Published online Sep 7, 2012. doi: 10.3748/wjg.v18.i33.4491
Revised: March 27, 2012
Accepted: March 29, 2012
Published online: September 7, 2012
The renewed interest in donation after cardio-circulatory death (DCD) started in the 1990s following the limited success of the transplant community to expand the donation after brain-death (DBD) organ supply and following the request of potential DCD families. Since then, DCD organ procurement and transplantation activities have rapidly expanded, particularly for non-vital organs, like kidneys. In liver transplantation (LT), DCD donors are a valuable organ source that helps to decrease the mortality rate on the waiting lists and to increase the availability of organs for transplantation despite a higher risk of early graft dysfunction, more frequent vascular and ischemia-type biliary lesions, higher rates of re-listing and re-transplantation and lower graft survival, which are obviously due to the inevitable warm ischemia occurring during the declaration of death and organ retrieval process. Experimental strategies intervening in both donors and recipients at different phases of the transplantation process have focused on the attenuation of ischemia-reperfusion injury and already gained encouraging results, and some of them have found their way from pre-clinical success into clinical reality. The future of DCD-LT is promising. Concerted efforts should concentrate on the identification of suitable donors (probably Maastricht category III DCD donors), better donor and recipient matching (high risk donors to low risk recipients), use of advanced organ preservation techniques (oxygenated hypothermic machine perfusion, normothermic machine perfusion, venous systemic oxygen persufflation), and pharmacological modulation (probably a multi-factorial biologic modulation strategy) so that DCD liver allografts could be safely utilized and attain equivalent results as DBD-LT.
- Citation: Le Dinh H, de Roover A, Kaba A, Lauwick S, Joris J, Delwaide J, Honoré P, Meurisse M, Detry O. Donation after cardio-circulatory death liver transplantation. World J Gastroenterol 2012; 18(33): 4491-4506
- URL: https://www.wjgnet.com/1007-9327/full/v18/i33/4491.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i33.4491
The first human liver transplantations (LT) were performed from donation after cardio-circulatory death (DCD) in the 1960s[1-4]. DCD-LT was nonetheless almost universally abandoned in the following two decades, given the well-recognized Harvard brain-dead concept in 1968 and given the better results of LT originating from donation after brain death (DBD)[5]. In 1983, LT was approved as a therapeutic modality for end-stage liver diseases after a long period considered as an experimental procedure. The renewed interest in DCD donors started in the 1990s following the limited success of the transplant community to expand the DBD organ supply and following the request of potential DCD families.
If DCD kidneys are increasingly accepted around the world[6], the use of DCD livers remains limited in experienced transplant centers due to higher risks of primary graft dysfunction and biliary complications as well as a lack of a reliable viability testing prior to liver implantation. However the number of DCD-LT increased rapidly over the past decade. In the United States, 276 DCD liver transplants were performed in 2008 compared to only 23 cases in 1999, making up 5% of the deceased donor (DD) liver transplants[7-10]. The same trend was observed in the United Kingdom[11-13], Spain[14], Netherlands[15] and Belgium[15,16]. Netherlands had the highest rate of DCD- over DD-LT in the world (22.5% in 2008)[15]. France has just initiated its DCD-LT program since 2010[17]. In Japan, although DCD donors were the essential DD source, its use was reserved mainly for kidney, pancreas and islet transplantation[18]. Using a mathematical model to analyze the potential impact of a DCD policy on LT programs, Chaib et al[19] reported if 1%, 5% and 10% of deceased individuals became DCD donors, there would be 8%, 27% and 37% relative reductions in the size of waiting list, respectively. The use of DCD livers could increase the supply of transplants by 53%[20]. Centers with active DCD-LT programs usually reported 4%-10% rates of LT from the DCD source[21]. The potential impact of DCD use on the DBD availability is also a controversial issue. Controlled DCD programs might negatively influenced DBD activity in Belgium, Netherlands and United Kingdom while uncontrolled DCD donors seemed to be a clear additional source of organs for transplantation in France and Spain[22].
Most countries use Maastricht-category-3 DCD donors for LT, except France and Spain, where categories 1 and 2 are exclusively used due to legal interdiction of discontinuation of therapy in irreversibly brain-injured individuals[17,23,24]. German law prohibits any DCD organ procurement and transplant activity. In Italy, death of a human being must be declared 20 min after cardiac arrest using continuous electrocardiography. The procedure therefore will enable, at best, retrieval of only a few marginal kidneys and some tissues, and will not be helpful for patients on LT waiting lists[25]. This article is aimed at reviewing mono- and multi-centric DCD-LT outcomes, experimental strategies on animal models to optimize the utilization of this donor source and its future development.
