Published online Nov 7, 2011. doi: 10.3748/wjg.v17.i41.4581
Revised: March 24, 2011
Accepted: March 31, 2011
Published online: November 7, 2011
AIM: To compare histological endpoint assessment using noninvasive alternatives to biopsy during treatment in a chronic hepatitis C virus (HCV) cohort.
METHODS: Patients with chronic HCV were randomized to receive interferon-based therapy for 24 (genotypes 2/3) or 48 (genotype 1) wk. FibroSURE™ (FS) was assessed at baseline and at week-12 post-treatment follow-up. Baseline biopsy for METAVIR was assessed by a single pathologist. FibroScan® transient elastography (TE) was performed during treatment in a patient subset.
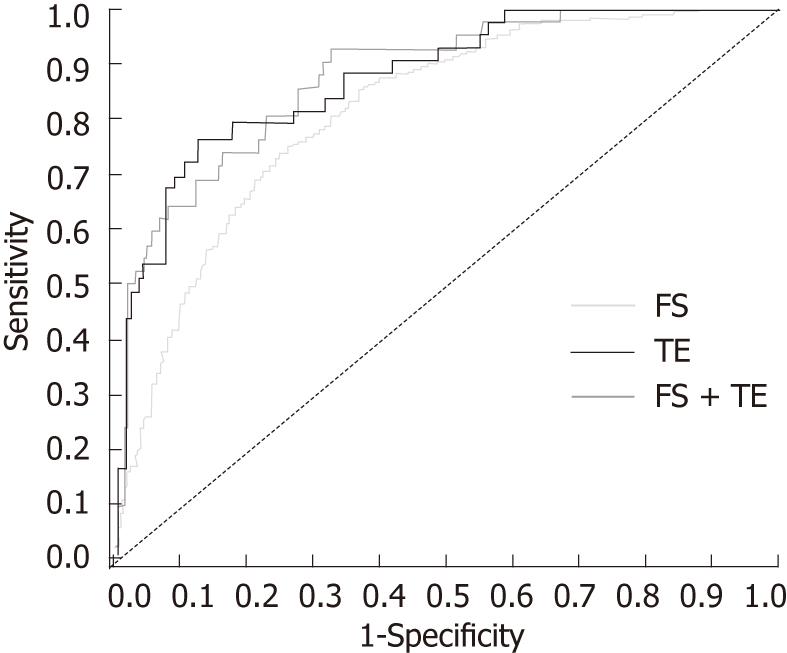
RESULTS: Two thousand and sixty patients (n = 253 in Asia) were classified as METAVIR F0-1 (n = 1682) or F2-4 (n = 378). For F2-4, FS (n = 2055) had sensitivity and specificity of 0.87 and 0.61, respectively, with area under the receiver-operating curve of 0.82; corresponding values for TE (n = 214) and combined FS/TE (n = 209) were 0.77, 0.88 and 0.88, and 0.93, 0.68 and 0.88. Overall FS/TE agreement for F2-4 was 71% (κ = 0.41) and higher in Asians vs non-Asians (κ = 0.86 vs 0.35; P < 0.001). Combined FS/TE had 97% accuracy in Asians (n = 33). Baseline FS (0.38 vs 0.51, P < 0.001) and TE (8.0 kPa vs 11.9 kPa, P = 0.006) scores were lower in patients with sustained virological response than in nonresponders, and were maintained through follow-up.
CONCLUSION: FS and TE may reliably differentiate mild from moderate-advanced disease, with a potential for high diagnostic accuracy in Asians with chronic HCV.
- Citation: Patel K, Friedrich-Rust M, Lurie Y, Grigorescu M, Stanciu C, Lee CM, Schiff ER, Häussinger D, Manns MP, Gerken G, Colle I, Torbenson M, Pulkstenis E, Subramanian GM, McHutchison JG, Zeuzem S. FibroSURE™ and FibroScan® in relation to treatment response in chronic hepatitis C virus. World J Gastroenterol 2011; 17(41): 4581-4589
- URL: https://www.wjgnet.com/1007-9327/full/v17/i41/4581.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i41.4581
Complications of chronic hepatitis C virus (HCV) infection occur as a consequence of progressive liver fibrosis, leading to cirrhosis, liver failure, and hepatocellular carcinoma. Histological assessment of liver injury and fibrosis is important for making treatment decisions, as well as for predicting prognosis and therapeutic outcome, in chronic liver disease[1]. Liver biopsy is, however, an invasive procedure, limited by issues relating to sampling, cost and morbidity, and only provides a static measure of fibrosis[2]. Accurate noninvasive methods of monitoring changes in fibrosis would be helpful in following the natural history of the disease and monitoring potential antifibrotic responses to antiviral or other treatment modalities. The past decade has seen the development of several noninvasive predictive indices for hepatic fibrosis based on direct and indirect serum markers, as well as imaging modalities to measure liver stiffness, such as transient elastography (TE) (FibroScan®, Echosens, Paris, France)[3]. Serum HCV FibroSURE™ (FS) (Laboratory Corporation of America, Raritan, NJ, United States) combines α2-macroglobulin, haptoglobin, γ-glutamyl transpeptidase, apolipoprotein A1, alanine transaminase, and total bilirubin into a proprietary algorithm for fibrosis and inflammatory activity[4]. Both noninvasive modalities have been extensively evaluated in viral hepatitis and other chronic liver diseases[5]. The combination of serum tests, such as FS or FibroMeters, and TE appears to improve the cross-sectional diagnostic accuracy for advanced-stage disease in chronic HCV[6,7]. The French Haute Autorité de Santé has approved FS and TE as first-line tests to detect cirrhosis in chronic HCV[8]. Few studies, however, have determined the utility of either biomarkers or TE to accurately follow longitudinal changes in fibrosis both during and after antiviral therapy to better define long-term histological outcomes in chronic HCV[9-12]. Although there are emerging studies of TE in Asian patients with chronic liver disease (mostly due to chronic hepatitis B virus), no studies have evaluated the utility of both FS and TE in Asian patients with chronic HCV[13,14].
The aims of the present study were to: (1) compare the diagnostic utility of FS and TE for fibrosis at baseline in treatment-naïve patients with chronic HCV; (2) determine concurrent changes in both FS and TE with virological responses during and after albinterferon alfa-2b (albIFN) combination therapy; and (3) evaluate the performance of these noninvasive tests for the detection of significant fibrosis in an Asian cohort.
