Published online Jun 14, 2012. doi: 10.3748/wjg.v18.i22.2832
Revised: September 18, 2011
Accepted: October 21, 2011
Published online: June 14, 2012
AIM: To re-evaluate the recent clinicopathological features of remnant gastric cancer (RGC) and to develop desirable surveillance programs.
METHODS: Between 1997 and 2008, 1149 patients underwent gastrectomy for gastric cancer at the Department of Digestive Surgery, Kyoto Prefectural University of Medicine, Japan. Of these, 33 patients underwent gastrectomy with lymphadenectomy for RGC. Regarding the initial gastric disease, there were 19 patients with benign disease and 14 patients with gastric cancer. The hospital records of these patients were reviewed retrospectively.
RESULTS: Concerning the initial gastric disease, the RGC group following gastric cancer had a shorter interval [P < 0.05; gastric cancer vs benign disease: 12 (2-22) vs 30 (4-51) years] and were more frequently reconstructed by Billroth-I procedure than those following benign lesions (P < 0.001). Regarding reconstruction, RGC following Billroth-II reconstruction showed a longer interval between surgical procedures [P < 0.001; Billroth-II vs Billroth-I: 32 (5-51) vs 12 (2-36) years] and tumors were more frequently associated with benign disease (P < 0.001) than those following Billroth-I reconstruction. In tumor location of RGC, after Billroth-I reconstruction, RGC occurred more frequently near the suture line and remnant gastric wall. After Billroth-II reconstruction, RGC occurred more frequently at the anastomotic site. The duration of follow-up was significantly associated with the stage of RGC (P < 0.05). Patients diagnosed with early stage RGC such as stage I-II tended to have been followed up almost every second year.
CONCLUSION: Meticulous follow-up examination and early detection of RGC might lead to a better prognosis. Based on the initial gastric disease and the procedure of reconstruction, an appropriate follow-up interval and programs might enable early detection of RGC.
- Citation: Komatsu S, Ichikawa D, Okamoto K, Ikoma D, Tsujiura M, Nishimura Y, Murayama Y, Shiozaki A, Ikoma H, Kuriu Y, Nakanishi M, Fujiwara H, Ochiai T, Kokuba Y, Otsuji E. Progression of remnant gastric cancer is associated with duration of follow-up following distal gastrectomy. World J Gastroenterol 2012; 18(22): 2832-2836
- URL: https://www.wjgnet.com/1007-9327/full/v18/i22/2832.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i22.2832
The incidence of remnant gastric cancer (RGC) following distal gastrectomy has been reported to account for 1%-2% of all gastric cancers in Japan[1,2]. Previously, RGC was reported to be caused by multiple factors, and the incidence, pathological features, and potential mechanisms have been extensively investigated[3-5]. Specifically, RGC is commonly found at an advanced stage, resulting in low rates of curative resection (38%-40%) and a consequently poor prognosis[6,7]. However, recently, the incidence and etiology of RGC have been changing[8] because of the long latency periods, decreasing prevalence of gastrectomy for benign disease[6,9], early detection and improved outcomes in patients with gastric cancers[10,11]. Moreover, recent advances in diagnostic and treatment techniques have led to a higher detection rate of early RGC following distal gastrectomy[12]. Consequently, endoscopic therapy such as endoscopic mucosal resection or endoscopic submucosal dissection is applicable for treatment of early-stage RGC[13,14]. Indeed, more than half of the RGC patients were treated for T1 or T2, node-negative and early stage cancer at our institution and almost 80% of patients with RGC were curatively resected. Therefore, it is necessary to re-evaluate the risk factors of RGC to develop an optimal new surveillance program and treatment guide. However, there is limited information available to help guide the treatment of patients with RGC. This study was designed to re-evaluate the clinicopathological characteristics and surgical outcomes of RGC and to develop desirable surveillance programs.
