Published online Jan 28, 2011. doi: 10.3748/wjg.v17.i4.409
Revised: December 1, 2010
Accepted: December 8, 2010
Published online: January 28, 2011
Nestin is a class VI intermediate filament protein that was originally described as a neuronal stem cell marker during central nervous system (CNS) development, and is currently widely used in that capacity. Nestin is also expressed in non-neuronal immature or progenitor cells in normal tissues. Under pathological conditions, nestin is expressed in repair processes in the CNS, muscle, liver, and infarcted myocardium. Furthermore, increased nestin expression has been reported in various tumor cells, including CNS tumors, gastrointestinal stromal tumors, pancreatic cancer, prostate cancer, breast cancer, malignant melanoma, dermatofibrosarcoma protuberances, and thyroid tumors. Nestin is reported to correlate with aggressive growth, metastasis, and poor prognosis in some tumors; however, the roles of nestin in cancer cells have not been well characterized. Furthermore, nestin is more specifically expressed in proliferating small-sized tumor vessels in glioblastoma and gastric, colorectal, and prostate cancers than are other tumor vessel markers. These findings indicate that nestin may be a marker for newly synthesized tumor vessels and a therapeutic target for tumor angiogenesis. It has received a lot of attention recently as a cancer stem cell marker in various cancer cells including brain tumors, malignant rhabdoid tumors, and uterine, cervical, prostate, bladder, head and neck, ovarian, testicular, and pancreatic cancers. The purpose of this review is to clarify the roles of nestin in cancer cells and in tumor angiogenesis, and to examine the association between nestin and cancer stem cells. Nestin has the potential to serve as a molecular target for cancers with nestin-positive cancer cells and nestin-positive tumor vasculature.
- Citation: Ishiwata T, Matsuda Y, Naito Z. Nestin in gastrointestinal and other cancers: Effects on cells and tumor angiogenesis. World J Gastroenterol 2011; 17(4): 409-418
- URL: https://www.wjgnet.com/1007-9327/full/v17/i4/409.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i4.409
Nestin is an intermediate filament (IF) protein that was originally described in 1990 as a neuronal stem cell/progenitor cell marker during central nervous system (CNS) development[1]. Cytoskeletal proteins mainly consist of microfilaments, IFs, and microtubules. The diameter of microfilaments (actin) is 5-7 nm and that of microtubules is 20-25 nm, while the diameter of IFs falls between the two at 10 nm, giving them their name. IFs are classified into six subtypes[2,3], and the IF protein members are expressed in specific cell types, for example, keratin in epithelial cells, vimentin in mesenchymal cells, desmin in muscular cells, neurofilament in neuronal cells, and glial fibrillar acidic protein in glial cells.
Nestin is expressed in dividing cells during the early stages of development in the CNS, peripheral nervous system, and myogenic and other tissues. With differentiation, nestin is downregulated and replaced by tissue-specific IF proteins, and therefore, is widely used as a neuronal stem cell marker. Nestin is also expressed in immature or progenitor cells in non-neuronal cells in normal tissues[4-7]. High levels of nestin expression have been detected in oligodendroglial lineage cells, ependymocytes, Sertoli cells, enteric glia, hair follicle cells, podocytes of renal glomeruli, pancreatic stellate cells, pericytes, islets, optic nerve, and odontoblasts[8-14]. Recent work has shown that nestin is also expressed in follicle stem cells and their immediate, differentiated progeny, and the hair follicle bulge area has been noted as an easily accessible source of actively growing pluripotent adult stem cells[15]. In adult organisms, nestin-expressing cells are restricted to defined locations, where they may function as a cellular reserve that is capable of proliferation, differentiation, and migration after reactivation[16].
In pathological conditions, nestin is expressed in repair processes in the CNS, muscle, liver[17-20], and infarcted myocardium[21]. Furthermore, increased nestin expression has been reported in various tumor cells, including CNS tumors, pancreatic cancer, gastrointestinal stromal tumors (GISTs), prostate cancer, breast cancer, malignant melanoma, dermatofibrosarcoma protuberances, and thyroid tumors[22-28] (Table 1). Expression of nestin in several tumors has been reported to be closely correlated with poor prognosis. Recently, nestin has also received attention as a cancer stem cell marker in various tumor cells including brain tumors, uterine and cervical cancers, prostate cancer, bladder cancer, head and neck cancers, ovarian cancer, testicular cancer, pancreatic cancer, and malignant rhabdoid tumors[29-36]. In the tumor tissues, proliferating vascular endothelial cells also highly express nestin, and nestin is therefore closely correlated with tumor angiogenesis[37-40] (Table 2). Detailed analyses of expression patterns of nestin in various tumor tissues and tumor angiogenesis, including gastrointestinal cancer, will be helpful for examining the roles of nestin in mechanisms of tumor growth and invasion and for finding novel therapeutic targets.
| Expression patterns | Roles | |
| Glioblastoma[66-69] | Tumor cells and tumor vessels | In vitro and in vivo growth |
| Glioma << glioblastoma | G1/S arrest | |
| Migration, invasion | ||
| Pancreatic cancer[87,90] | 30% of PDAC | Nerve invasion, migration |
| Initiation of PanIN | ||
| GIST[25,96] | Tumor cells and interstitial cells of Cajal | |
| Prostate cancer[27] | Androgen-insensitive cancer cells | Migration, invasion in vitro |
| 75% of lethal androgen-independent prostate cancer | Lung metastasis | |
| Breast cancer[26,101-103] | Basal breast cancer subtype | Shorter survival |
| Triple-negative breast cancer | Independent prognostic factor | |
| Lymphovascular embolus of inflammatory breast cancer | ||
| Malignant melanoma[24,106-108,112,113] | Tumor cells and endothelial cells | Advanced stage |
| Ulceration of primary tumors | Metastasis | |
| Infiltrating front of tumors | Shorter survival | |
| Primary tumor << metastatic tumor | ||
| Stage IV >> III/IV with no evidence of disease in blood |
| Expression patterns | Roles | |
| Gastric adenocarcinoma[37] | Tumor blood vessels | Shorter survival |
| Colorectal cancer[38] | Small-sized and proliferating tumor vessels | Shorter survival |
| Prostate cancer[39,40] | Proliferating vascular cells | Shorter survival |
| Endothelial cells in metastatic bone | Recurrence | |
| Skeletal metastasis | ||
| Glioblastoma[131] | Proliferating endothelial cells | |
| Malignant melanoma[108] | Endothelial cells | Shorter survival |
Nestin is a large protein (> 1600 amino acids) with a highly conserved α-helical core domain of 300-330 amino acids flanked by N- and C-terminal domains and classified into type VI IFs[1,3,41]. Nestin contains a short N terminus and an unusually long C terminus, which interacts with other IFs including vimentin, desmin, or internexin, forming heterodimers and mixed polymers[42-44], but in contrast to other IFs, nestin cannot form homopolymers[2]. Nestin is known to contribute to the disassembly of vimentin during mitosis[45]. It has been suggested that the long C-terminal portion of nestin protrudes from the filament body and may function as a linker or cross-bridge between IFs and microtubules[2]. The assembly and disassembly of cytoskeletal IFs modulate a variety of signaling cascades, and several lines of evidence suggest that nestin participates in this regulation[46]. Nestin may thus play a role in connecting the three components of the cytoskeleton and coordinate changes in cell dynamics.
