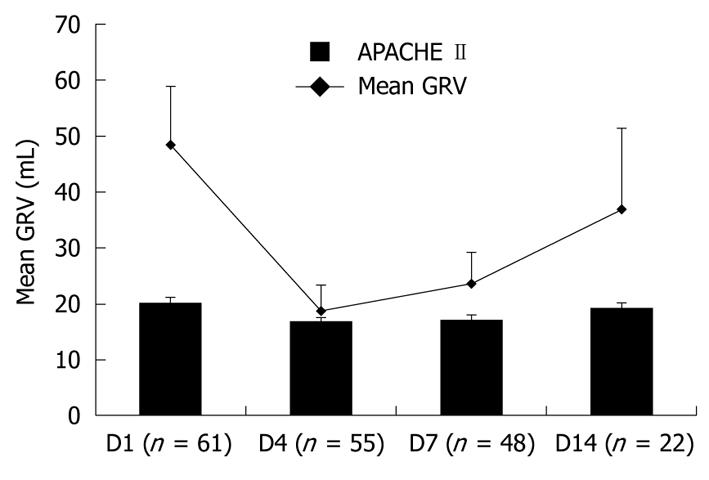
During the study period, the mean APACHE II scores and mean daily GRV of all patients on the first, fourth, seventh, and fourteenth day are shown in Figure 2. The mean APACHE II scores of all patients on the first, fourth, seventh, and fourteenth day were 20.2 ± 0.9, 16.8 ± 0.7, 17.0 ± 0.9 and 19.1 ± 1.1, respectively. The mean daily GRVs of all patients on the first, fourth, seventh, and fourteenth day were 48.4 ± 10.4, 18.6 ± 4.6, 23.6 ± 5.5 and 36.9 ± 14.4 mL, respectively. The mean daily GRV fluctuated simultaneously with the mean APACHE II scores during the study period. There was a significant correlation between daily GRV and APACHE II score (P = 0.011, Pearson correlation = 0.338).
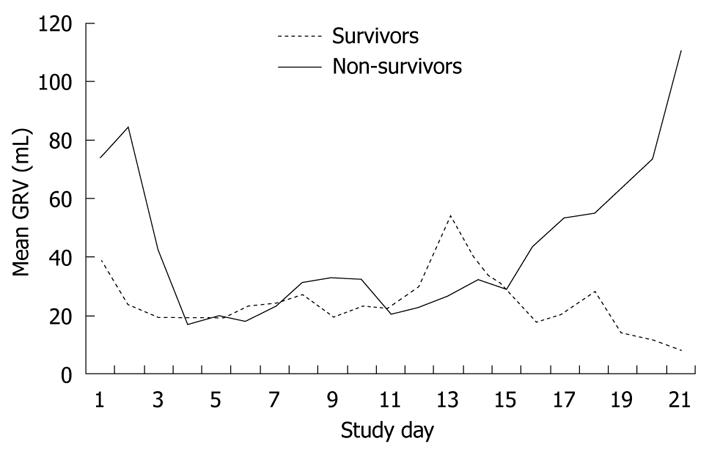
We divided study patients into survivors and non-survivors. There were 43 patients in the survivor group and 18 patients in the non-survivor group. Figure 3 shows the mean daily GRV of survivors and non-survivors during the study period. The mean daily GRV of non-survivors was higher than that of survivors in the early and late stages of the study period. Non-survivors had a trend of increasing mean daily GRV in the first 2 d, while survivors had a decreasing trend of GRV.
Table 2 demonstrates that the mean GRV1 (daily GRV on the first day) was 74.4 ± 31.0 mL in non-survivors and 38.0 ± 6.9 mL in survivors. Additionally, the mean [GRV1-GRV2 (daily GRV on the second day)]/GRV1 was -1.1 ± 0.6 mL in non-survivors and 0.3 ± 0.1 mL in survivors. If we define the percentage of daily GRV change from day 1 to day 2 (PDay12) as (GRV1-GRV2)/GRV1, non-survivors had a negative PDay12 while survivors had a positive PDay12.