Between 1997 and 2008, 1149 patients underwent gastrectomy for gastric cancer. Of these, 33 consecutive patients with primary RGC were treated in the Department of Digestive Surgery, Kyoto Prefectural University of Medicine. All patients underwent gastrectomy with lymphadenectomy for RGC. The clinicopathologic findings of these patients were determined retrospectively based on their hospital records. Macroscopic, microscopic and histopathological classifications of gastric cancers were based on the Japanese Classification of Gastric Carcinomas[15] and tumor-node-metastasis staging system[16]. Histologic types were classified as differentiated (papillary, moderately or well-differentiated adenocarcinoma) and undifferentiated (poorly or undifferentiated adenocarcinoma, signet-ring cell carcinoma, and mucinous adenocarcinoma).
The follow-up program after initial gastrectomy at our institution is comprised of a regular physical examination and laboratory blood tests, chest X rays, an upper gastrointestinal series or endoscopy and ultrasonography or computer tomography for the first 5 years, and yearly endoscopy thereafter, if possible.
The correlations between clinical factors and an initial factor such as previous disease or method of reconstruction in initial surgery were examined. Moreover, the follow-up interval is very important for screening recurrence and second primary gastric cancers. Therefore, correlation between follow-up periods and progression was evaluated in RGC.
The patient was included as a cause-specific death when the cause of death was specified as recurrent RGC. χ2 test and Fisher’s exact probability test were performed for categorical variables, while Student’s t-test and Mann-Whitney U-test for unpaired data with continuous variables were performed to compare the clinicopathological characteristics between two groups. Kruskal-Wallis H test was used as a nonparametric procedure that can be used to compare more than two groups for analyses of follow-up interval. A P value less than 0.05 was considered significant.
The mean patient age was 68 years, and the male:female ratio was 2.7:1. Regarding the initial gastric disease, there were 19 patients with benign disease and 14 patients with gastric cancer. The median interval between the 1st and 2nd surgery was 20 years. Reconstruction during the 1st surgery was mainly Billroth-I or Billroth-II. En bloc resection of the tumor by total remnant gastrectomy was performed with jejunal mesentery and D2 lymphadenectomy and concomitant organ resection. Eighteen patients additionally received splenectomy, four patients received distal pancreatectomy, two patients received partial colon resection and two patients received liver resection. Reconstructions were performed in 16 patients by Billroth-I, 16 patients by Billroth-II and one by Roux-en-Y procedure for all resected RGC tumors. Tumors were located at the anastomotic site in 16 (61%) patients, corpus and/or cardia in nine (34%), and throughout the whole remnant in one (4%) patient. Consequently, more than half of the RGC patients demonstrated T1 or T2, undifferentiated, node-negative and early stage cancer. In 78.8% (26/33) of patients, resections were performed with curative intent.
Clinicopathologic findings of 33 patients with primary RGC are listed in Table 1 according to the nature of the primary disease. Patients with RGC following gastric cancer showed a significantly shorter interval between the 1st and 2nd surgery [P < 0.05, gastric cancer vs benign disease: 12 (2-22) vs 30 (4-51) years] and were more frequently reconstructed by the Billroth-I method than those following benign disease (P < 0.005). Other factors did not significantly differ between the two groups.