Adult patients with chronic HCV genotype (Gt) 1 or 2/3 (n = 2225) who had not previously received interferon (IFN)-α therapy were enrolled in two global phase III studies of albIFN conducted at 136 centers worldwide between December 2006 and October 2008 (ClinicalTrials. gov nos. NCT00402428 and NCT00411385)[15,16]. Patients were excluded if they had decompensated liver disease or other causes of chronic liver disease, including co-infection with hepatitis B virus or human immunodeficiency virus; a significant co-existing medical condition; Gilbert’s disease; or alcohol or drug dependence. Patients were randomized in a 1:1:1 ratio to one of three open-label treatment groups: albIFN 900 or 1200 μg every 2 wk, or PEGinterferon alfa-2a (PEGASYS®, Hoffmann-La Roche Inc., Nutley, NJ) 180 μg
once weekly. All patients also received oral ribavirin (RIBASPHERE®, 3 Rivers Pharmaceuticals®, Warrendale, PA, United States) 800 mg/d (Gt 2/3) or 1000-1200 mg/d
(Gt 1) in two divided doses. Treatment duration was 24 (Gt 2/3) or 48 (Gt 1) wk, with follow-up at week 48 or 72, respectively, for sustained virological response (SVR) assessment. Patients with detectable HCV RNA at post-treatment week-12 follow-up were determined to be nonresponders (NRs) and were not required to complete final follow-up at post-treatment week 24. Serum HCV-RNA levels were measured by real-time polymerase chain reaction assay (COBAS® Ampliprep/COBAS® Taqman® HCV Test, Hoffman-La Roche): limit of detection was 15 IU/mL and lower limit of quantitation 43 IU/mL.
All patients provided written informed consent and the institutional review boards of all participating centers approved the studies, which were performed in accordance with the Helsinki Declaration of 1975. The authors accept full responsibility for the accuracy of the whole content, including findings, citations, and references contained in this manuscript.
Pretreatment liver biopsies were available in 2060 patients, and evaluated for METAVIR fibrosis stage and activity grade by a single pathologist (Torbenson M) who was blinded to study assignments or results. Adequate biopsy quality was based on assessment by the pathologist of specimens ≥ 15 mm in length and/or including ≥ 6 portal tracts. The METAVIR scoring system classifies fibrosis on a five-point scale: F0 = no fibrosis; F1 = portal fibrosis without septa; F2 = few septa; F3 = numerous septa without cirrhosis; and F4 = cirrhosis; necro-inflammatory activity is graded on a 4-point scale: A0 = none; A1 = mild; A2 = moderate; and A3 = severe[17].
Fasting serum samples were frozen at -70 °C within 2 h of collection. Assessment with FibroSURE, a commercial serum marker panel assay, was performed independently, with blinding of clinical and pathologic assessments at a central laboratory (Laboratory Corporation of America), at baseline and 12 wk after the end of treatment. The TE measurements were obtained using FibroScan at baseline, weeks 12, 24 and 48, and 12 wk after the end of treatment in patients with HCV Gt 1, and weeks 12, 24 and 36 with Gt 2/3, at 40 study sites as part of a protocol-specified substudy. Results of TE with ≥10
acquisitions, a success rate ≥ 60%, and an interquartile range < 30% of the median value were considered valid measurements, as per manufacturer’s recommendation and prior studies[3,18,19].
Patient demographic and clinical laboratory characteristics were descriptively summarized, and reported as mean ± SD and range. All tests were two-sided, and statistical significance was assessed at the 0.05 level. Performance characteristics differentiating mild (F0-1) from moderate-severe (F2-4) fibrosis at baseline were determined for FS. Performance of this assay for F2-4 was determined by area under the receiver-operating characteristic curve (AUROC) using the DeLong method[20]. Values for AUROC were standardized relative to a uniform prevalence distribution, and an adjusted AUROC was calculated to account for spectrum bias, using the difference between the mean stage of advanced fibrosis minus the mean stage of nonadvanced fibrosis[21]. The FS modality provides a continuous regression index with a corresponding predicted individual fibrosis stage[22]. An FS index < 0.32 was used for stage F0-1. For two-stage predictive indices with FS for F0-1, F1-2, and F3-4, the midpoint index value was used as a threshold for assignment of stage for analysis. The TE cut-off values were chosen via AUROC analysis as the point at which sensitivity and specificity were maximized. Recommended thresholds for TE in chronic HCV of > 7 kPa and > 12.5 kPa for F2 and F4, respectively, were also assessed[6]. For assessing the utility of combined FS and TE, prediction was based on a logistic-regression model containing both indices, as well as their pairwise interaction. The measure of agreement chosen was Cohen’s κ. Differences between continuous variables were assessed by Student’s t test, assuming unequal variance. All statistical analyses were performed using SAS® 9.2 (SAS Institute, Cary, NC).
Baseline biopsy (mean length 17 mm ± 9 mm) results were available from 2060 patients with chronic HCV. Patients were mostly men (58.1%) and caucasian (77.6%), with a mean age of 45.2 ± 11.4 years and a prevalence of significant fibrosis of 18.3% (Table 1).