Nestin has a high molecular weight (about 240 kDa), which differs among organs because of modifications to the protein[47]. It has multiple phosphorylation sites and glycosylated side chains, and the phosphorylated and glycosylated forms of nestin may affect intracellular localization or act as a means of functional regulation in specific cell types or brain regions[48]. Nestin is known to be phosphorylated at Thr316 by cdc2 kinase[49] and/or cyclin-dependent kinase 5[50], and modulates mitosis-associated cytoplasmic reorganization during mitosis. However, the roles of glycosylation have not been closely examined[51].
The minimal promoter of the mouse nestin gene resides in the region -11 to +183 of the 5’ non-coding and upstream flanking regions, and two adjacent Sp1-binding sites are necessary for promoter activity. Sp1 and Sp3 proteins are reported to regulate the expression of the mouse nestin gene[52]. The nestin gene has four exons and three introns, and neural cell-specific expression is reported to be regulated by the second intron, whereas nestin expression in tumor endothelium is enhanced by the first intron[53]. Nestin expression in muscle precursor cells is regulated by temporally and spatially restricted enhancer elements in the first intron[54]. Furthermore, the epigenetic regulation of nestin transcripts has been reported; histone acetylation is sufficient to mediate the activation of nestin transcription, but DNA demethylation is not[55]. Tissue- and cell-specific and spatiotemporal regulation of nestin is important for cell behavior during development or in pathological conditions. These observations also suggest that nestin is more than just a structural protein that serves as a progenitor stem cell marker.
Nestin has been implicated in the rapid proliferation of progenitor cells during neurogenesis[56]. However, when precursor cells differentiate into neural or glial cell types, nestin expression is downregulated or disappears[54,57]. Nestin mRNA is expressed at a high level in the cerebrum of the developing rat embryo on embryonic day 15, and the level decreases toward postnatal day 12, disappearing from postnatal day 18 to the adult stage[1]. Cells that express nestin have been found at the ventricular border in mammalian brains, and these cells give rise to neurons and glia in avian models[58,59]. Nestin expression has not been detected in normal astrocytes but is transiently detected in reactive astrocytes accompanying, for example, trauma, tumor growth, or neurodegenerative diseases in brain tissue[19,60-62].
Nestin expression has been reported in tumor cells originating from the CNS, including astrocytoma, ependymoma, oligodendroglioma, glioblastoma, and primitive neuroectodermal tumors[63-66]. Nestin has been detected in human gliomas, including low and high grade, but its expression has been observed more frequently in high-grade than in low-grade gliomas, such as pilocytic astrocytomas[66,67]. The downregulation of nestin in glioblastomas induces cell cycle arrest at the G1/S phase[68]. However, the roles of nestin in glial cell tumors have not been well clarified. Recent work has shown that nestin expression does not influence the in vitro proliferation of glioblastoma cell lines, while subclones characterized by high levels of nestin form larger tumors in vivo than those with low expression. Furthermore, blocking the expression of nestin in glioblastoma tumors via intratumoral injection of short hairpin RNA (shRNA) significantly slows tumor growth and volume[69]. We have also found that expression of nestin correlates with cell growth, migration, and invasion in low- and high-grade gliomas. These findings demonstrate that nestin plays important roles in the development of glioblastomas and may potentially be a target for treatment of the disease.
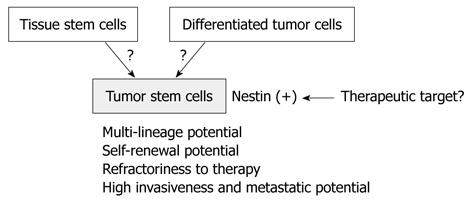
Brain tumor stem cells (BTSCs), obtained by cell sorting of dissociated suspensions of tumor cells for the NSC marker CD133[70,71], also express nestin but not differentiated neural lineage markers. These CD133+, nestin+ cells represent a minority fraction of the entire brain tumor cell population, exclusively generate clonal tumor spheres in suspension culture, and exhibit increased self-renewal capacity. These findings suggest that nestin serves as a BTSC marker. Furthermore, it has been reported that tumor stem cells play crucial roles in tumor proliferation, invasion, and metastasis; therefore, nestin may be closely associated with these tumor stem cell functions. The origin of tumor stem cells has been controversial, but nestin may be a novel therapeutic target to suppress them (Figure 1).
During early embryonic development, neuronal and islet cells in the pancreas share many phenotypic properties, and developing islet cells express several neuronal-specific markers[72-74]. In the adult pancreas, nestin-positive cells were initially described as a specific subpopulation of cells located in the endocrine islets, with a possible stem cell function[75]. Nestin-expressing cells also reside in the pancreatic ducts, where they may function as possible progenitor cells[76]. Nestin has been used as a selection marker for neuronal and pancreatic endocrine precursor cells[77,78] during differentiation assays using embryonic and adult stem cells. Additionally, the isolation of nestin-expressing cells from rat and human islets, and their in vitro differentiation into pancreatic endocrine and exocrine cells, has led to the suggestion that nestin-positive cells have a role as multipotent pancreatic stem cells[76]. Moreover, nestin-positive cells do not necessarily serve as endocrine precursors during pancreas development in mice, rats and humans, or in a regenerating model of adult rat pancreas[11,79-81].
Lineage-tracing experiments have indicated that exocrine cells are derived from nestin-expressing progenitor cells in the pancreas[82-85]. In adult pancreas, localization of nestin has been reported in vascular endothelial cells and acinar cells at different levels but not in endocrine cells[86-89]. In the regenerative process of a rat acute pancreatitis model, nestin expression was observed in proliferating capillary endothelial cells, stellate cells surrounding ductular structures, and submesothelial cells[82].
Concerning nestin and pancreatic cancer, it has been demonstrated that activation of oncogenic K-ras in the nestin cell lineage is sufficient for initiation of premalignant pancreatic intraepithelial neoplasia in mice[90]. We have reported that nestin immunoreactivity is present in the cancer cells in about 30% of pancreatic ductal adenocarcinoma (PDAC) cases, and nestin expression in pancreatic cancer cells correlates with nerve invasion and the presence of cancer cells at the tumor resection margins[87]. We recently found that nestin expression also correlates with migration, invasion, and metastasis of pancreatic cancer cells.
Regarding pancreatic tumors other than PDAC, nestin expression has been reported in acinar cell carcinoma, pancreatoblastoma, solid-pseudopapillary neoplasm, and serous cystadenoma[91]. However, nestin is rarely detected in intraductal papillary-mucinous neoplasms, mucinous cystic neoplasm, or undifferentiated carcinoma cases.
Mesenchymal tumors consisting of spindle-shaped cells develop in the gastrointestinal tract, and they were at first believed to originate from smooth muscle or neuronal cells. However, subsequent studies have shown that most of the tumors do not have the typical features of smooth muscle or neuronal cells; therefore, the most common mesenchymal tumors that differ from leiomyomas or schwannomas are designated as GISTs. Most GISTs express c-kit receptor tyrosine kinase (KIT) and CD34; both of which are expressed in hematopoietic stem cells[92-95]. In some studies, nestin expression has been identified in most GIST cases examined but not in leiomyomas[25,96]. However, a subsequent study from the same group has shown that nestin is also highly expressed in gastrointestinal schwannomas; thus, nestin may not be a definitive marker for GIST[96]. Nestin expression has also been reported in granular cell tumors, considered to be benign neoplasms of Schwann cell origin in the gastrointestinal tract[97].