| Initial disease | ||||
| Variables | n | Benign (n= 19) | Cancer (n= 14) | Pvalue |
| Age (yr) (mean) | 70 (51) | 66 (49) | 0.26 | |
| Gender | ||||
| Male | 24 | 15 (63) | 9 (38) | |
| Female | 9 | 4 (44) | 5 (56) | 0.35 |
| Interval from initial surgery | ||||
| Year (median) | 30 (4-51) | 12 (2-22) | < 0.05 | |
| Reconstruction of 1st surgery | ||||
| Billroth-I | 16 | 4 (25) | 12 (75) | |
| Billroth-II | 16 | 15 (94) | 1 (6) | |
| R-Y | 1 | 0 (0) | 1 (100) | < 0.001 |
| Location of RGC | ||||
| Anastomotic site | 11 | 9 (82) | 2 (18) | |
| Suture line | 7 | 2 (29) | 5 (71) | |
| Others | 15 | 8 (53) | 7 (47) | 0.08 |
| Histological type | ||||
| Differentiated | 13 | 8 (62) | 5 (38) | |
| Undifferentiated | 20 | 11 (55) | 9 (45) | 0.71 |
| Lymphatic invasion | ||||
| Negative | 16 | 8 (50) | 8 (50) | |
| Positive | 17 | 11 (65) | 6 (35) | 0.39 |
| Venous invasion | ||||
| Negative | 16 | 8 (50) | 8 (50) | |
| Positive | 17 | 11 (65) | 6 (35) | 0.39 |
| Tumor size | ||||
| cm (mean) | 51 (46) | 61 (54) | 0.40 | |
| Depth of tumor | ||||
| T1 | 10 | 4 (40) | 6 (60) | |
| T2, 3, 4 | 23 | 15 (65) | 8 (35) | 0.18 |
| Lymph node metastasis | ||||
| Negative | 20 | 10 (50) | 10 (50) | |
| Positive | 13 | 9 (69) | 4 (31) | 0.27 |
| Stage | ||||
| I | 17 | 8 (47) | 9 (53) | |
| II, III, IV | 16 | 11 (69) | 5 (31) | 0.21 |
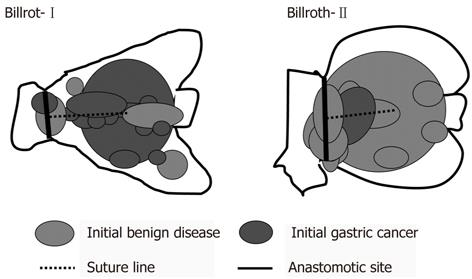
Table 2 shows details of 33 RGC patients according to the method of reconstruction. RGC following Billroth-II reconstruction showed a longer interval between surgical procedures (P < 0.001) and tumors were more frequently associated with benign disease (P < 0.001) than those following Billroth-I reconstruction. Figure 1 shows the tumor location of 32 RGC following distal gastrectomy according to the method of reconstruction. After Billroth-I reconstruction, RGC occurred more frequently near the suture line and remnant gastric wall. After Billroth-II reconstruction, RGC occurred more frequently at the anastomotic site.
| Reconstruction at first surgery | |||||
| Variables | n | Billroth-I(n= 16) | Billroth-II(n= 16) | Pvalue | |
| Age (yr) (mean) | 68 (50) | 69 (50) | 0.64 | ||
| Gender | |||||
| Male | 24 | 13 (54) | 11 (46) | ||
| Female | 8 | 3 (38) | 5 (63) | 0.69 | |
| Interval from initial surgery | |||||
| Year (median) | 12 (2-36) | 32 (5-51) | < 0.001 | ||
| Initial gastric disease | |||||
| Benign | 19 | 4 (21) | 15 (79) | ||
| Cancer | 13 | 12 (92) | 1 (8) | < 0.001 | |
| Location of RGC | |||||
| Anastomotic site | 11 | 2 (18) | 9 (82) | ||
| Suture line | 7 | 5 (71) | 2 (29) | ||
| Others | 14 | 9 (64) | 5 (36) | 0.11 | |
| Histological type | |||||
| Differentiated | 13 | 8 (62) | 5 (38) | ||
| Undifferentiated | 19 | 8 (42) | 11 (58) | 0.47 | |
| Lymphatic invasion | |||||
| Negative | 15 | 6 (40) | 9 (60) | ||
| Positive | 17 | 10 (59) | 7 (41) | 0.48 | |
| Venous invasion | |||||
| Negative | 16 | 8 (50) | 8 (50) | ||
| Positive | 16 | 8 (50) | 8 (50) | 0.72 | |
| Tumor size | |||||
| mm (mean) | 51 | 56 | 0.67 | ||
| Depth of tumor | |||||
| T1 | 10 | 6 (60) | 4 (40) | ||
| T2, 3, 4 | 22 | 10 (45) | 12 (55) | 0.76 | |
| Lymph node metastasis | |||||
| Negative | 14 | 9 (47) | 10 (53) | ||
| Positive | 13 | 7 (54) | 6 (46) | 1 | |
| Stage | |||||
| I | 17 | 9 (53) | 8 (47) | ||
| II, III, IV | 15 | 7 (47) | 8 (53) | 1 | |
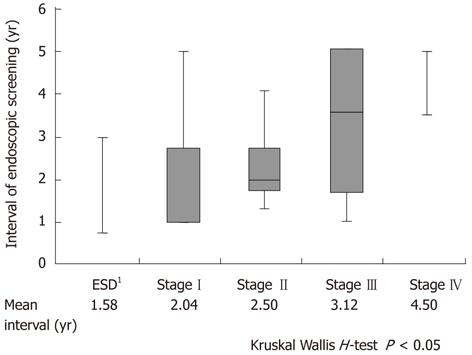
The duration of follow-up was significantly associated with the stage of progression in RGC (P < 0.05). Patients diagnosed with early stage RGC such as stage I-II tended to have been followed up almost every second year (Figure 2).