| Host characteristics | All(n = 2060) | Patients with TE (n = 214) |
| Mean age ± SD (yr) | 45.2 ± 11.4 | 45.7 ± 11.7 |
| Race | ||
| Caucasian | 1599 (77.6) | 169 (79.0) |
| Black | 98 (4.8) | 5 (2.3) |
| Asian | 322 (15.6) | 39 (18.2) |
| Other | 41 (2.0) | 1 (0.5) |
| Genotype | ||
| 1 | 1186 (57.6) | 120 (56.1) |
| 2/3 | 874 (42.4) | 94 (43.9) |
| Male sex | 1196 (58.1) | 105 (49.1) |
| Mean BMI ± SD | 26.6 ± 5.1 | 25.1 ± 4.2 |
| ALT > 1.5 × ULN | 911 (44.2) | 98 (45.8) |
| Mean biopsy length ± SD (mm) | 17.0 ± 9.1 | 15.8 ± 8.2 |
| METAVIR fibrosis stage | ||
| F0 | 740 (35.9) | 80 (37.4) |
| F1 | 942 (45.7) | 91 (42.5) |
| F2 | 159 (7.7) | 16 (7.5) |
| F3 | 101 (4.9) | 9 (4.2) |
| F4 | 118 (5.7) | 18 (8.4) |
| METAVIR activity | ||
| A0-1 | 1125 (54.6) | 126 (58.9) |
| A2-3 | 935 (45.4) | 88 (41.1) |
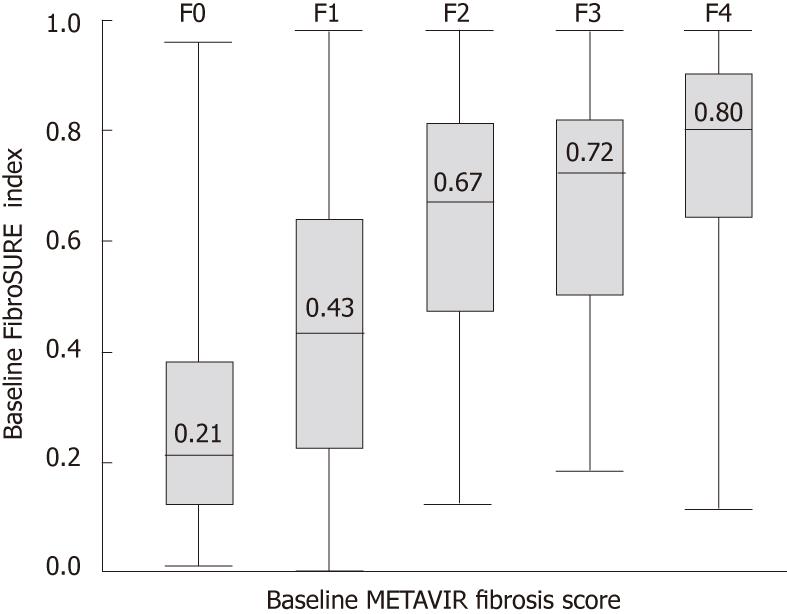
Results for FS and biopsy were available in 2055 patients. For stages F2-4, FS had a sensitivity of 0.87, a specificity of 0.61, and an AUROC of 0.82 [95% confidence interval (CI) 0.80-0.84, Figure 1]; the corresponding adjusted AUROC relative to a uniform prevalence distribution was 0.84. For F4, sensitivity was 0.63, specificity was 0.85, and AUROC was 0.83 (95% CI 0.79-0.86). The misclassification rate for FS was 34% (n = 703/2055), and most of these patients (93%; n = 653) were false-positive F2-4 (Figure 2). For biopsy specimens > 15 mm and F2-4 (46.0%; n = 948/2055), sensitivity was 0.86, specificity was 0.61, and AUROC was 0.83 (95% CI 0.80-0.86). The FS misclassification rate, however, remained 34% in these patients with longer biopsy specimens. For biopsies > 15 mm and F4, sensitivity was 0.67, specificity was 0.84, and AUROC was 0.86 (95% CI 0.81-0.91). For moderate-severe necro-inflammatory activity of A2-3, sensitivity and specificity were both 0.66, and AUROC was 0.71 (95% CI 0.69-0.73).
Results of TE and biopsy were available in 214 patients. For stage F2, TE > 10.1 kPa had a sensitivity of 0.77, a specificity of 0.88, an AUROC of 0.88 (95% CI 0.82-0.93), and an adjusted AUROC of 0.88 (Figure 1). For F4, TE > 11.7 kPa had a sensitivity of 0.94, a specificity of 0.88, and an AUROC of 0.93 (95% CI 0.88-0.98). The misclassification rate for TE was 14% (n = 31), with approximately two-thirds of these patients (68%; n = 21) classified as false-positive F2-4. For F2-4 with biopsy specimens > 15 mm (39.3%, n = 84; F2-4 prevalence 22.6%, n = 19), sensitivity was 0.63, specificity was 0.91, and AUROC was 0.83 (95% CI 0.72-0.93).
Performance characteristics of TE, using a previously recommended threshold of > 7 kPa for stages F2-4[6], indicated a higher sensitivity and lower specificity of 0.88 and 0.65, respectively, with a lower overall accuracy of 0.70. For stage F4 at a TE threshold of > 12.5 kPa, sensitivity was lower at 0.72, but with a similar specificity of 0.89.
Both FS and TE results were available in 209 patients before therapy. For this subset, prediction of stages F2-4 using FS and TE in combination had a sensitivity of 0.93, a specificity of 0.68, an AUROC of 0.88 (95% CI 0.82-0.94), and an adjusted AUROC of 0.88 (Figure 1). Agreement between FS and TE, however, for F2-4 was 0.71 (95% CI 0.65-0.77), with a Cohen’s κ of 0.41 (95% CI 0.30-0.52). Among 61 patients with nonconcordance for FS and TE, 88% (n = 54) were F2-4 by FS and F0-1 by TE; biopsy indicated mild-stage disease in most of these 54 patients [F0-1 in 88.9% (n = 48); F2-4 in 11.1% (n = 6)]. Conversely, only seven of the 61 patients were F0-1 by FS and F2-4 by TE; four of these patients were F0-1 by biopsy.
For the 148 patients with agreement between FS and TE, 68% (n = 101) were stages F0-1 and 32% (n = 47) were F2-4 by both noninvasive tests. Biopsy results, however, indicated agreement with both FS and TE in 86% (n = 128), with 3% and 11% misclassified by both noninvasive tests as F0-1 and F2-4, respectively. Biopsies were > 15 mm in 56 of the patients for which there was agreement between FS and TE, and concordance results for the noninvasive tests were compared with the biopsy results: there was a slight reduction in the proportion of patients misclassified by the combination of FS and TE (from 14.0% to 10.7%), although the sample size was relatively small. Agreement between FS and TE for stage F4 increased to 0.85 (95% CI 0.80-0.90; κ = 0.53), and compared with biopsy, misclassification rates (biopsy < F4) for both FS and TE were 7.3%, with all cases being false positives.