In the normal gastrointestinal tract, intestinal cells of Cajal (ICCs), which are localized between the circular and longitudinal muscle layers, express KIT and CD34. ICCs are assumed to originate from mesenchymal progenitor cells that can also differentiate into smooth muscle cells[98,99]. Expression of nestin in the ICCs and GIST supports the hypothesis that GIST is derived from ICCs.
Nestin is highly expressed in androgen-insensitive prostate cancer cell lines, but it has not been detected in androgen-dependent prostate cancer cells[27]. Furthermore, nestin has been localized in 75% of lethal androgen-independent prostate cancer cases, but is undetectable in localized androgen-deprived tumors and in metastases without prior androgen deprivation. Work using shRNA against nestin has shown a marked decrease of migration and invasion of prostate cancer cells in vitro, and nestin knockdown in prostate cancer cells inhibits lung metastasis of the cells[27]. Furthermore, it has been reported that nestin is a tumor stem cell marker of prostate cancer[36,100]. The underlying mechanisms have not been well examined, but nestin may be a novel therapeutic target for preventing the metastatic and cancer stem cell potential of prostate cancer.
In normal human breast tissues, nestin is expressed in the cells within the basal/myoepithelial layers[26,101,102] and may be used as a myoepithelial marker. In one of these cell types, nestin is co-expressed with ΔN-p63. This finding, coupled with the role of ΔN-p63 in preservation of self-renewal, suggests that nestin may be expressed in the regenerative compartment within the mammary gland. Furthermore, nestin and ΔN-p63 are coordinately expressed during pregnancy in the murine mammary gland[26].
Among the breast cancer subtypes, nestin is highly expressed in basal breast cancer subtype (ERα-/PR-/Her2-) but not in the Her2 subtype (ERα-/PR-/Her2+) or luminal epithelial phenotype (ERα+/PR+)[26]. The other group showed that triple-negative breast cancers, which do not express ER, PR, and Her2, have higher expression rates for nestin than other breast cancers[103]. Furthermore, nestin expression has been associated with shorter survival and shown to be an independent prognostic factor of breast cancer[103]. Another group has reported significantly high nestin expression in basal-like and triple-negative breast cancers in a cohort of 245 patients with invasive breast cancer treated with surgery followed by anthracycline-based chemotherapy[104]. These findings indicate that nestin is a selective marker of the basal breast cancer subtype (triple negative), which displays aggressive growth and has a poor prognosis. Co-expression of nestin and melatonin receptor 1 (MT 1) in breast cancer cells has been reported in patients with higher stages (T II/III) and with a high risk of relapse. Co-expression of nestin and MT 1 may correlate with invasive breast cancer and advanced tumors[102]. Lymphovascular emboli of inflammatory breast cancer, a particularly lethal form of breast cancer characterized by exaggerated lymphovascular invasion, express stem cell markers including nestin[105]. These data indicate that nestin correlates with an aggressive growth phenotype and lymphatic invasion.
Nestin is expressed in benign nevi and melanoma but not in basal cell carcinoma[106]. Nestin expression is higher at the advanced stage in melanoma and in metastatic foci of melanoma cells[24,107]. Nestin staining in stage I and II melanoma patients significantly predicts poor survival, with lower survival rates in cases with nestin positivity in tumoral and endothelial cells[108]. Furthermore, the 5-year survival rate exceeded 80% in nestin-negative melanoma at all stages of tumor development[109]. Nestin and SOX9 and SOX10 transcription factors are co-expressed in melanoma cells, and downregulation of SOX9 and SOX10 markedly decreases nestin levels[110,111]. Furthermore, nestin has been significantly associated with the presence of ulceration in primary tumors of melanoma and with SOX9 in the more advanced state. These findings indicate that nestin and SOX9 may be negative prognostic markers in melanoma. Nestin protein has been shown to occur most abundantly at the infiltrating front of the tumors, suggesting that nestin plays important roles in melanoma cell migration and invasion[106].
Nestin expression in the peripheral blood of melanoma patients has been examined using flow cytometry, and expression is higher in stage IV patients as compared with stage III/IV with no evidence of the disease[112,113]. Nestin thus may be an additional marker of interest for circulating melanoma cells in the future.
Immunohistochemically, melanoma antigen-encoding-1 (MAGE-1), melanocyte-specific transcription factor, tyrosinase, and Melan-A have been reported as useful markers in the diagnosis of melanotic parts when HMB-45 is negative[114]. However, nestin has been more specifically detected in HMB-45-negative melanoma cells in the dermal portions of melanotic and amelanotic nodular melanomas[115]. Nestin thus also may be a useful diagnostic marker for HMB-45-negative melanoma.
Few reports have addressed nestin expression in other types of tumor cells. Its expression has been reported in various kinds of thyroid tumors, and nestin mRNA has been detected in differentiated thyroid tumors but not in anaplastic carcinoma[22]. Nestin mRNA is also expressed in normal thyroid tissues, therefore, the authors of the above study have suggested that nestin mRNA is not associated with the malignant characteristics of thyroid tumors. Dermatofibrosarcoma protuberans (DFSP) is a dermal and subcutaneous neoplasm of intermediate malignancy that is invasive and locally aggressive with frequent recurrence. Histopathologically, the differential diagnosis between DFSP and dermatofibroma (DF) is important because DF is benign[28]. In one study, nestin was found to be strongly expressed in DFSP, while all DFs examined were nestin negative. Based on these findings, nestin may serve as an additional marker for DFSP and for surgical margin evaluation of DFSP.
Tumor angiogenesis is an important factor in the proliferation, metastasis, and drug sensitivity of human neoplasms. A possible explanation of this metastatic mechanism is that the increased number of tumor vessels increases the chances for tumor cells to enter the circulation. Newly formed tumor vessels or capillaries have leaky and weak basement membranes; thus, tumor cells can penetrate these more easily than they can mature vessels[116]. Furthermore, increased tumor vessels supply abundant oxygen and nutrition to the tumor cells. Angiogenesis in malignant tumors, as measured by microvessel density (MVD), has been reported to correlate with clinicopathological factors or survival in breast, ovarian, esophageal, gastric, colorectal, and prostate cancers, malignant melanoma, and non-small-cell lung carcinoma[117-124]. CD34, CD31, and factor-VIII-related antigen are commonly used as endothelial cell markers of tumor vessels, and MVD is determined based on staining of blood vessels with these markers[125-127]. However, the markers identify not only newly formed small tumor blood vessels but also pre-existing large blood vessels[128].