| Non-survivors (n = 18) | Survivors (n = 43) | |
| GRV1 (mL) | 74.4 ± 31.0 | 38.0 ± 6.9 |
| PDay12 | -1.1 ± 0.6 | 0.3 ± 0.1 |
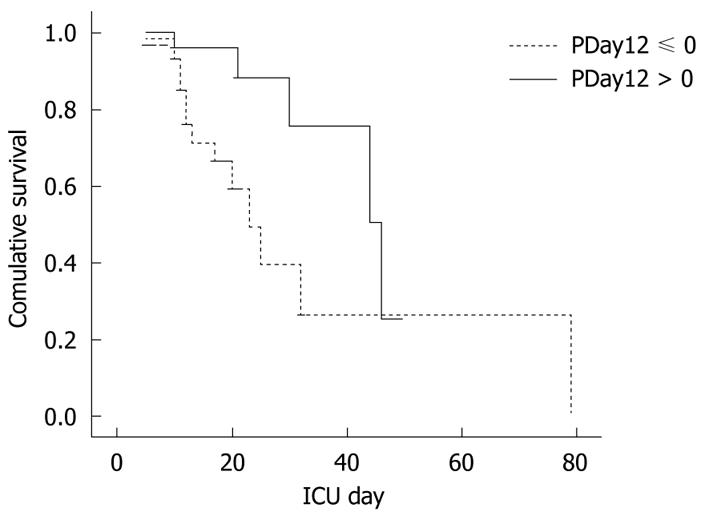
We categorized patients into 2 groups; one group was patients without a decreasing daily GRV (PDay12 ≤ 0) in the first 2 d, and the other group was patients with a decreasing daily GRV in the first 2 d (PDay12 > 0). Thirty patients did not have a decreasing daily GRV (PDay12 ≤ 0) and 31 patients had a decreasing daily GRV (PDay12 > 0). Patients with PDay12 > 0 had a significantly higher ICU survival rate than patients with PDay12 ≤ 0 (P = 0.017) (Figure 4). Relative risk among the percentage change of daily GRV in the first 2 d was constructed by Cox model. The relative risk is equal to exp {-1.211x} where x represents the percentage change of daily GRV in the first 2 d. If the daily GRV decreased by 10% in the second day, the relative risk is equal to 0.886 (= exp {-0.1211}). If the daily GRV increased by 10% in the second day, the relative risk is equal to 1.129 (= exp {0.1211}). There was no evidence indicating that the proportional hazards assumption was not fixed in the data set.
In this prospective study, we found disease severity was associated with GRV in these medical ICU patients. This study showed patients with higher SOFA scores had higher GRV, i.e. GRV tended to be higher in more severely ill patients. These results are similar to those of the study by Mentec et al[8], in which patients with high gastric aspirate volume had a higher ICU mortality rate. Slow GE may partly explain why patients with more severe illness had higher GRV. GRV is determined by the balance between the amount of infused formula plus endogenous secretions (saliva and gastric secretions), and the amount of fluid emptied from the stomach[9]. GE is influenced by many factors, including admission diagnosis, nature of illness, age, medications and mechanical ventilation[5,6,8,9]. Nguyen et al[6] reported that slow GE was more common in patients who were older, had higher admission APACHE II scores, admission blood glucose and bilirubin concentrations, and were ventilated with synchronized intermittent mandatory ventilation. Of these, APACHE II scores correlated best with GE, suggesting that illness severity is an important determinant of GE in critically ill patients. Patients with severe illnesses have high levels of circulating catecholamines which are likely to have an impact on GI motor function. Adrenaline reduces GE via a β-adrenergic effect[13].
During the study period, we also found a significant correlation between mean APACHE II scores and mean GRV. There was a trend that patients with higher APACHE II scores had higher daily GRV. Our results also demonstrated that illness severity was related to GRV, and illness severity varied during the ICU course. Concurrent day-to-day variation in illness severity and daily GRV were seen in this study. GRV was the greatest in the first few days of tube feedings. Some of the severely ill patients with high APACHE II scores expired in the first few days, thus the overall illness severity of patients declined from the first day to the fourth day, so mean APACHE II score also decreased from the first day to the fourth day. Mean daily GRV decreased as mean APACHE II score decreased. As the study went on, some patients improved, they were extubated, and their enteral feeding stopped; therefore the number of study patients decreased gradually. The rest of the study patients were more complicated or severely ill and they had higher APACHE II scores, thus the mean APACHE IIscore increased gradually after the seventh day of the study. From that time on, mean daily GRV also increased gradually. Mean daily GRV exhibited a good correlation with APACHE II scores (P < 0.05).