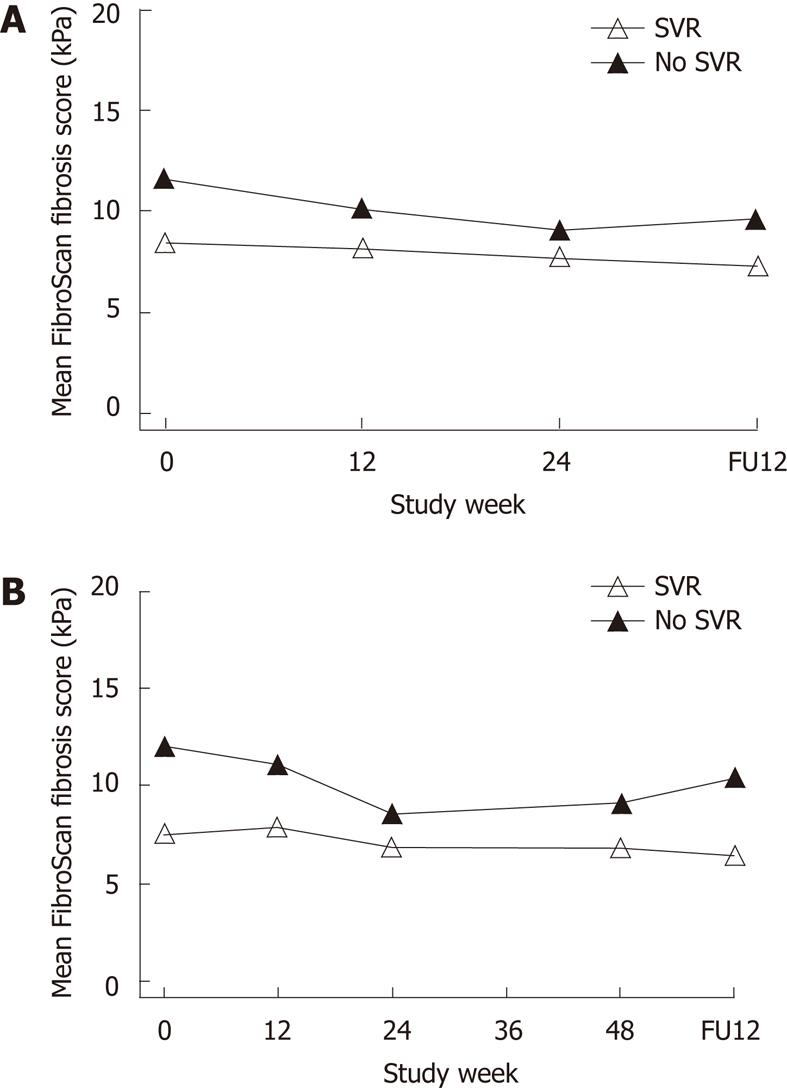
Results for TE were available in a subset of 217 patients who completed treatment (HCV Gt 1, n = 122; Gt 2/3, n = 95). Mean TE scores were lower at baseline in patients who achieved an SVR compared with NRs (8.0 vs 11.9 kPa; P = 0.006). Further multivariate modeling showed no association with Gt, race, or body mass index, but significant increases in liver stiffness in older patients (P < 0.001) and men (P = 0.03). In addition, TE scores were higher in patients with METAVIR grades 2-3 inflammatory activity score at baseline (11.8 vs 7.3; P < 0.001), with further minimal declines in TE measurements during therapy for patients with an SVR and NRs. The difference at baseline remained significant only at week 12 (P = 0.03) and lost significance at later on-treatment time points (Figure 3). At the follow-up visit, overall mean changes in TE from baseline were -1.3 kPa (P < 0.001) and -2.7 (P = 0.04) for patients with an SVR and NRs, respectively, reflecting declines to levels that remained different (6.9 vs 10.1; P = 0.049). There was no correlation between changes in TE and alanine transaminase during or after therapy.
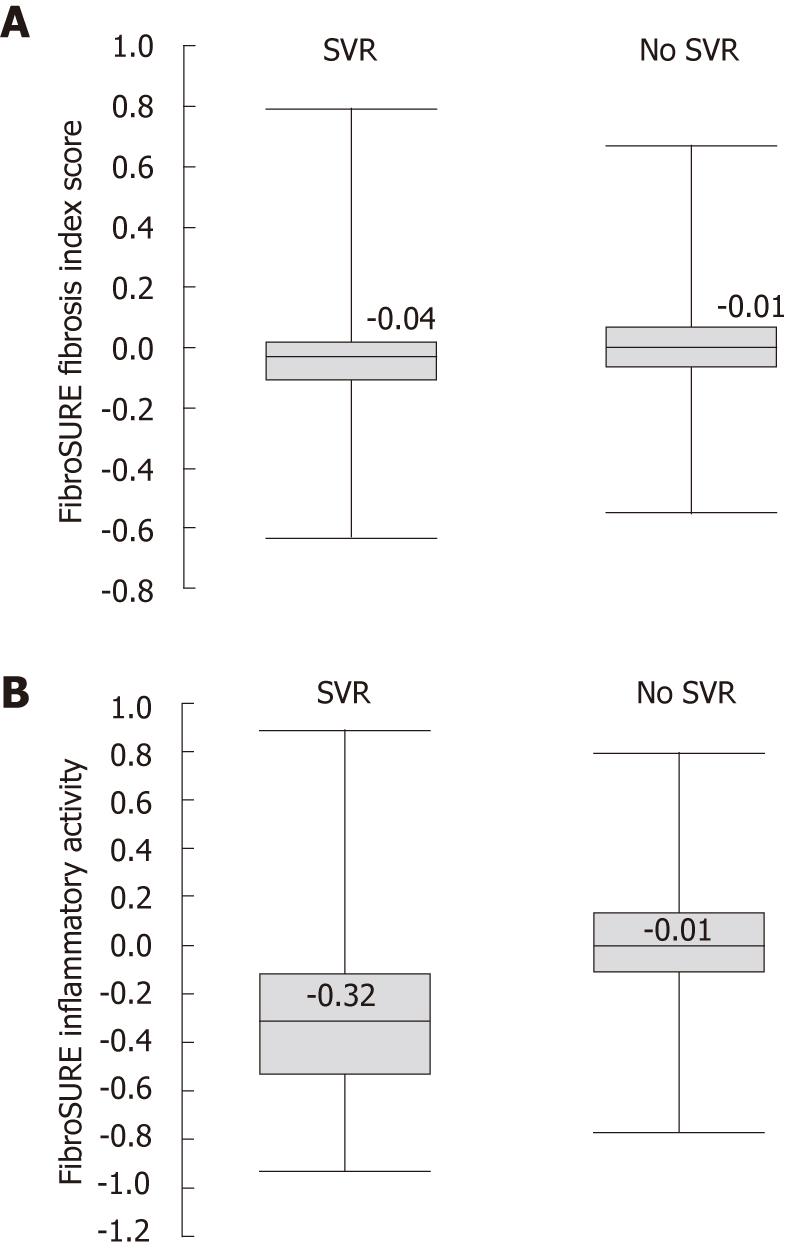
Baseline FS scores were available in 2082 patients (HCV Gt 1, n = 1200; Gt 2/3, n = 882), with 1731 also available at post-treatment follow-up. Patients who achieved an SVR (n = 1305) had lower mean baseline FS fibrosis index scores compared with NRs (n = 777; 0.38 vs 0.51; P < 0.001). Baseline FS necro-inflammatory activity scores were not significantly lower in patients with an SVR (0.45 vs 0.47; P = 0.06). At post-treatment follow-up week 12, there was a significant reduction in FS fibrosis scores from baseline in patients with an SVR compared with NRs (Δ = -0.06 vs 0.0; P < 0.001; Figure 4). As expected with a biochemical response that accompanies viral clearance, there was a significant reduction in FS necro-inflammatory activity scores in patients with an SVR compared with NRs following antiviral therapy (Δ = -0.35 vs -0.02, P < 0.001) (Figure 4).