Generally results of DCD-LT are inferior to those from DBD-LT with regard to both short-and long-term graft and patient survival as well as post-transplant morbidity. Expected DCD-LT outcomes could be explained by inherent differences between DCD and DBD donors in circumstances of death, warm ischemia time (WIT) and donor cause of death. Consequently, a different strategy of DCD use in terms of logistics of organ retrieval and preservation, allocation and recipient selection appears necessary to guarantee acceptable results. These differences will be briefly discussed prior to considering results of DCD-LT in detail.
In DCD, donor death is diagnosed on the basis of irreversible cessation of cardio-pulmonary function instead of conventional neurologic criteria. As a result, organs from DCD donors are subjected to a period of hypotension, hypoxia and a circulation prior to organ procurement and this WIT adversely affects tissue viability and graft function after transplantation[26]. An international classification of DCD donors into 4 categories was first proposed in 1995 and widely accepted up to now[27]. New DCD categories have been recently suggested in Spain[28,29], Italy[30] and Belgium[31]. The length of WIT varies greatly according to the type of DCD process. It is longest among uncontrolled categories 1 and 2 (usually 90-120 min) and shorter among controlled categories 3 and 4 DCD donors (usually 20-30 min). In brain death, issues related to donor warm ischemia are eliminated because DBD donors have an effective natural organ perfusion and a potentially well-preserved organ function and WIT is thus nearly equal to zero.
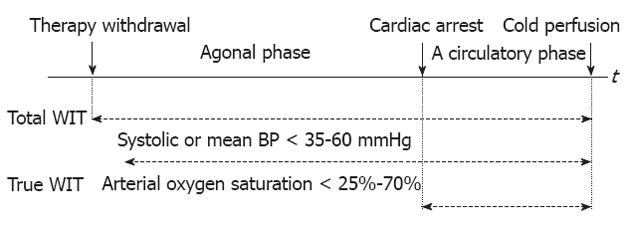
However, WIT is heterogeneously defined among authors[32]. In the controlled DCD context, the commonest definition is the time interval between withdrawal of both ventilator and cardiac support to start of cold flushing of the organ[33,34]. This definition includes the no-touch period and the time of death declaration and is proposed to have two phases (withdrawal and acirculatory phases). Other authors used a blood pressure (BP) or oxygen saturation threshold below which would be defined as the beginning of true WIT (systolic or mean BP < 35-60 mmHg, oxygen saturation < 25%-70% or unreadable)[35-42]. de Vera et al[34] did not use a BP threshold to define the start of WIT because tissues are still hypoxic in a DCD donor who maintains a BP but has ceased to ventilate. It is unknown at what BP or oxygen saturation the liver parenchyma and biliary system undergo irrecoverable injury[43]. The first international Non-Heart Beating Donor workshop in Maastricht in 1995 suggested WIT should be calculated from the moment of cardiac arrest until the start of hypothermic flush-out[44]. This definition may be useful for consistency but is inaccurate at the cellular level. Hypoxia starts when the blood flow or oxygenation no longer meets cellular metabolic needs[37]. The start of WIT may be chosen prior to asystole, and the end of WIT may be at or after aortic flushing[45]. Apparently a well-accepted definition of donor organ ischemic times is needed to standardize nomenclature and allow accurate comparisons of individual DCD studies[46,47] (Figure 1).