Gastric cancer is the second leading cause of cancer-related death in the world[17]. However, recent advances in diagnostic methods, less invasive treatment techniques and better peri-operative management have increased the early detection of gastric cancer and decreased the mortality and morbidity rates[18-20]. Consequently, the number of cured patients has been increasing and some of these patients are at risk of acquiring second primary cancer in the remnant stomach. This implies that more cases of RGC will be encountered in the future.
In previous reports, RGC was commonly found at an advanced stage, resulting in low rates of curative resection (38%-40%) and a consequent poor prognosis[6,7]. However, recently, the incidence and etiology of RGC following distal gastrectomy may be changing due to diagnostic and technological advances. In our study, more than half of the RGC patients were treated for T1 or T2, node-negative and early stage cancer, contrary to that in previous series (Table 1). Almost 80% of patients were curatively resected with intensive lymphadenectomy. Thereby, the survival curves of primary proximal gastric cancer (PGC) and RGC were similar and without a significant difference, although patients with RGC tended to have a higher incidence of undifferentiated cancer, vascular invasion, and T4 component than patients with PGC (data not shown). Therefore, RGC is not always advanced at diagnosis and if so, intensive surgery for RGC does not necessarily mean a poor prognosis in comparison to that for primary gastric cancer. Therefore, it is necessary to re-evaluate the risk factors of RGC to develop an optimal new endoscopic surveillance program and treatment guide.
Regarding surveillance systems for early detection and curative treatment of RGC, periodic endoscopic examinations of the gastric remnant are shown to be extremely important in our study (Figure 1). However, a follow-up program that is too intensive may not be beneficial to the patient. The initial gastric disease and the interval between the 1st and 2nd surgery could affect the incidence of RGC. In our study, RGC following gastric cancer had a significantly shorter interval between 1st and 2nd surgery than that following benign disease [P < 0.05; gastric cancer vs benign disease: 12 (2-22) years vs 30 (4-51) years]. However, surveillance systems for gastric cancer should be especially considered because of decreasing gastrectomy for benign disease. Furthermore, 86% of all initial gastric cancer patients underwent Billroth-I reconstruction at our institution and their median interval between 1st and 2nd surgery was 12 (2-36) years. Moreover, the duration of follow-up was significantly associated with the stage of RGC progression and an early detection of RGC led to better prognosis (Figure 2). Taken together, annual surveillance endoscopic screening should be required for at least 12 years following distal gastrectomy. Furthermore, after 12 years of follow-up, surveillance endoscopy should be recommended every second year because we found that patients diagnosed with early stage RGC such as stage I-II tended to have been followed almost every second year. In particular, meticulous endoscopy examination should be performed near the suture line and remnant gastric wall after Billroth-I reconstruction and also should be performed at the anastomotic site after Billroth-II reconstruction.