There were 253 Asian patients with a baseline biopsy (mean length 13.5 ± 8.4 mm), classified as stages F0-1 in 75% (n = 190) and F2-F4 in 26% (n = 63). For F2-4 with FS (n = 253), sensitivity was 0.90, specificity was 0.60, and AUROC was 0.83. These results were comparable to those in patients from a non-Asian region (NAR; n = 1802), with a sensitivity of 0.86, a specificity of 0.61, and an AUROC of 0.82 (Table 2). In comparison to FS, TE had a similar sensitivity (0.88), but a higher specificity (0.92) and AUROC (0.92, P = 0.11); however, there were only 33 Asian patients with available TE. There was no difference in F2-4 prevalence between Asian patients with available TE (25%) and FS (24%). Despite the small cohort, TE results for F2-4 in Asian patients were comparable to those in NAR patients (n = 181, AUROC 0.92 vs 0.87, P = 0.35).
| Test | Region (n) | F2-4 (%) | Sens | Spec | PPV (%) | NPV (%) | Accuracy (%) | AUROC |
| FS | Asia (253) | 24.9 | 0.90 | 0.60 | 42.9 | 95.0 | 67.6 | 0.83 |
| NAR (1802) | 17.4 | 0.86 | 0.61 | 31.9 | 95.4 | 65.5 | 0.82 | |
| TE | Asia (33) | 24.2 | 0.88 | 0.92 | 77.8 | 95.8 | 90.9 | 0.92 |
| NAR (181) | 19.3 | 0.77 | 0.87 | 58.7 | 94.1 | 85.1 | 0.87 | |
| FS + TE | Asia (33) | 24.2 | 0.88 | 1.00 | 100.0 | 96.2 | 97.0 | 0.97 |
| NAR (176) | 19.3 | 0.91 | 0.68 | 40.8 | 97.0 | 72.7 | 0.88 |
For patients with both noninvasive tests available (Asian, n = 33; NAR, n = 176), agreement between FS and TE for stages F2-4 was higher in Asian than in NAR patients [94% (κ = 0.86) vs 67% (κ = 0.35); P < 0.001]. The combination of FS and TE improved the accuracy for F2-4 in both Asian (97.0%) and NAR patients (72.7%), with AUROC values of 0.97 and 0.88, respectively (Table 2).
Most patients from the Asian region with available FS or TE achieved an SVR (85%; n = 216) and thus comparisons with NRs were not feasible. Baseline FS fibrosis scores, however, were higher in Asian than in NAR patients with an SVR (0.45 vs 0.36; P < 0.001), likely reflecting differences in F2-4 prevalence between the two study populations. Changes in FS fibrosis scores at week-12 follow-up were also comparable between Asian and NAR patients with an SVR (Δ = -0.04 vs -0.06; P = 0.09). In addition, mean TE measurements were comparable at baseline between Asian and NAR patients with an SVR (8.8 vs 7.8 kPa; P = 0.56), with no significant differences in changes at week 12 (-0.14 kPa vs -0.25 kPa, P = 0.91) or after therapy (-1.4 kPa vs -1.3 kPa, P = 0.98).
This large prospective cohort study in patients with chronic HCV provides validation of the diagnostic utility of serum markers and TE in relation to biopsy and IFN-based therapy. Few studies have addressed longitudinal changes in either serum markers or TE with therapy and, importantly, the present global study also provides the first evaluation of both noninvasive tests in patients from the Asia-Pacific region with chronic HCV. One limitation of this study was that TE data could only be obtained at 40 non-US study sites, as this device is not yet approved for use in the United States. Despite the limited cohort size for TE (n = 214) compared with FS (n = 2055), in accordance with prior observations, the overall results of this study indicate that both FS and TE have potential utility in the detection of moderate-severe-stage disease. However, the performance characteristics of these noninvasive tests (particularly TE) may be somewhat better for exclusion of cirrhosis[6,23]. For stages F2-4, FS and TE were effective in both Asian and NAR patients, but the agreement and accuracy of combined FS and TE were higher in the limited cohort of Asian patients with both tests (n = 33). Changes in FS and TE in relation to SVR were similar for both Asian and NAR patients.
This study shows that both pretreatment TE (n = 217) and FS (n = 2082) scores were lower in patients who achieved an SVR than in NRs, and that these differences were maintained through week 12 of therapy. Multivariate modeling indicated that older age and male sex (both predictive of lower virological responses in chronic HCV) were also independently associated with higher TE measurements at baseline. Other smaller studies, however, have failed to demonstrate similar baseline associations. A recent study from France evaluated TE and FS in 112 patients with chronic HCV receiving antiviral therapy, but did not include baseline biopsy or evaluation during therapy[11]. That study did not find any significant differences at baseline between patients with an SVR and NRs. Another study assessed TE alone before and after therapy in a Japanese chronic HCV cohort of 145 patients, and noted no differences at baseline between patients with an SVR and NRs[12]. Similar findings from another small French cohort evaluating TE alone have also been reported[24]. A recent meta-analysis of longitudinal studies in viral hepatitis indicated that both FS and TE could estimate treatment effect on fibrosis progression, although TE appeared to have early variability on treatment due to possible changes in necro-inflammatory activity[25]. The present study suggests that FS and TE could provide useful adjunctive information for the prediction of virological response prior to IFN-based therapy for chronic HCV. These noninvasive tests, however, likely reflect baseline differences in inflammatory response, but could complement established host-viral predictors of virological response to IFN-based therapy, such as HCV-RNA levels, viral Gt, race, and host IL28B polymorphism[26,27].
At follow-up, both FS and TE declined in patients who achieved an SVR. This is in accordance with prior observations that successful treatment with a biochemical response was associated with a decline in serum fibrosis marker indices or TE measurements in patients with chronic HCV[9-12,28,29]. A limitation of this study is that post-treatment biopsies were not required as part of these two clinical registration trials, which would have allowed for correlation between the observed declines in noninvasive test scores and changes in fibrosis or necro-inflammation. TE measurements may vary significantly with immune-mediated inflammatory responses in patients with chronic hepatitis B virus[30]. In contrast to other studies in patients with chronic HCV[6], however, this study also demonstrated a significant association between TE and histological necro-inflammatory activity at baseline. This association may have implications for establishing TE thresholds for different fibrosis stages in chronic HCV and may also explain the decline in liver stiffness measurements in patients with an established SVR in the present cohort. In this study, 80% of patients had no or mild fibrosis (stages F0-1) prior to treatment and thus were incapable of achieving a significant regression in fibrosis.