In the interim analysis, we also found that patients with the same SOFA score had widely different daily GRV. This suggests that there was wide variability of daily GRV among patients with the same disease severity, since GRV is not only influenced by disease severity but also by other factors such as GE, medications, size of the feeding tube and timing of measurement.
GE itself is influenced by many factors. Admission diagnosis had modest impact on GE in critically ill patients[6]. Most patients with increased intracranial pressure after a head injury have been found to have slow GE, and elevated intracranial pressure is thought to be the main mediator of impaired gastric motility and emptying[14]. Other diseases associated with delayed GE include burns, multiple trauma, sepsis, chronic liver disease, and renal disease[6,15]. Patients with myocardial injury and non-intestinal post-operative respiratory failure have the lowest incidence of delayed GE[6]. Hyperglycemia has also been shown to result in delayed GE[16].
Medications used routinely in ICU patients are also likely to have clinically important effects on GI motor function. Opioids and benzodiazepines can impair gastric motility and reduce GE[17,18]. High gastric aspirate volume was more frequently seen in patients who received at least 1 d of sedation[8]. Both endogenous and administered opiates, acting viaμ receptors, may contribute to abnormal upper GI motor function[19,20]. Dopamine slows GE by reducing antral contractions[21]. Its negative effect on GI motility can be seen at doses as low as 5 μg/kg per minute, and the effect increases with increasing rates of infusion[22]. Proton pump inhibitors[23] and cimetidine[24] can delay GE. Other medications such as phenothiazines, diltiazem, verapamil and anticholinergic drugs also cause GI hypomotility[5]. Use of promotility agents is associated with reduced GRV[25], e.g. erythromycin is a powerful stimulator of gastric contractions[26].
The size of enteral feeding tube has also been shown to influence the measurement of GRV. Higher GRVs are found in patients with larger enteral feeding tubes[27]. Timing of measurement of GRV also affects the value of GRV. GRV typically increases and then plateaus in the first 3 to 6 h after feeding. The highest GRV tends to occur in the 2 to 4 h after initiation of feedings. Additionally, GRV may vary with different infusion rate. To avoid this confounding factor, we stopped feeding for 30 min prior to measurement of GRV. This is the reason why the measured GRV values in this study are lower than in previous studies[28,29].
As discussed above, there are many factors that influence GRV. This results in wide variability of daily GRV in patients with the same illness severity. For this reason, we used daily GRV change rather than a single daily GRV to predict a patient’s ICU outcome. This may avoid some of the confounding factors such as admission diagnosis, nature of illness, medications and age, as it reflects the change of illness severity more accurately. In addition, we also found that there was a different trend of GRV change between survivors and non-survivors in the first 2 d. Daily GRV tended to increase in non-survivors and decrease in survivors. We found that patients with a decreasing change of daily GRV had better ICU survival than those without a decreasing change of daily GRV in the first 2 study days. Because daily GRV correlated with illness severity, decreasing changes of daily GRV in the earlier ICU days represented decreasing illness severity; thus could indicate a positive sign for the medical ICU patients.
There are limitations in this study. Firstly, this was not a double-blind study. However, nurses who measured GRV were not aware of the study purpose. Secondly, all patients in this study were mechanically ventilated and enrolled from a medical ICU; thus, the results may not be applicable to all critically ill patients. Further larger studies including all critically ill patients are needed. Thirdly, we did not know how many patients had diabetes with gastroparesis which is characterized by severely slow GE; such patients may have increased GRVs secondary to gastroparesis[30].
In conclusion, illness severity has an impact on daily GRV. There was a trend that more severely ill medical ICU patients had higher daily GRV. Patients with decreasing change of daily GRV in the earlier ICU days had better ICU survival than patients without decreasing change of daily GRV.
Disordered upper gastrointestinal tract motility occurs frequently in critical patients, resulting in intolerance to nasogastric delivery of feeding. Upper digestive intolerance and enteral feeding intolerance are linked to adverse outcomes, suggesting that decreased gastric emptying (GE) is related to clinical deterioration. It is easier to measure gastric residual volume (GRV) than GE, but it is not clear whether illness severity has an impact on GRV.
GE was influenced by age, Acute Physiology and Chronic Health Evaluation (APACHE) II score, nature of illness, medication and mechanical ventilation. Of these, APACHE II score correlated best with GE. This study found disease severity had an impact on GRV. Concurrent day-to-day variation in illness severity and daily GRV were seen during hospitalization course. There is a trend that GRV increases when disease severity increases, and vice versa.