In conclusion, due to recent advances in diagnostic and treatment technologies, the etiology of RGC has been changing. Meticulous follow-up examination and early detection of RGC might lead to a better prognosis. Considering both the initial gastric disease and the procedure of reconstruction, an appropriate follow-up interval and programs should facilitate the detection of early RGC.
Recently, the incidence and etiology of remnant gastric cancer (RGC) have been changing because of the long latency periods, decreasing prevalence of gastrectomy for benign disease, early detection and improved outcomes in patients with gastric cancers. Moreover, recent advances in diagnostic and treatment technique have led to a higher detection rate of early RGC following distal gastrectomy.
It is necessary to re-evaluate the risk factors of RGC and develop an optimal new surveillance program and treatment guide. However, there is limited information available to help guide the treatment of patients with RGC. In this study, the authors re-evaluated the clinicopathological characteristics and surgical outcomes of RGC and developed desirable surveillance programs.
In this study, more than half of the RGC patients were demonstrated to have T1 or T2, undifferentiated, node-negative and early stage cancer. The duration of follow-up was significantly associated with the stage of progression in RGC. Patients diagnosed with early stage RGC such as stage I-II tended to have been followed almost every second year. After Billroth-I reconstruction, RGC occurred more frequently near the suture line and remnant gastric wall. After Billroth-II reconstruction, RGC occurred more frequently at the anastomotic site.
RGC following gastric cancer had a significantly shorter interval between 1st and 2nd surgery than those following benign disease. Annual surveillance endoscopic screening should be required for at least 12 years following distal gastrectomy. Furthermore, after 12 years of follow-up, surveillance endoscopy should be recommended every second year.
The incidence of RGC following distal gastrectomy has been reported to account for 1%-2% of all gastric cancers in Japan. In previous reports, RGC was commonly found at an advanced stage, resulting in low rates of curative resection (38%-40%) and a consequent poor prognosis.
Authors have given new thoughts while designing this study. The paper is nicely written.
Peer reviewers: Dr. Ashok Kumar, MD, Department of Surgical Gastroenterology, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow 226014, India; Liang-Shun Wang, MD, Professor, Vice-superintendent, Shuang-Ho Hospital, Taipei Medical University, No. 291, Jhongjheng Rd., Jhonghe City, New Taipei City 237, Taiwan, China; Takaaki Arigami, MD, PhD, Department of Surgical Oncology and Digestive Surgery, Field of Oncology, Kagoshima University Graduate School of Medical and Dental Sciences, 8-35-1 Sakuragaoka, Kagoshima 891-0175, Japan
S- Editor Cheng JX L- Editor Logan S E- Editor Zhang DN
| 1. | Ohashi M, Katai H, Fukagawa T, Gotoda T, Sano T, Sasako M. Cancer of the gastric stump following distal gastrectomy for cancer. Br J Surg. 2007;94:92-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 2. | Kaneko K, Kondo H, Saito D, Shirao K, Yamaguchi H, Yokota T, Yamao G, Sano T, Sasako M, Yoshida S. Early gastric stump cancer following distal gastrectomy. Gut. 1998;43:342-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 67] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Kaminishi M, Shimizu N, Shiomoyama S, Yamaguchi H, Ogawa T, Sakai S, Kuramoto S, Oohara T. Etiology of gastric remnant cancer with special reference to the effects of denervation of the gastric mucosa. Cancer. 1995;75:1490-1496. [PubMed] [Cited in This Article: ] |
| 4. | Tersmette AC, Offerhaus GJ, Tersmette KW, Giardiello FM, Moore GW, Tytgat GN, Vandenbroucke JP. Meta-analysis of the risk of gastric stump cancer: detection of high risk patient subsets for stomach cancer after remote partial gastrectomy for benign conditions. Cancer Res. 1990;50:6486-6489. [PubMed] [Cited in This Article: ] |