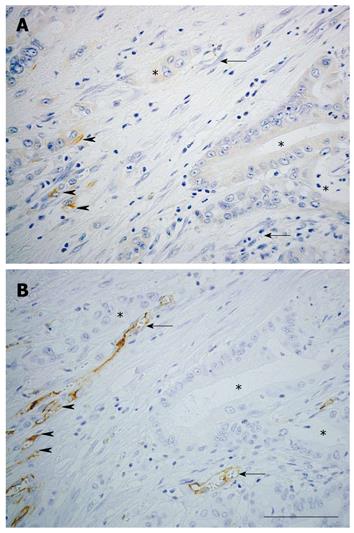
Nestin expression in endothelial cells accompanying the process of angiogenesis has been reported[129]. In pathological conditions, nestin is strongly expressed in proliferating endothelial cells in acute pancreatitis[82] and vascular malformations[130]. Furthermore, nestin has been reported to be a marker of proliferating endothelial cells in brain tumor tissues[131] (Table 2). In gastric adenocarcinoma, MVD determined by nestin correlates better with patient survival than MVD determined by CD34 when the size of the carcinoma exceeds 5 cm[37]. We examined the effectiveness of nestin as an angiogenic marker in colorectal cancer[38]. Diameters of nestin-positive and CD34-positive vessels were compared in subcutaneous colorectal cancer tumors formed in nude mice. Nestin was localized in the endothelial cells in small tumor blood vessels, whereas CD34 was localized in large blood vessels in nude mice. Furthermore, the diameter of nestin-positive vessels was smaller than that of CD34-positive vessels in human colorectal cancer. The ratio of proliferating cell nuclear antigen (PCNA)-positive to nestin-positive vascular endothelial cells was higher than that of PCNA-positive to CD34-positive cells. These findings indicate that nestin is expressed in small-sized and proliferating tumor vessels in colorectal cancer. Further, prognosis is worse in the highly nestin-positive MVD population of colorectal cancer patients. In pancreatic cancer tissues, CD31 is expressed in endothelial cells of most blood vessels, while nestin is specifically expressed in those of small-sized blood vessels (Figure 2).
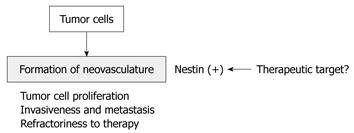
Recently, co-expression of nestin and Ki-67 has been shown to be a vascular proliferation marker in prostate cancer[39], and vascular proliferation is of independent prognostic importance among prostate cancer. Furthermore, vascular proliferation is significantly increased in castration-resistant cases and metastatic lesions compared with localized cancers. Very recently, nestin expression has been reported in the endothelial cells of bone metastatic lesions of prostate cancer[40]. These results indicate that nestin correlates with tumor angiogenesis in primary and metastatic lesions, and that nestin may be a novel molecular target for inhibition of tumor angiogenesis, as are vascular endothelial growth factor receptors (Figure 3).
Nestin is highly expressed in various kinds of cancer cells and proliferating tumor vasculature. The roles of nestin in cancer cells have not been clarified fully, although nestin correlates with growth, migration, invasion, and metastasis of some cancers. Nestin is also highly expressed in proliferating vascular endothelial cells in cancer tissues and metastatic lesions. These findings indicate that this protein may become a new molecular target for nestin-positive cancer cells and tumor vessels. Furthermore, nestin is highly expressed in tumor stem cells in various tissues, and research concerning its expression and roles in various tumors may provide us important information about the origin of cancer stem cells and differentiation of cancer stem cells into cancer cells.
Peer reviewer: Cornelis FM Sier, PhD, Department of Surgery and Gastroenterology, Leiden University Medical Center, Albinusdreef 2, Bldg 1, D6-18, 2333 ZA Leiden, The Netherlands
S- Editor Tian L L- Editor Kerr C E- Editor Ma WH
| 1. | Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585-595. [Cited in This Article: ] |
| 2. | Herrmann H, Aebi U. Intermediate filaments and their associates: multi-talented structural elements specifying cytoarchitecture and cytodynamics. Curr Opin Cell Biol. 2000;12:79-90. [Cited in This Article: ] |
| 3. | Prasad S, Soldatenkov VA, Srinivasarao G, Dritschilo A. Intermediate filament proteins during carcinogenesis and apoptosis (Review). Int J Oncol. 1999;14:563-570. [Cited in This Article: ] |
| 4. | Kachinsky AM, Dominov JA, Miller JB. Myogenesis and the intermediate filament protein, nestin. Dev Biol. 1994;165:216-228. [Cited in This Article: ] |
| 5. | Sejersen T, Lendahl U. Transient expression of the intermediate filament nestin during skeletal muscle development. J Cell Sci. 1993;106:1291-1300. [Cited in This Article: ] |
| 6. | Fröjdman K, Pelliniemi LJ, Lendahl U, Virtanen I, Eriksson JE. The intermediate filament protein nestin occurs transiently in differentiating testis of rat and mouse. Differentiation. 1997;61:243-249. [Cited in This Article: ] |
| 7. | Terling C, Rass A, Mitsiadis TA, Fried K, Lendahl U, Wroblewski J. Expression of the intermediate filament nestin during rodent tooth development. Int J Dev Biol. 1995;39:947-956. [Cited in This Article: ] |
| 8. | Yang J, Bian W, Gao X, Chen L, Jing N. Nestin expression during mouse eye and lens development. Mech Dev. 2000;94:287-291. [Cited in This Article: ] |
| 9. | Almazán G, Vela JM, Molina-Holgado E, Guaza C. Re-evaluation of nestin as a marker of oligodendrocyte lineage cells. Microsc Res Tech. 2001;52:753-765. [Cited in This Article: ] |
| 10. | Amoh Y, Li L, Yang M, Moossa AR, Katsuoka K, Penman S, Hoffman RM. Nascent blood vessels in the skin arise from nestin-expressing hair-follicle cells. Proc Natl Acad Sci USA. 2004;101:13291-13295. [Cited in This Article: ] |
| 11. | Lardon J, Rooman I, Bouwens L. Nestin expression in pancreatic stellate cells and angiogenic endothelial cells. Histochem Cell Biol. 2002;117:535-540. [Cited in This Article: ] |
| 12. | Takano T, Rutka JT, Becker LE. Overexpression of nestin and vimentin in ependymal cells in hydrocephalus. Acta Neuropathol. 1996;92:90-97. [Cited in This Article: ] |
| 13. | Eaker EY, Sallustio JE. The distribution of novel intermediate filament proteins defines subpopulations of myenteric neurons in rat intestine. Gastroenterology. 1994;107:666-674. [Cited in This Article: ] |
| 14. | Ishizaki M, Ishiwata T, Adachi A, Tamura N, Ghazizadeh M, Kitamura H, Sugisaki Y, Yamanaka N, Naito Z, Fukuda Y. Expression of nestin in rat and human glomerular podocytes. J Submicrosc Cytol Pathol. 2006;38:193-200. [Cited in This Article: ] |
| 15. | Hoffman RM. The potential of nestin-expressing hair follicle stem cells in regenerative medicine. Expert Opin Biol Ther. 2007;7:289-291. [Cited in This Article: ] |