Patients with decreasing change of GRV in the earlier intensive care unit (ICU) had better ICU outcome than patients without decreasing change. We emphasized that change of daily GRV, not a single daily GRV, can predict ICU outcome. Since GRV was influenced by many factors, change of daily GRV can avoid some confounding factors other than illness severity.
Increasing change of daily GRV is a negative sign for critical patients. In the clinical situation, we should be careful if a patient has increasing change of daily GRV day by day as it may indicate the patient getting gradually worse.
GRV is determined by the balance between the amount of infused formula plus endogenous secretion and the amount of fluid emptied from the stomach. APACHE II score, a system for classifying disease severity, is widely used to predict hospital mortality based on a number of laboratory values and patient characteristics. Sequential Organ Failure Assessment scores were assessed as presence or absence of cardiovascular, respiratory, renal, hepatic, hematologic and central nervous systems dysfunction. A higher score means more severe illness.
The manuscript is of good quality.
Peer reviewer: Maxim Petrov, MD, MPH, Department of Surgery, University of Auckland, Private Bag 921019, Auckland 1142, New Zealand
S- Editor Sun H L- Editor Logan S E- Editor Zheng XM
| 1. | Dempsey DT, Mullen JL, Buzby GP. The link between nutritional status and clinical outcome: can nutritional intervention modify it? Am J Clin Nutr. 1988;47:352-356. [Cited in This Article: ] |
| 2. | Marik PE, Zaloga GP. Early enteral nutrition in acutely ill patients: a systematic review. Crit Care Med. 2001;29:2264-2270. [Cited in This Article: ] |
| 3. | Kattelmann KK, Hise M, Russell M, Charney P, Stokes M, Compher C. Preliminary evidence for a medical nutrition therapy protocol: enteral feedings for critically ill patients. J Am Diet Assoc. 2006;106:1226-1241. [Cited in This Article: ] |
| 4. | MacLaren R. Intolerance to intragastric enteral nutrition in critically ill patients: complications and management. Pharmacotherapy. 2000;20:1486-1498. [Cited in This Article: ] |
| 5. | Mutlu GM, Mutlu EA, Factor P. GI complications in patients receiving mechanical ventilation. Chest. 2001;119:1222-1241. [Cited in This Article: ] |
| 6. | Nguyen NQ, Ng MP, Chapman M, Fraser RJ, Holloway RH. The impact of admission diagnosis on gastric emptying in critically ill patients. Crit Care. 2007;11:R16. [Cited in This Article: ] |
| 7. | Wolf SE, Jeschke MG, Rose JK, Desai MH, Herndon DN. Enteral feeding intolerance: an indicator of sepsis-associated mortality in burned children. Arch Surg. 1997;132:1310-1313; discussion 1313-1314. [Cited in This Article: ] |
| 8. | Mentec H, Dupont H, Bocchetti M, Cani P, Ponche F, Bleichner G. Upper digestive intolerance during enteral nutrition in critically ill patients: frequency, risk factors, and complications. Crit Care Med. 2001;29:1955-1961. [Cited in This Article: ] |
| 9. | Metheny NA, Schallom ME, Edwards SJ. Effect of gastrointestinal motility and feeding tube site on aspiration risk in critically ill patients: a review. Heart Lung. 2004;33:131-145. [Cited in This Article: ] |
| 10. | Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707-710. [Cited in This Article: ] |
| 11. | Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818-829. [Cited in This Article: ] |
| 12. | McClave SA, DeMeo MT, DeLegge MH, DiSario JA, Heyland DK, Maloney JP, Metheny NA, Moore FA, Scolapio JS, Spain DA. North American Summit on Aspiration in the Critically Ill Patient: consensus statement. JPEN J Parenter Enteral Nutr. 2002;26:S80-S85. [Cited in This Article: ] |
| 13. | Gáti T, Gelencsér F, Hideg J. The role of adrenergic receptors in the regulation of gastric motility in the rat. Z Exp Chir. 1975;8:179-184. [Cited in This Article: ] |