| 5. | Ahn HS, Kim JW, Yoo MW, Park do J, Lee HJ, Lee KU, Yang HK. Clinicopathological features and surgical outcomes of patients with remnant gastric cancer after a distal gastrectomy. Ann Surg Oncol. 2008;15:1632-1639. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Sasako M, Maruyama K, Kinoshita T, Okabayashi K. Surgical treatment of carcinoma of the gastric stump. Br J Surg. 1991;78:822-824. [PubMed] [Cited in This Article: ] |
| 7. | Newman E, Brennan MF, Hochwald SN, Harrison LE, Karpeh MS. Gastric remnant carcinoma: just another proximal gastric cancer or a unique entity? Am J Surg. 1997;173:292-297. [PubMed] [Cited in This Article: ] |
| 8. | Tanigawa N, Nomura E, Lee SW, Kaminishi M, Sugiyama M, Aikou T, Kitajima M. Current state of gastric stump carcinoma in Japan: based on the results of a nationwide survey. World J Surg. 2010;34:1540-1547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Kodera Y, Yamamura Y, Torii A, Uesaka K, Hirai T, Yasui K, Morimoto T, Kato T, Kito T. Gastric stump carcinoma after partial gastrectomy for benign gastric lesion: what is feasible as standard surgical treatment? J Surg Oncol. 1996;63:119-124. [PubMed] [Cited in This Article: ] |
| 10. | Maruyama K, Kaminishi M, Hayashi K, Isobe Y, Honda I, Katai H, Arai K, Kodera Y, Nashimoto A. Gastric cancer treated in 1991 in Japan: data analysis of nationwide registry. Gastric Cancer. 2006;9:51-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 189] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 11. | Kitano S, Shiraishi N, Uyama I, Sugihara K, Tanigawa N. A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg. 2007;245:68-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 519] [Cited by in F6Publishing: 509] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 12. | Nakayoshi T, Tajiri H, Matsuda K, Kaise M, Ikegami M, Sasaki H. Magnifying endoscopy combined with narrow band imaging system for early gastric cancer: correlation of vascular pattern with histopathology (including video). Endoscopy. 2004;36:1080-1084. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 328] [Cited by in F6Publishing: 321] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 13. | Takenaka R, Kawahara Y, Okada H, Tsuzuki T, Yagi S, Kato J, Ohara N, Yoshino T, Imagawa A, Fujiki S. Endoscopic submucosal dissection for cancers of the remnant stomach after distal gastrectomy. Gastrointest Endosc. 2008;67:359-363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Hirasaki S, Kanzaki H, Matsubara M, Fujita K, Matsumura S, Suzuki S. Treatment of gastric remnant cancer post distal gastrectomy by endoscopic submucosal dissection using an insulation-tipped diathermic knife. World J Gastroenterol. 2008;14:2550-2555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 25] [Cited by in F6Publishing: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma. 13th ed. Tokyo: Kanehara & Co 1999; . [Cited in This Article: ] |
| 16. | Sobin LH, Wittekind CH. TNM Classification of Malignant Tumors. 6th ed. John Wiley: New York 2002; 170-173. [Cited in This Article: ] |
| 17. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [PubMed] [Cited in This Article: ] |
| 18. | Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10:1-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 479] [Cited by in F6Publishing: 476] [Article Influence: 28.0] [Reference Citation Analysis (1)] |
| 19. | Degiuli M, Sasako M, Ponti A. Morbidity and mortality in the Italian Gastric Cancer Study Group randomized clinical trial of D1 versus D2 resection for gastric cancer. Br J Surg. 2010;97:643-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 217] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 20. | Katai H, Sasako M, Fukuda H, Nakamura K, Hiki N, Saka M, Yamaue H, Yoshikawa T, Kojima K. Safety and feasibility of laparoscopy-assisted distal gastrectomy with suprapancreatic nodal dissection for clinical stage I gastric cancer: a multicenter phase II trial (JCOG 0703). Gastric Cancer. 2010;13:238-244. [PubMed] [Cited in This Article: ] |