| 16. | Wiese C, Rolletschek A, Kania G, Blyszczuk P, Tarasov KV, Tarasova Y, Wersto RP, Boheler KR, Wobus AM. Nestin expression--a property of multi-lineage progenitor cells? Cell Mol Life Sci. 2004;61:2510-2522. [Cited in This Article: ] |
| 17. | Niki T, Pekny M, Hellemans K, Bleser PD, Berg KV, Vaeyens F, Quartier E, Schuit F, Geerts A. Class VI intermediate filament protein nestin is induced during activation of rat hepatic stellate cells. Hepatology. 1999;29:520-527. [Cited in This Article: ] |
| 18. | Aärimaa V, Kääriäinen M, Vaittinen S, Tanner J, Järvinen T, Best T, Kalimo H. Restoration of myofiber continuity after transection injury in the rat soleus. Neuromuscul Disord. 2004;14:421-428. [Cited in This Article: ] |
| 19. | Lin RC, Matesic DF, Marvin M, McKay RD, Brüstle O. Re-expression of the intermediate filament nestin in reactive astrocytes. Neurobiol Dis. 1995;2:79-85. [Cited in This Article: ] |
| 20. | Holmin S, Almqvist P, Lendahl U, Mathiesen T. Adult nestin-expressing subependymal cells differentiate to astrocytes in response to brain injury. Eur J Neurosci. 1997;9:65-75. [Cited in This Article: ] |
| 21. | El-Helou V, Dupuis J, Proulx C, Drapeau J, Clement R, Gosselin H, Villeneuve L, Manganas L, Calderone A. Resident nestin+ neural-like cells and fibers are detected in normal and damaged rat myocardium. Hypertension. 2005;46:1219-1225. [Cited in This Article: ] |
| 22. | Yamada H, Takano T, Ito Y, Matsuzuka F, Miya A, Kobayashi K, Yoshida H, Watanabe M, Iwatani Y, Miyauchi A. Expression of nestin mRNA is a differentiation marker in thyroid tumors. Cancer Lett. 2009;280:61-64. [Cited in This Article: ] |
| 23. | Strojnik T, Røsland GV, Sakariassen PO, Kavalar R, Lah T. Neural stem cell markers, nestin and musashi proteins, in the progression of human glioma: correlation of nestin with prognosis of patient survival. Surg Neurol. 2007;68:133-143; discussion 143-144. [Cited in This Article: ] |
| 24. | Brychtova S, Fiuraskova M, Hlobilková A, Brychta T, Hirnak J. Nestin expression in cutaneous melanomas and melanocytic nevi. J Cutan Pathol. 2007;34:370-375. [Cited in This Article: ] |
| 25. | Tsujimura T, Makiishi-Shimobayashi C, Lundkvist J, Lendahl U, Nakasho K, Sugihara A, Iwasaki T, Mano M, Yamada N, Yamashita K. Expression of the intermediate filament nestin in gastrointestinal stromal tumors and interstitial cells of Cajal. Am J Pathol. 2001;158:817-823. [Cited in This Article: ] |
| 26. | Li H, Cherukuri P, Li N, Cowling V, Spinella M, Cole M, Godwin AK, Wells W, DiRenzo J. Nestin is expressed in the basal/myoepithelial layer of the mammary gland and is a selective marker of basal epithelial breast tumors. Cancer Res. 2007;67:501-510. [Cited in This Article: ] |
| 27. | Kleeberger W, Bova GS, Nielsen ME, Herawi M, Chuang AY, Epstein JI, Berman DM. Roles for the stem cell associated intermediate filament Nestin in prostate cancer migration and metastasis. Cancer Res. 2007;67:9199-9206. [Cited in This Article: ] |
| 28. | Sellheyer K, Nelson P, Krahl D. Dermatofibrosarcoma protuberans: a tumour of nestin-positive cutaneous mesenchymal stem cells? Br J Dermatol. 2009;161:1317-1322. [Cited in This Article: ] |
| 29. | Jimeno A, Feldmann G, Suárez-Gauthier A, Rasheed Z, Solomon A, Zou GM, Rubio-Viqueira B, García-García E, López-Ríos F, Matsui W. A direct pancreatic cancer xenograft model as a platform for cancer stem cell therapeutic development. Mol Cancer Ther. 2009;8:310-314. [Cited in This Article: ] |
| 30. | Bentivegna A, Conconi D, Panzeri E, Sala E, Bovo G, Viganò P, Brunelli S, Bossi M, Tredici G, Strada G. Biological heterogeneity of putative bladder cancer stem-like cell populations from human bladder transitional cell carcinoma samples. Cancer Sci. 2010;101:416-424. [Cited in This Article: ] |
| 31. | Singh SK, Clarke ID, Hide T, Dirks PB. Cancer stem cells in nervous system tumors. Oncogene. 2004;23:7267-7273. [Cited in This Article: ] |
| 32. | Bortolomai I, Canevari S, Facetti I, De Cecco L, Castellano G, Zacchetti A, Alison MR, Miotti S. Tumor initiating cells: Development and critical characterization of a model derived from the A431 carcinoma cell line forming spheres in suspension. Cell Cycle. 2010;9:1194-1206. [Cited in This Article: ] |
| 33. | Okuno K, Ohta S, Kato H, Taga T, Sugita K, Takeuchi Y. Expression of neural stem cell markers in malignant rhabdoid tumor cell lines. Oncol Rep. 2010;23:485-492. [Cited in This Article: ] |
| 34. | Hombach-Klonisch S, Paranjothy T, Wiechec E, Pocar P, Mustafa T, Seifert A, Zahl C, Gerlach KL, Biermann K, Steger K. Cancer stem cells as targets for cancer therapy: selected cancers as examples. Arch Immunol Ther Exp (Warsz). 2008;56:165-180. [Cited in This Article: ] |
| 35. | Kasper S. Exploring the origins of the normal prostate and prostate cancer stem cell. Stem Cell Rev. 2008;4:193-201. [Cited in This Article: ] |
| 36. | Guzmán-Ramírez N, Völler M, Wetterwald A, Germann M, Cross NA, Rentsch CA, Schalken J, Thalmann GN, Cecchini MG. In vitro propagation and characterization of neoplastic stem/progenitor-like cells from human prostate cancer tissue. Prostate. 2009;69:1683-1693. [Cited in This Article: ] |
| 37. | Kim HS, Kang HS, Messam CA, Min KW, Park CS. Comparative evaluation of angiogenesis in gastric adenocarcinoma by nestin and CD34. Appl Immunohistochem Mol Morphol. 2002;10:121-127. [Cited in This Article: ] |
| 38. | Teranishi N, Naito Z, Ishiwata T, Tanaka N, Furukawa K, Seya T, Shinji S, Tajiri T. Identification of neovasculature using nestin in colorectal cancer. Int J Oncol. 2007;30:593-603. [Cited in This Article: ] |
| 39. | Gravdal K, Halvorsen OJ, Haukaas SA, Akslen LA. Proliferation of immature tumor vessels is a novel marker of clinical progression in prostate cancer. Cancer Res. 2009;69:4708-4715. [Cited in This Article: ] |
| 40. | Eaton CL, Colombel M, van der Pluijm G, Cecchini M, Wetterwald A, Lippitt J, Rehman I, Hamdy F, Thalman G. Evaluation of the frequency of putative prostate cancer stem cells in primary and metastatic prostate cancer. Prostate. 2010;70:875-882. [Cited in This Article: ] |
| 41. | Dahlstrand J, Zimmerman LB, McKay RD, Lendahl U. Characterization of the human nestin gene reveals a close evolutionary relationship to neurofilaments. J Cell Sci. 1992;103:589-597. [Cited in This Article: ] |
| 42. | Marvin MJ, Dahlstrand J, Lendahl U, McKay RD. A rod end deletion in the intermediate filament protein nestin alters its subcellular localization in neuroepithelial cells of transgenic mice. J Cell Sci. 1998;111:1951-1961. [Cited in This Article: ] |