Both Asian and NAR cohorts demonstrated comparable performance characteristics for FS and TE. The observed accuracy and specificity for prediction of stages F2-4 in Asians, however, was higher in the small TE cohort. The combination of FS and TE in Asian patients resulted in a high accuracy for prediction of F2-4, with no false-positive results. Thus, biopsies for staging F2-4 could have been avoided in almost all Asian patients in this small cohort of 33 patients. There was excellent agreement between FS and TE in Asian patients, and this may partly relate to a slightly higher prevalence of advanced-stage disease and lower body mass index in the Asian cohort. Furthermore, increased waist circumference appears to be a common reason for failure of TE in European cohorts[19]. Although there are potential issues in obtaining adequate TE measurements in Asian patients due to a narrow intercostal space, this was not a limiting factor in the present study[13]. Thus, the combination of FS and TE in Asian patients with chronic HCV merits further evaluation.
One of the strengths of this study is that sample collections were standardized per protocol for the two phase III clinical trials, laboratory assessments were performed centrally, and all biopsies were evaluated by a single experienced liver histopathologist. Standardization significantly reduced the heterogeneity observed in prior studies comparing results across different geographic populations[23]. Biopsy sampling and observer error, however, are inherent limitations to the development and validation of all fibrosis biomarkers[31]. The experience of the pathologist may be more important than biopsy characteristics[32]. Furthermore, prior studies have indicated that the accuracy of liver biopsy (and noninvasive tests) is dependent on sample size[9,33-35]. In contrast to these prior observations, no significant change in the diagnostic accuracy of FS for stages F2-4 in > 900 patients with biopsies > 15 mm was found in the present study. Of note, TE accuracy for F2-4 appeared to decline in > 80 patients with this optimal biopsy length, although only 19 with biopsy F2-4 were in this cohort.
Prior studies have suggested that noninvasive performance indices for stages F2-4 and F4 are improved using sequential algorithms of FS and TE[6], or aspartate aminotransferase-to-platelet ratio index and FS[36]. In the present study, the F2-4 results for combined FS and TE indicated a comparable AUROC and agreement (0.88 and 71%, respectively) to those observed in a recent study from France in 302 patients with chronic HCV (0.91 and 72%, respectively) with a higher prevalence of advanced-stage disease[37]. Although discordance rates between sequential FS and TE with biopsy were not reported in the French study, prior data from that cohort indicated 84% concordance between biopsy and FS/TE agreement. This observation is similar to the 86% agreement noted in the present study; however, in contrast to the French cohort, cases of discordance between biopsy and FS/TE agreement were due to false positives with the noninvasive tests. In practical terms, agreement between FS and TE regarding prediction of F2-4 in the present study cohort could have avoided 71% of biopsies, although 10% of patients would still have been misclassified as having significant fibrosis. The discordance rate between FS and TE was 29%, with biopsy and TE agreement in most of the cases that appeared to have mild-stage disease. As expected, misclassification rates and discordance between FS and TE with biopsy were significantly reduced for prediction of F4.
With a broader range of available therapeutic options for patients with chronic HCV in the future, noninvasive measures that can accurately exclude advanced-stage disease will likely assume a more significant clinical role in the treatment decision process. Recent mathematical modeling indicates that a perfect biomarker of stages F2-4 may not exceed an AUROC of 0.9[38], and thus various issues regarding biopsy sampling error and noninvasive test discordance should be individualized when using these tests to predict a threshold of F2 in clinical practice. The observed heterogeneity among studies (including the present one) for optimized TE cutoffs indicates that a range of liver stiffness measurements for each fibrosis threshold in patients with chronic HCV may be more appropriate[39]. Standardization of AUROC curves or other methods to reduce effects of spectrum bias in disease prevalence allows for comparison of FS across studies, including selected cohorts within studies, but not for TE due to variable optimal thresholds[21]. No significant differences between observed and standardized AUROC values were found in the present study for either noninvasive measure.
In summary, this study demonstrates that a combination of serum and imaging noninvasive tests can be used for prediction of at least moderate-stage disease in a global cohort of patients with chronic HCV, including the potential of higher accuracy for the combination of FS and TE in Asian patients. Furthermore, some baseline differences in index values for both FS and TE were dependent on virological response and merit further evaluation in the context of IFN-based therapy.
Marx G of BioScience Communications, New York, NY, United States, provided editorial assistance, supported by Human Genome Sciences and Novartis Pharma AG. The authors also wish to thank all research staff and technicians who participated in this study.
ACHIEVE-1 investigators: Benhamou Y, Hôpital Pitié-Salpêtrière, Paris, France; Berg T, University Hospital Charite Berlin/Virchow Klinikum, Berlin, Germany; Bourliere M, Hopital Saint-Joseph, Marseille, France; Chang TT, National Cheng Kung University Hospital, Tainan, Taiwan, China; Couzigou P, Hopital Haut-Leveque, Pessac, France; Davis G, Baylor University Medical Center, Dallas, TX, United States; Encke J, Universitätsklinikum Heidelberg, Germany; Forns X, Hospital Clinic i Provincial, Barcelona, Spain; Gerken G, University Hospital of Essen, Germany; Ghalib R, Liver Institute at Methodist Dallas, Texas; Gladysz A, Przychodnia przy Łowieckiej, Wrocław, Poland; Grigorescu M, Spitalul Clinic de Urgenta, Cluj-Napoca, Romania; Häussinger D, Universitätsklinik Düsseldorf, Germany; LurieY, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel; Manns M, Medizinische Hochschule Hannover, Germany; Marcellin P, Hopital Beaujon, Clichy, France; Moreno R, Hospital Universitario La Princesa, Madrid, Spain; Pawlotsky JM, Hopital Henri Mondor, Creteil, France; Plisek S, Klinika Infekcních Nemocí, Hradec Kralove, Czech Republic; Poupon R, Hôpital Saint-Antoine, Paris, France;
Rodriquez-Torres M, Fundacion de Investigacion de Diego, Santurce, Puerto Rico; Safadi R, Holy Family Hospital, Nazareth, Israel; Schiff E, University of Miami, Florida, United States; Stanciu C, Institutul de Gastroenterologie si Hepatologie, Iasi, Romania; Trepo C, CHU de Lyon, Hôpital de l’Hôtel Dieu, France; Zeuzem S, J.W. Goethe University Hospital, Frankfurt, Germany.