In transplant practice, WIT should be minimized as much as possible. For controlled DCD donors, the possibility to predict whether a potential donor will or will not expire in a time frame consistent with donation is extremely important, because prolonged time to asystole, likely resulting in suboptimal organ perfusion, is a common reason for non procurement of DCD grafts[48,49]. Time between therapy withdrawal and cardiac arrest usually does not exceed 1 h in most DCD donors. However, if a DCD donor has a period of relatively hemodynamic stability after life-support withdrawal, this period may be extended beyond 1 h without additional warm injury to the organs[50]. Some authors emphasized during the withdrawal phase, time to a systolic BP < 50 mmHg should be < 30 min[20] and the hypotensive period (mean BP < 50 mmHg) < 15 min[51]. Manara et al[52] proposed the so-called functional WIT, which is measured from the donor’s systolic BP < 50 mmHg, the arterial oxygen saturation < 70%, or both, to the start of cold perfusion, should not exceed 30 min and may be limited to 20 min in suboptimal donors. Several factors have been identified as predictors of rapid death following treatment withdrawal and include the DCD tool of University of Wisconsin[53], donor Glasgow coma scale, inotropic use, BP at treatment discontinuation, high FiO2 and mode of ventilation[54,55]. Withdrawal of therapy is preferably occurred in the operating room with a donor surgical team immediately available. Prior to cessation of the ventilator and organ perfusion support, the donor may be already prepared and draped, and the surgical instruments, preservation solution and tubing are set up to facilitate rapid organ recovery. The super rapid recovery technique is preferable and organs may be removed en bloc[39,50]. For uncontrolled donors, in vivo organ preservation techniques, like in-situ intravascular cooling using a double balloon and triple lumen catheter or hypo- and normo-thermic cardiopulmonary bypass with extracorporeal membrane oxygenation (ECMO), should be employed. With regard to the logistic organization, two frequently mentioned initiatives are the “Maastricht’s box” and the “Madrid’s rapid identification and response system”[6].
DCD donors do not experience the brain dead process. Brain death provokes a cascade of changes in hemodynamics, hormones, and immune response, which negatively affect donor organ viability and transplant outcomes[56,57]. Hemodynamic instability may have deleterious effects on liver function, although the liver has a high tolerance to marked hypotension and a large physiological reserve. Only a few histological changes were observed in the liver both on light and electron microscopic examination during the brain dead process[58,59]. The most important changes are the increased liver immunogenicity with subsequent increased host allo-responsiveness and the occurrence of apoptosis of hepatocytes[60]. Clinical findings in livers from DBD donors revealed significantly higher leukocyte infiltrates, up-regulation of adhesion molecules [intercellular adhesion molecule (ICAM), vascular cell adhesion molecule] and pro-inflammatory cytokines [interleukin-6 (IL-6), IL-10, IL-1β, interferon γ and tumor necrosis factor-alpha (TNF-α)], along with an increased expression of major histo-compatibility complex-II relative to livers from living donors[61,62]. The peak time of cytokine expression and cell infiltration is during brain death and organ procurement but not after reperfusion[61]. These changes may amplify ischemia-reperfusion injury (IRI) during the transplant procedure and accelerate graft rejection after transplant[63]. In reality, donor brain-death mechanisms are quite varied and large differences may exist in the degree of impaired organ quality and transplant outcomes. The impact of donor cause of death on transplant outcomes has been recently confirmed in a United Network for Organ Sharing (UNOS) registry analysis, in which the cerebro-vascular accident presented as a predictor of worse graft survival across all organs relative to other donor modes of death[64].
Uncontrolled DCD donors whose cause of death is usually other than neurologic do not undergo the process of brain death, while most controlled DCD donors have sustained irreversible cerebral injuries. As a result, organs from controlled DCD donors are likely to suffer more from the harmful immunologic and inflammatory effects of acute brain injury than those from uncontrolled DCD donors[65].
It is reported that organs that have already subjected to warm ischemic injury have an increased susceptibility to damage during cold storage[66]. The incidence of primary non-function (PNF) was 2.5 times less in patients with cold ischemia time (CIT) ≤ 8 h vs those with CIT > 8 h (5% vs 13%)[34]. The incidence of graft failure within 60 d of transplantation was 10.8% if CIT < 8 h and substantially increased to 30.4% and 58.3% if CIT > 8 h and > 12 h, respectively[67]. Proper and rapid allocation of DCD livers thus appears pivotal to minimize CIT. One-year graft survival of DCD livers shared regionally was less good than those shared locally (67% vs 77%)[68] and the relative risk of graft failure from nationally shared DCD livers was 31% higher than locally or regionally shared ones[69]. Thus a policy to favor local use of DCD livers seems reasonable[67,68]. However, parallel (backup) offers should also be made to expedite organ placement[33]. The exchange of DCD livers between transplant centers has been successfully done but requires a more efficient and rapid referral system due to a lower tolerance of these allografts to cold storage[70].