| 43. | Sjöberg G, Edström L, Lendahl U, Sejersen T. Myofibers from Duchenne/Becker muscular dystrophy and myositis express the intermediate filament nestin. J Neuropathol Exp Neurol. 1994;53:416-423. [Cited in This Article: ] |
| 44. | Steinert PM, Chou YH, Prahlad V, Parry DA, Marekov LN, Wu KC, Jang SI, Goldman RD. A high molecular weight intermediate filament-associated protein in BHK-21 cells is nestin, a type VI intermediate filament protein. Limited co-assembly in vitro to form heteropolymers with type III vimentin and type IV alpha-internexin. J Biol Chem. 1999;274:9881-9890. [Cited in This Article: ] |
| 45. | Chou YH, Khuon S, Herrmann H, Goldman RD. Nestin promotes the phosphorylation-dependent disassembly of vimentin intermediate filaments during mitosis. Mol Biol Cell. 2003;14:1468-1478. [Cited in This Article: ] |
| 46. | Michalczyk K, Ziman M. Nestin structure and predicted function in cellular cytoskeletal organisation. Histol Histopathol. 2005;20:665-671. [Cited in This Article: ] |
| 47. | Lobo MV, Arenas MI, Alonso FJ, Gomez G, Bazán E, Paíno CL, Fernández E, Fraile B, Paniagua R, Moyano A. Nestin, a neuroectodermal stem cell marker molecule, is expressed in Leydig cells of the human testis and in some specific cell types from human testicular tumours. Cell Tissue Res. 2004;316:369-376. [Cited in This Article: ] |
| 48. | Messam CA, Hou J, Major EO. Coexpression of nestin in neural and glial cells in the developing human CNS defined by a human-specific anti-nestin antibody. Exp Neurol. 2000;161:585-596. [Cited in This Article: ] |
| 49. | Sahlgren CM, Mikhailov A, Hellman J, Chou YH, Lendahl U, Goldman RD, Eriksson JE. Mitotic reorganization of the intermediate filament protein nestin involves phosphorylation by cdc2 kinase. J Biol Chem. 2001;276:16456-16463. [Cited in This Article: ] |
| 50. | Sahlgren CM, Mikhailov A, Vaittinen S, Pallari HM, Kalimo H, Pant HC, Eriksson JE. Cdk5 regulates the organization of Nestin and its association with p35. Mol Cell Biol. 2003;23:5090-5106. [Cited in This Article: ] |
| 51. | Grigelioniené G, Blennow M, Török C, Fried G, Dahlin I, Lendahl U, Lagercrantz H. Cerebrospinal fluid of newborn infants contains a deglycosylated form of the intermediate filament nestin. Pediatr Res. 1996;40:809-814. [Cited in This Article: ] |
| 52. | Cheng L, Jin Z, Liu L, Yan Y, Li T, Zhu X, Jing N. Characterization and promoter analysis of the mouse nestin gene. FEBS Lett. 2004;565:195-202. [Cited in This Article: ] |
| 53. | Aihara M, Sugawara K, Torii S, Hosaka M, Kurihara H, Saito N, Takeuchi T. Angiogenic endothelium-specific nestin expression is enhanced by the first intron of the nestin gene. Lab Invest. 2004;84:1581-1592. [Cited in This Article: ] |
| 54. | Zimmerman L, Parr B, Lendahl U, Cunningham M, McKay R, Gavin B, Mann J, Vassileva G, McMahon A. Independent regulatory elements in the nestin gene direct transgene expression to neural stem cells or muscle precursors. Neuron. 1994;12:11-24. [Cited in This Article: ] |
| 55. | Han DW, Do JT, Araúzo-Bravo MJ, Lee SH, Meissner A, Lee HT, Jaenisch R, Schöler HR. Epigenetic hierarchy governing Nestin expression. Stem Cells. 2009;27:1088-1097. [Cited in This Article: ] |
| 56. | Frederiksen K, McKay RD. Proliferation and differentiation of rat neuroepithelial precursor cells in vivo. J Neurosci. 1988;8:1144-1151. [Cited in This Article: ] |
| 57. | Loo DT, Althoen MC, Cotman CW. Down regulation of nestin by TGF-beta or serum in SFME cells accompanies differentiation into astrocytes. Neuroreport. 1994;5:1585-1588. [Cited in This Article: ] |
| 58. | Doetsch F, García-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046-5061. [Cited in This Article: ] |
| 59. | Gray GE, Sanes JR. Migratory paths and phenotypic choices of clonally related cells in the avian optic tectum. Neuron. 1991;6:211-225. [Cited in This Article: ] |
| 60. | Frisén J, Johansson CB, Török C, Risling M, Lendahl U. Rapid, widespread, and longlasting induction of nestin contributes to the generation of glial scar tissue after CNS injury. J Cell Biol. 1995;131:453-464. [Cited in This Article: ] |
| 61. | Clarke SR, Shetty AK, Bradley JL, Turner DA. Reactive astrocytes express the embryonic intermediate neurofilament nestin. Neuroreport. 1994;5:1885-1888. [Cited in This Article: ] |
| 62. | Eng LF, Ghirnikar RS. GFAP and astrogliosis. Brain Pathol. 1994;4:229-237. [Cited in This Article: ] |
| 63. | Almqvist PM, Mah R, Lendahl U, Jacobsson B, Hendson G. Immunohistochemical detection of nestin in pediatric brain tumors. J Histochem Cytochem. 2002;50:147-158. [Cited in This Article: ] |
| 64. | Duggal N, Hammond RR. Nestin expression in ganglioglioma. Exp Neurol. 2002;174:89-95. [Cited in This Article: ] |
| 65. | Schiffer D, Manazza A, Tamagno I. Nestin expression in neuroepithelial tumors. Neurosci Lett. 2006;400:80-85. [Cited in This Article: ] |
| 66. | Dahlstrand J, Collins VP, Lendahl U. Expression of the class VI intermediate filament nestin in human central nervous system tumors. Cancer Res. 1992;52:5334-5341. [Cited in This Article: ] |
| 67. | Ma YH, Mentlein R, Knerlich F, Kruse ML, Mehdorn HM, Held-Feindt J. Expression of stem cell markers in human astrocytomas of different WHO grades. J Neurooncol. 2008;86:31-45. [Cited in This Article: ] |
| 68. | Reimer R, Helmbold H, Szalay B, Hagel C, Hohenberg H, Deppert W, Bohn W. Nestin modulates glucocorticoid receptor function by cytoplasmic anchoring. PLoS One. 2009;4:e6084. [Cited in This Article: ] |
| 69. | Lu WJ, Lan F, He Q, Lee A, Tang CZ, Dong L, Lan B, Ma X, Wu JC, Shen L. Inducible expression of stem cell associated intermediate filament nestin reveals an important role in glioblastoma carcinogenesis. Int J Cancer. 2011;128:343-351. [Cited in This Article: ] |
| 70. | Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396-401. [Cited in This Article: ] |
| 71. | Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821-5828. [Cited in This Article: ] |
| 72. | Alpert S, Hanahan D, Teitelman G. Hybrid insulin genes reveal a developmental lineage for pancreatic endocrine cells and imply a relationship with neurons. Cell. 1988;53:295-308. [Cited in This Article: ] |