ACHIEVE-2/3 investigators: Baruch Y, Rambam Health Care Campus, Haifa, Israel; Benhamou Y, Hopital Pitie-Salpetriere, Paris, France; Berg T, University Hospital Charite Berlin/Virchow Klinikum, Germany; Bourliere M, Hopital Saint-Joseph, Marseille, France; Bronowiki JP, Hôpital de Brabois, Vandoeuvre, France; Lee CM, Chang Gung Memorial Hospital-Kaohsiung Medical Center, Chang Gung University College of Medicine, Kaohsiung, Taiwan; Cho M, Pusan National University Hospital, Busan, South Korea; Colle I, Ghent University Hospital, Ghent, Belgium; Delwaide J, CHU Sart Tilman, Liège, Belgium; Encke J, Universitätsklinikum Heidelberg, Germany; Gerken G, University Hospital Essen, Germany; Han KH, Severance Hospital, Yonsei University College of Medicine, Seoul, South Korea; Häussinger D, Universitätsklinik Düsseldorf, Germany; Lee KS, Yongdong Severance Hospital, Seoul, South Korea; Langlet P, CHU Brugmann ULB-Site Victor Horta, Brussels, Belgium; Manns M, Medizinische Hochschule Hannover, Germany; Marcellin P, Hopital Beaujon, Clichy, France; Michielsen P, University Hospital Antwerp, Edegem, Belgium; Pawlotsky JM, Hopital Henri Mondor, Creteil, France; Poupon R, Hôpital Saint-Antoine, Paris, France; Schiff E, University of Miami, Florida; Trepo C, CHU de Lyon, Hôpital de l’Hôtel Dieu, France; Tur Kaspa R, Rabin Medical Center Beilinson Hospital, Petach Tikva, Israel; Um SH, Korea University Medical Center, Anam Hospital, Seoul, South Korea; Zeuzem S, J.W. Goethe University Hospital, Frankfurt, Germany.
Liver biopsy is an invasive procedure associated with significant costs and risk of complications. Noninvasive alternatives to biopsy for the determination of fibrosis stage include serum [FibroSURE (FS)] or imaging-based [transient elastography (TE) FibroScan] tests. The combination of these two modalities appears to have a good predictive value for excluding cirrhosis. Fibrosis is a predictor of virological response to chronic hepatitis C virus (HCV) therapy, and noninvasive tests may also be useful in this regard. Test values may vary with antiviral therapy for chronic HCV, perhaps due to changes in hepatic inflammation or with body habitus. Few studies have evaluated the utility of both these noninvasive modalities in patients with chronic HCV during interferon-based therapy, and there are limited data for these tests in Asian patients, particularly in comparison with non-Asian cohorts.
Both FS and TE are validated measures for the noninvasive assessment of fibrosis. One important research issue is the determination of their utility in following changes in fibrosis or inflammation, e.g., as part of the natural history of disease or during and after antiviral therapy.
In this study, both FS and TE demonstrated good potential utility in the detection of moderate-severe-stage disease, but the performance characteristics of these noninvasive tests (particularly TE) may be somewhat better for exclusion of cirrhosis. Agreement between these tests and their accuracy for predicting disease stage may be higher in Asian than in non-Asian patients with chronic HCV. Patients that achieved a sustained virological response with interferon therapy appeared to have lower noninvasive test values at baseline.
Both FS and TE appear to have good clinical utility in predicting moderate-severe fibrosis prior to therapy, in both Asian and non-Asian patients with chronic HCV. Lower index scores at baseline may signify a better chance of responding to antiviral therapy.
FS comprises a combination of simple biochemical blood tests that predict fibrosis. TE is an ultrasound-based imaging method to measure liver stiffness, which also predicts fibrosis.
The authors investigated FS vs Fibroscan in treatment-naive patients with HCV. This article is unique and interesting.
Peer reviewers: Atsushi Nakajima, Professor, Division of Gastroenterology, Yokohama City University Graduate School of Medicine, 3-9 Fuku-ura, Kanazawa-ku, Yokohama 236-0004, Japan; A Mithat Bzodayi, MD, PhD, Hepatology Institute, Department of Gastroenterology, Ankara Medical Faculty, Ankara University, 06100 Cebeci Ankara, Turkey
S- Editor Lv S L- Editor Stewart GJ E- Editor Xiong L
| 1. | Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2252] [Cited by in F6Publishing: 2192] [Article Influence: 146.1] [Reference Citation Analysis (1)] |
| 2. | Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology. 2009;49:1017-1044. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1449] [Cited by in F6Publishing: 1419] [Article Influence: 94.6] [Reference Citation Analysis (0)] |
| 3. | Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, de Lédinghen V, Marcellin P, Dhumeaux D, Trinchet JC. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1090] [Cited by in F6Publishing: 1062] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 4. | Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069-1075. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1066] [Cited by in F6Publishing: 1113] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 5. | Smith JO, Sterling RK. Systematic review: non-invasive methods of fibrosis analysis in chronic hepatitis C. Aliment Pharmacol Ther. 2009;30:557-576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1796] [Cited by in F6Publishing: 1760] [Article Influence: 92.6] [Reference Citation Analysis (0)] |
| 7. | Boursier J, Vergniol J, Sawadogo A, Dakka T, Michalak S, Gallois Y, Le Tallec V, Oberti F, Fouchard-Hubert I, Dib N. The combination of a blood test and Fibroscan improves the non-invasive diagnosis of liver fibrosis. Liver Int. 2009;29:1507-1515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Fontaine H, Petitprez K, Roudot-Thoraval F, Trinchet JC. Guidelines for the diagnosis of uncomplicated cirrhosis. Gastroenterol Clin Biol. 2007;31:504-509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Poynard T, McHutchison J, Manns M, Myers RP, Albrecht J. Biochemical surrogate markers of liver fibrosis and activity in a randomized trial of peginterferon alfa-2b and ribavirin. Hepatology. 2003;38:481-492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 197] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 10. | Patel K, Benhamou Y, Yoshida EM, Kaita KD, Zeuzem S, Torbenson M, Pulkstenis E, Subramanian GM, McHutchison JG. An independent and prospective comparison of two commercial fibrosis marker panels (HCV FibroSURE and FIBROSpect II) during albinterferon alfa-2b combination therapy for chronic hepatitis C. J Viral Hepat. 2009;16:178-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Vergniol J, Foucher J, Castéra L, Bernard PH, Tournan R, Terrebonne E, Chanteloup E, Merrouche W, Couzigou P, de Lédinghen V. Changes of non-invasive markers and FibroScan values during HCV treatment. J Viral Hepat. 2009;16:132-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Ogawa E, Furusyo N, Toyoda K, Takeoka H, Maeda S, Hayashi J. The longitudinal quantitative assessment by transient elastography of chronic hepatitis C patients treated with pegylated interferon alpha-2b and ribavirin. Antiviral Res. 2009;83:127-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Chang PE, Lui HF, Chau YP, Lim KH, Yap WM, Tan CK, Chow WC. Prospective evaluation of transient elastography for the diagnosis of hepatic fibrosis in Asians: comparison with liver biopsy and aspartate transaminase platelet ratio index. Aliment Pharmacol Ther. 2008;28:51-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Wong GL, Wong VW, Choi PC, Chan AW, Chum RH, Chan HK, Lau KK, Chim AM, Yiu KK, Chan FK. Assessment of fibrosis by transient elastography compared with liver biopsy and morphometry in chronic liver diseases. Clin Gastroenterol Hepatol. 2008;6:1027-1035. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 15. | Nelson DR, Benhamou Y, Chuang WL, Lawitz EJ, Rodriguez-Torres M, Flisiak R, Rasenack JW, Kryczka W, Lee CM, Bain VG. Albinterferon Alfa-2b was not inferior to pegylated interferon-α in a randomized trial of patients with chronic hepatitis C virus genotype 2 or 3. Gastroenterology. 2010;139:1267-1276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Zeuzem S, Sulkowski MS, Lawitz EJ, Rustgi VK, Rodriguez-Torres M, Bacon BR, Grigorescu M, Tice AD, Lurie Y, Cianciara J. Albinterferon Alfa-2b was not inferior to pegylated interferon-α in a randomized trial of patients with chronic hepatitis C virus genotype 1. Gastroenterology. 2010;139:1257-1266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology. 1994;20:15-20. [PubMed] [Cited in This Article: ] |