| 14. | Kao CH, ChangLai SP, Chieng PU, Yen TC. Gastric emptying in head-injured patients. Am J Gastroenterol. 1998;93:1108-1112. [Cited in This Article: ] |
| 15. | Hu OY, Ho ST, Wang JJ, Ho W, Wang HJ, Lin CY. Evaluation of gastric emptying in severe, burn-injured patients. Crit Care Med. 1993;21:527-531. [Cited in This Article: ] |
| 16. | Björnsson ES, Urbanavicius V, Eliasson B, Attvall S, Smith U, Abrahamsson H. Effects of hyperglycemia on interdigestive gastrointestinal motility in humans. Scand J Gastroenterol. 1994;29:1096-1104. [Cited in This Article: ] |
| 17. | Nimmo WS, Heading RC, Wilson J, Tothill P, Prescott LF. Inhibition of gastric emptying and drug absorption by narcotic analgesics. Br J Clin Pharmacol. 1975;2:509-513. [Cited in This Article: ] |
| 18. | Steyn PF, Twedt D, Toombs W. The effect of intravenous diazepam on solid phase gastric emptying in normal cats. Vet Radiol Ultrasound. 1997;38:469-473. [Cited in This Article: ] |
| 19. | Stanghellini V, Malagelada JR, Zinsmeister AR, Go VL, Kao PC. Effect of opiate and adrenergic blockers on the gut motor response to centrally acting stimuli. Gastroenterology. 1984;87:1104-1113. [Cited in This Article: ] |
| 20. | Murphy DB, Sutton JA, Prescott LF, Murphy MB. Opioid-induced delay in gastric emptying: a peripheral mechanism in humans. Anesthesiology. 1997;87:765-770. [Cited in This Article: ] |
| 21. | Dive A, Foret F, Jamart J, Bulpa P, Installé E. Effect of dopamine on gastrointestinal motility during critical illness. Intensive Care Med. 2000;26:901-907. [Cited in This Article: ] |
| 22. | Levein NG, Thörn SE, Lindberg G, Wattwill M. Dopamine reduces gastric tone in a dose-related manner. Acta Anaesthesiol Scand. 1999;43:722-725. [Cited in This Article: ] |
| 23. | Rasmussen L, Oster-Jørgensen E, Qvist N, Pedersen SA. The effects of omeprazole on intragastric pH, intestinal motility, and gastric emptying rate. Scand J Gastroenterol. 1999;34:671-675. [Cited in This Article: ] |
| 24. | Scarpignato C, Bertaccini G. Different effects of cimetidine and ranitidine on gastric emptying in rats and man. Agents Actions. 1982;12:172-173. [Cited in This Article: ] |
| 25. | Pinilla JC, Samphire J, Arnold C, Liu L, Thiessen B. Comparison of gastrointestinal tolerance to two enteral feeding protocols in critically ill patients: a prospective, randomized controlled trial. JPEN J Parenter Enteral Nutr. 2001;25:81-86. [Cited in This Article: ] |
| 26. | Dive A, Miesse C, Galanti L, Jamart J, Evrard P, Gonzalez M, Installé E. Effect of erythromycin on gastric motility in mechanically ventilated critically ill patients: a double-blind, randomized, placebo-controlled study. Crit Care Med. 1995;23:1356-1362. [Cited in This Article: ] |
| 27. | Metheny NA, Stewart J, Nuetzel G, Oliver D, Clouse RE. Effect of feeding-tube properties on residual volume measurements in tube-fed patients. JPEN J Parenter Enteral Nutr. 2005;29:192-197. [Cited in This Article: ] |
| 28. | Lin HC, Van Citters GW. Stopping enteral feeding for arbitrary gastric residual volume may not be physiologically sound: results of a computer simulation model. JPEN J Parenter Enteral Nutr. 1997;21:286-289. [Cited in This Article: ] |
| 29. | Davies AR, Froomes PR, French CJ, Bellomo R, Gutteridge GA, Nyulasi I, Walker R, Sewell RB. Randomized comparison of nasojejunal and nasogastric feeding in critically ill patients. Crit Care Med. 2002;30:586-590. [Cited in This Article: ] |
| 30. | Samsom M, Bharucha A, Gerich JE, Herrmann K, Limmer J, Linke R, Maggs D, Schirra J, Vella A, Wörle HJ. Diabetes mellitus and gastric emptying: questions and issues in clinical practice. Diabetes Metab Res Rev. 2009;25:502-514. [Cited in This Article: ] |