| 73. | Madsen OD, Jensen J, Petersen HV, Pedersen EE, Oster A, Andersen FG, Jørgensen MC, Jensen PB, Larsson LI, Serup P. Transcription factors contributing to the pancreatic beta-cell phenotype. Horm Metab Res. 1997;29:265-270. [Cited in This Article: ] |
| 74. | St-Onge L, Wehr R, Gruss P. Pancreas development and diabetes. Curr Opin Genet Dev. 1999;9:295-300. [Cited in This Article: ] |
| 75. | Hunziker E, Stein M. Nestin-expressing cells in the pancreatic islets of Langerhans. Biochem Biophys Res Commun. 2000;271:116-119. [Cited in This Article: ] |
| 76. | Zulewski H, Abraham EJ, Gerlach MJ, Daniel PB, Moritz W, Müller B, Vallejo M, Thomas MK, Habener JF. Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes. 2001;50:521-533. [Cited in This Article: ] |
| 77. | Lee SH, Lumelsky N, Studer L, Auerbach JM, McKay RD. Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat Biotechnol. 2000;18:675-679. [Cited in This Article: ] |
| 78. | Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 2001;292:1389-1394. [Cited in This Article: ] |
| 79. | Piper K, Ball SG, Turnpenny LW, Brickwood S, Wilson DI, Hanley NA. Beta-cell differentiation during human development does not rely on nestin-positive precursors: implications for stem cell-derived replacement therapy. Diabetologia. 2002;45:1045-1047. [Cited in This Article: ] |
| 80. | Selander L, Edlund H. Nestin is expressed in mesenchymal and not epithelial cells of the developing mouse pancreas. Mech Dev. 2002;113:189-192. [Cited in This Article: ] |
| 81. | Humphrey RK, Bucay N, Beattie GM, Lopez A, Messam CA, Cirulli V, Hayek A. Characterization and isolation of promoter-defined nestin-positive cells from the human fetal pancreas. Diabetes. 2003;52:2519-2525. [Cited in This Article: ] |
| 82. | Ishiwata T, Kudo M, Onda M, Fujii T, Teduka K, Suzuki T, Korc M, Naito Z. Defined localization of nestin-expressing cells in L-arginine-induced acute pancreatitis. Pancreas. 2006;32:360-368. [Cited in This Article: ] |
| 83. | Esni F, Stoffers DA, Takeuchi T, Leach SD. Origin of exocrine pancreatic cells from nestin-positive precursors in developing mouse pancreas. Mech Dev. 2004;121:15-25. [Cited in This Article: ] |
| 84. | Delacour A, Nepote V, Trumpp A, Herrera PL. Nestin expression in pancreatic exocrine cell lineages. Mech Dev. 2004;121:3-14. [Cited in This Article: ] |
| 85. | Ueno H, Yamada Y, Watanabe R, Mukai E, Hosokawa M, Takahashi A, Hamasaki A, Fujiwara H, Toyokuni S, Yamaguchi M. Nestin-positive cells in adult pancreas express amylase and endocrine precursor Cells. Pancreas. 2005;31:126-131. [Cited in This Article: ] |
| 86. | Street CN, Lakey JR, Seeberger K, Helms L, Rajotte RV, Shapiro AM, Korbutt GS. Heterogenous expression of nestin in human pancreatic tissue precludes its use as an islet precursor marker. J Endocrinol. 2004;180:213-225. [Cited in This Article: ] |
| 87. | Kawamoto M, Ishiwata T, Cho K, Uchida E, Korc M, Naito Z, Tajiri T. Nestin expression correlates with nerve and retroperitoneal tissue invasion in pancreatic cancer. Hum Pathol. 2009;40:189-198. [Cited in This Article: ] |
| 88. | Treutelaar MK, Skidmore JM, Dias-Leme CL, Hara M, Zhang L, Simeone D, Martin DM, Burant CF. Nestin-lineage cells contribute to the microvasculature but not endocrine cells of the islet. Diabetes. 2003;52:2503-2512. [Cited in This Article: ] |
| 89. | Klein T, Ling Z, Heimberg H, Madsen OD, Heller RS, Serup P. Nestin is expressed in vascular endothelial cells in the adult human pancreas. J Histochem Cytochem. 2003;51:697-706. [Cited in This Article: ] |
| 90. | Carrière C, Seeley ES, Goetze T, Longnecker DS, Korc M. The Nestin progenitor lineage is the compartment of origin for pancreatic intraepithelial neoplasia. Proc Natl Acad Sci USA. 2007;104:4437-4442. [Cited in This Article: ] |
| 91. | Ohike N, Sato M, Hisayuki T, Imataka H, Sato S, Wada Y, Saito K, Takahashi M, Tajiri T, Kunimura T. Immunohistochemical analysis of nestin and c-kit and their significance in pancreatic tumors. Pathol Int. 2007;57:589-593. [Cited in This Article: ] |
| 92. | Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577-580. [Cited in This Article: ] |
| 93. | Sarlomo-Rikala M, Kovatich AJ, Barusevicius A, Miettinen M. CD117: a sensitive marker for gastrointestinal stromal tumors that is more specific than CD34. Mod Pathol. 1998;11:728-734. [Cited in This Article: ] |
| 94. | van de Rijn M, Hendrickson MR, Rouse RV. CD34 expression by gastrointestinal tract stromal tumors. Hum Pathol. 1994;25:766-771. [Cited in This Article: ] |
| 95. | Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1-12. [Cited in This Article: ] |
| 96. | Sarlomo-Rikala M, Tsujimura T, Lendahl U, Miettinen M. Patterns of nestin and other intermediate filament expression distinguish between gastrointestinal stromal tumors, leiomyomas and schwannomas. APMIS. 2002;110:499-507. [Cited in This Article: ] |
| 97. | Parfitt JR, McLean CA, Joseph MG, Streutker CJ, Al-Haddad S, Driman DK. Granular cell tumours of the gastrointestinal tract: expression of nestin and clinicopathological evaluation of 11 patients. Histopathology. 2006;48:424-430. [Cited in This Article: ] |
| 98. | Young HM, Ciampoli D, Southwell BR, Newgreen DF. Origin of interstitial cells of Cajal in the mouse intestine. Dev Biol. 1996;180:97-107. [Cited in This Article: ] |
| 99. | Torihashi S, Nishi K, Tokutomi Y, Nishi T, Ward S, Sanders KM. Blockade of kit signaling induces transdifferentiation of interstitial cells of cajal to a smooth muscle phenotype. Gastroenterology. 1999;117:140-148. [Cited in This Article: ] |
| 100. | Liu T, Xu F, Du X, Lai D, Liu T, Zhao Y, Huang Q, Jiang L, Huang W, Cheng W. Establishment and characterization of multi-drug resistant, prostate carcinoma-initiating stem-like cells from human prostate cancer cell lines 22RV1. Mol Cell Biochem. 2010;340:265-273. [Cited in This Article: ] |
| 101. | Kishaba Y, Matsubara D, Niki T. Heterogeneous expression of nestin in myofibroblasts of various human tissues. Pathol Int. 2010;60:378-385. [Cited in This Article: ] |
| 102. | Rögelsperger O, Ekmekcioglu C, Jäger W, Klimpfinger M, Königsberg R, Krenbek D, Sellner F, Thalhammer T. Coexpression of the melatonin receptor 1 and nestin in human breast cancer specimens. J Pineal Res. 2009;46:422-432. [Cited in This Article: ] |
| 103. | Liu C, Chen B, Zhu J, Zhang R, Yao F, Jin F, Xu H, Lu P. Clinical implications for nestin protein expression in breast cancer. Cancer Sci. 2010;101:815-819. [Cited in This Article: ] |