| 18. | Castéra L, Foucher J, Bernard PH, Carvalho F, Allaix D, Merrouche W, Couzigou P, de Lédinghen V. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology. 2010;51:828-835. [PubMed] [Cited in This Article: ] |
| 19. | DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837-845. [PubMed] [Cited in This Article: ] |
| 20. | Fraquelli M, Rigamonti C, Casazza G, Conte D, Donato MF, Ronchi G, Colombo M. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007;56:968-973. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13220] [Cited by in F6Publishing: 13268] [Article Influence: 368.6] [Reference Citation Analysis (0)] |
| 21. | Poynard T, Halfon P, Castera L, Munteanu M, Imbert-Bismut F, Ratziu V, Benhamou Y, Bourlière M, de Ledinghen V. Standardization of ROC curve areas for diagnostic evaluation of liver fibrosis markers based on prevalences of fibrosis stages. Clin Chem. 2007;53:1615-1622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 201] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 22. | Poynard T, Imbert-Bismut F, Munteanu M, Messous D, Myers RP, Thabut D, Ratziu V, Mercadier A, Benhamou Y, Hainque B. Overview of the diagnostic value of biochemical markers of liver fibrosis (FibroTest, HCV FibroSure) and necrosis (ActiTest) in patients with chronic hepatitis C. Comp Hepatol. 2004;3:8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 269] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 23. | Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, Herrmann E. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960-974. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1046] [Cited by in F6Publishing: 1011] [Article Influence: 63.2] [Reference Citation Analysis (1)] |
| 24. | Hezode C, Castera L, Roudot-Thoraval F, Rosa I, Roulot D, Leroy V, Bouvier-Alias M, Mallat A, Pawlotsky JM. Liver stiffness dynamics in HCV-infected patients with and without an SVR to peginterferon alpha-ribavirin treatment. Final results of a prospective study. J Hepatol. 2010;52:S164, Abstract 401. [Cited in This Article: ] |
| 25. | Poynard T, Ngo Y, Munteanu M, Thabut D, Massard J, Moussalli J, Varaud A, Benhamou Y, Ratziu V. Biomarkers of liver injury for hepatitis clinical trials: a meta-analysis of longitudinal studies. Antivir Ther. 2010;15:617-631. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Foster GR, Fried MW, Hadziyannis SJ, Messinger D, Freivogel K, Weiland O. Prediction of sustained virological response in chronic hepatitis C patients treated with peginterferon alfa-2a (40KD) and ribavirin. Scand J Gastroenterol. 2007;42:247-255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, Heinzen EL, Qiu P, Bertelsen AH, Muir AJ. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399-401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2776] [Cited by in F6Publishing: 2666] [Article Influence: 177.7] [Reference Citation Analysis (0)] |
| 28. | Nøjgaard C, Johansen JS, Krarup HB, Holten-Andersen M, Møller A, Bendtsen F. Effect of antiviral therapy on markers of fibrogenesis in patients with chronic hepatitis C. Scand J Gastroenterol. 2003;38:659-665. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Abe S, Tabaru A, Ono M, Tai M, Narita R, Moriyama A, Otsuki M. High-dose interferon-alpha therapy lowers the levels of serum fibrogenesis markers over 5 years in chronic hepatitis C. Hepatol Res. 2003;25:22-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Oliveri F, Coco B, Ciccorossi P, Colombatto P, Romagnoli V, Cherubini B, Bonino F, Brunetto MR. Liver stiffness in the hepatitis B virus carrier: a non-invasive marker of liver disease influenced by the pattern of transaminases. World J Gastroenterol. 2008;14:6154-6162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 97] [Cited by in F6Publishing: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 31. | Rockey DC, Bissell DM. Noninvasive measures of liver fibrosis. Hepatology. 2006;43:S113-S120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 240] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 32. | Rousselet MC, Michalak S, Dupré F, Croué A, Bedossa P, Saint-André JP, Calès P. Sources of variability in histological scoring of chronic viral hepatitis. Hepatology. 2005;41:257-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 409] [Cited by in F6Publishing: 426] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 33. | Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449-1457. [PubMed] [Cited in This Article: ] |
| 34. | Poynard T, Halfon P, Castera L, Charlotte F, Le Bail B, Munteanu M, Messous D, Ratziu V, Benhamou Y, Bourlière M. Variability of the area under the receiver operating characteristic curves in the diagnostic evaluation of liver fibrosis markers: impact of biopsy length and fragmentation. Aliment Pharmacol Ther. 2007;25:733-739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 35. | Kettaneh A, Marcellin P, Douvin C, Poupon R, Ziol M, Beaugrand M, de Lédinghen V. Features associated with success rate and performance of FibroScan measurements for the diagnosis of cirrhosis in HCV patients: a prospective study of 935 patients. J Hepatol. 2007;46:628-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 208] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 36. | Sebastiani G, Halfon P, Castera L, Pol S, Thomas DL, Mangia A, Di Marco V, Pirisi M, Voiculescu M, Guido M. SAFE biopsy: a validated method for large-scale staging of liver fibrosis in chronic hepatitis C. Hepatology. 2009;49:1821-1827. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 135] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 37. | Castéra L, Sebastiani G, Le Bail B, de Lédinghen V, Couzigou P, Alberti A. Prospective comparison of two algorithms combining non-invasive methods for staging liver fibrosis in chronic hepatitis C. J Hepatol. 2010;52:191-198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 152] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 38. | Mehta SH, Lau B, Afdhal NH, Thomas DL. Exceeding the limits of liver histology markers. J Hepatol. 2009;50:36-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 39. | Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835-847. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 972] [Cited by in F6Publishing: 974] [Article Influence: 60.9] [Reference Citation Analysis (0)] |