| 104. | Parry S, Savage K, Marchiò C, Reis-Filho JS. Nestin is expressed in basal-like and triple negative breast cancers. J Clin Pathol. 2008;61:1045-1050. [Cited in This Article: ] |
| 105. | Xiao Y, Ye Y, Yearsley K, Jones S, Barsky SH. The lymphovascular embolus of inflammatory breast cancer expresses a stem cell-like phenotype. Am J Pathol. 2008;173:561-574. [Cited in This Article: ] |
| 106. | Flørenes VA, Holm R, Myklebost O, Lendahl U, Fodstad O. Expression of the neuroectodermal intermediate filament nestin in human melanomas. Cancer Res. 1994;54:354-356. [Cited in This Article: ] |
| 107. | Klein WM, Wu BP, Zhao S, Wu H, Klein-Szanto AJ, Tahan SR. Increased expression of stem cell markers in malignant melanoma. Mod Pathol. 2007;20:102-107. [Cited in This Article: ] |
| 108. | Piras F, Perra MT, Murtas D, Minerba L, Floris C, Maxia C, Demurtas P, Ugalde J, Ribatti D, Sirigu P. The stem cell marker nestin predicts poor prognosis in human melanoma. Oncol Rep. 2010;23:17-24. [Cited in This Article: ] |
| 109. | Tanabe K, Amoh Y, Kanoh M, Takasu H, Sakai N, Sato Y, Katsuoka K. Prognostic significance of the hair follicle stem cell marker nestin in patients with malignant melanoma. Eur J Dermatol. 2010;20:283-288. [Cited in This Article: ] |
| 110. | Flammiger A, Besch R, Cook AL, Maier T, Sturm RA, Berking C. SOX9 and SOX10 but not BRN2 are required for nestin expression in human melanoma cells. J Invest Dermatol. 2009;129:945-953. [Cited in This Article: ] |
| 111. | Bakos RM, Maier T, Besch R, Mestel DS, Ruzicka T, Sturm RA, Berking C. Nestin and SOX9 and SOX10 transcription factors are coexpressed in melanoma. Exp Dermatol. 2010;19:e89-e94. [Cited in This Article: ] |
| 112. | Fusi A, Ochsenreither S, Busse A, Rietz A, Keilholz U. Stem cell marker nestin expression in peripheral blood of patients with melanoma. Br J Dermatol. 2010;Epub ahead of print. [Cited in This Article: ] |
| 113. | Fusi A, Ochsenreither S, Busse A, Rietz A, Keilholz U. Expression of the stem cell marker nestin in peripheral blood of patients with melanoma. Br J Dermatol. 2010;163:107-114. [Cited in This Article: ] |
| 114. | Xu X, Chu AY, Pasha TL, Elder DE, Zhang PJ. Immunoprofile of MITF, tyrosinase, melan-A, and MAGE-1 in HMB45-negative melanomas. Am J Surg Pathol. 2002;26:82-87. [Cited in This Article: ] |
| 115. | Kanoh M, Amoh Y, Tanabe K, Maejima H, Takasu H, Katsuoka K. Nestin is expressed in HMB-45 negative melanoma cells in dermal parts of nodular melanoma. J Dermatol. 2010;37:505-511. [Cited in This Article: ] |
| 116. | Liotta LA, Saidel MG, Kleinerman J. The significance of hematogenous tumor cell clumps in the metastatic process. Cancer Res. 1976;36:889-894. [Cited in This Article: ] |
| 117. | Hansen S, Grabau DA, Rose C, Bak M, Sørensen FB. Angiogenesis in breast cancer: a comparative study of the observer variability of methods for determining microvessel density. Lab Invest. 1998;78:1563-1573. [Cited in This Article: ] |
| 118. | Schoell WM, Pieber D, Reich O, Lahousen M, Janicek M, Guecer F, Winter R. Tumor angiogenesis as a prognostic factor in ovarian carcinoma: quantification of endothelial immunoreactivity by image analysis. Cancer. 1997;80:2257-2262. [Cited in This Article: ] |
| 119. | Srivastava A, Laidler P, Hughes LE, Woodcock J, Shedden EJ. Neovascularization in human cutaneous melanoma: a quantitative morphological and Doppler ultrasound study. Eur J Cancer Clin Oncol. 1986;22:1205-1209. [Cited in This Article: ] |
| 120. | Macchiarini P, Fontanini G, Hardin MJ, Squartini F, Angeletti CA. Relation of neovascularisation to metastasis of non-small-cell lung cancer. Lancet. 1992;340:145-146. [Cited in This Article: ] |
| 121. | Wakui S, Furusato M, Itoh T, Sasaki H, Akiyama A, Kinoshita I, Asano K, Tokuda T, Aizawa S, Ushigome S. Tumour angiogenesis in prostatic carcinoma with and without bone marrow metastasis: a morphometric study. J Pathol. 1992;168:257-262. [Cited in This Article: ] |
| 122. | Tanigawa N, Matsumura M, Amaya H, Kitaoka A, Shimomatsuya T, Lu C, Muraoka R, Tanaka T. Tumor vascularity correlates with the prognosis of patients with esophageal squamous cell carcinoma. Cancer. 1997;79:220-225. [Cited in This Article: ] |
| 123. | Tanigawa N, Amaya H, Matsumura M, Shimomatsuya T. Association of tumour vasculature with tumour progression and overall survival of patients with non-early gastric carcinomas. Br J Cancer. 1997;75:566-571. [Cited in This Article: ] |
| 124. | Takahashi Y, Tucker SL, Kitadai Y, Koura AN, Bucana CD, Cleary KR, Ellis LM. Vessel counts and expression of vascular endothelial growth factor as prognostic factors in node-negative colon cancer. Arch Surg. 1997;132:541-546. [Cited in This Article: ] |
| 125. | Uzzan B, Nicolas P, Cucherat M, Perret GY. Microvessel density as a prognostic factor in women with breast cancer: a systematic review of the literature and meta-analysis. Cancer Res. 2004;64:2941-2955. [Cited in This Article: ] |
| 126. | Vieira SC, Zeferino LC, Da Silva BB, Aparecida Pinto G, Vassallo J, Carasan GA, De Moraes NG. Quantification of angiogenesis in cervical cancer: a comparison among three endothelial cell markers. Gynecol Oncol. 2004;93:121-124. [Cited in This Article: ] |
| 127. | Tomoda M, Maehara Y, Kakeji Y, Ohno S, Ichiyoshi Y, Sugimachi K. Intratumoral neovascularization and growth pattern in early gastric carcinoma. Cancer. 1999;85:2340-2346. [Cited in This Article: ] |
| 128. | Meert AP, Paesmans M, Martin B, Delmotte P, Berghmans T, Verdebout JM, Lafitte JJ, Mascaux C, Sculier JP. The role of microvessel density on the survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br J Cancer. 2002;87:694-701. [Cited in This Article: ] |
| 129. | Mokrý J, Cízková D, Filip S, Ehrmann J, Osterreicher J, Kolár Z, English D. Nestin expression by newly formed human blood vessels. Stem Cells Dev. 2004;13:658-664. [Cited in This Article: ] |
| 130. | Shimizu T, Sugawara K, Tosaka M, Imai H, Hoya K, Takeuchi T, Sasaki T, Saito N. Nestin expression in vascular malformations: a novel marker for proliferative endothelium. Neurol Med Chir (Tokyo). 2006;46:111-117. [Cited in This Article: ] |
| 131. | Sugawara K, Kurihara H, Negishi M, Saito N, Nakazato Y, Sasaki T, Takeuchi T. Nestin as a marker for proliferative endothelium in gliomas. Lab Invest. 2002;82:345-351. [Cited in This Article: ] |