Regarding recipient selection criteria, DCD livers could be routinely discussed and offered to all recipients on the waiting list[20,70,71] or selectively reserved to uncomplicated cases to ensure short CIT (by avoiding cases with extensive history of abdominal surgery or portal-vein thrombosis)[20,35]. An expected long surgical procedure exceeding 8 h of CIT, logistical reasons for an extended CIT, combined organ transplantation, recipients with high Model for End-Stage Liver Disease (MELD) scores or a large age difference between donors and recipients could all result in the refusal of a DCD liver[51]. Patients with stable cholestatic liver disease or re-transplantation were also excluded from DCD programs because of problems related to the quality of life in primary biliary cirrhosis and to the fear that pre-existent warm ischemic biliary damage could trigger the recurrence of primary sclerosing cholangitis[72]. Using DCD livers in re-transplanted patients might increase the CIT associated with a difficult hepatectomy. Recently LaMattina has demonstrated the feasibility of simultaneous liver and kidney (SLK) transplantation using DCD donors and shown short-term results comparable to those of SLK transplantation using DBD donors, making it a valid approach to safely expanding the donor organ pool for patients with end-stage liver and kidney disease[73].
It is still controversial whether it is better to transplant such grafts into healthy or sicker recipients (i.e., according to the recipient liver disease severity). UNOS database reviews advocated utilizing DCD livers in “low-risk” recipients[32,67,74]. de Vera et al[34] also observed better graft survival when DCD livers were utilized in patients with MELD scores ≤ 30, but simultaneously could demonstrate that “sicker”, high-risk recipients (at MELD scores > 30 or on organ-perfusion support, like mechanic ventilation or hemodialysis) had a greater patient and graft survival benefit from the transplantation of DCD livers compared to patients who are not as critically ill. Risk classification for DCD donors and DCD-LT recipients is summarized in Table 1. Other groups of patients that may have a true survival benefit from DCD-LT include MELD “disadvantaged” patients (hepato-cellular carcinoma patients beyond the Milan criteria or who are listed in areas with long waiting times, patients with low MELD scores that do not adequately reflect their level of illness and their critical need for a transplant)[34,72].
| Authors | Donors | Recipients | |
| Mateo et al[74] | Low risk | Both WIT ≤ 30 min and CIT ≤ 10 h | RCRR ≤ 1.5 |
| High risk | WIT > 30 min and/or CIT > 10 h | RCRR > 1.5 | |
| Re-transplantation and/or | |||
| On life-support and/or | |||
| A combination of ≥ 3 risk factors: | |||
| Hospitalization or in an intensive care unit | |||
| Serum creatinine > 2 mg/dL | |||
| On dialysis | |||
| Age > 60 yr | |||
| Lee et al[32] | Low risk | Donors with no identified donor risk factors | Recipients with no identified recipient risk factors |
| High risk | Donors with at least one identified donor risk factor: | Recipients with at least one identified recipient risk factor: | |
| Donor age > 45 yr | Previous transplantation | ||
| WIT > 15 min | Life support at transplantation | ||
| CIT > 10 h | |||
| de Vera et al[34] | Low risk | - | MELD scores ≤ 30 |
| High risk | - | MELD scores > 30 | |
| On life support (mechanical ventilation, hemodialysis) | |||
Studies about the effect of DCD liver grafts on hepatitis-C virus positive (HCV+) recipients transplant outcomes were inconsistent. Nguyen et al[45] and recently Hernandez-Alejandro et al[75] found a negative effect of HCV on DCD livers, but a formal contraindication for the use of DCD liver allografts in HCV+ recipients was not justified except for older donors. In fact, while single-center series reported no significant difference in graft and patient survival rates of HCV+ recipients and graft loss from HCV recurrence between DCD and DBD groups[20,34,76,77], as well as no deleterious effects of DCD liver grafts on the disease progression (fibrosis) in comparison with DBD liver grafts in HCV+ recipients[77], the most recent UNOS registry data showed inferior graft survival but similar patient survival of HCV+ recipients with DCD donors compared to ones with DBD donors. Furthermore, DCD livers on HCV disease do not fare worse than DCD livers on non-HCV disease. DCD livers thus appeared to be important source of LT for HCV patients[78]. Split livers from DCD donors have also been reported in recent years with acceptable results[79,80].
Currently one-year patient survival after DBD-LT and to a certain extent after controlled DCD-LT is about 85%-90% in comparison to 60% in the early eighties and around 30% in the early days of LT and at 5 years post-transplant patient survival rate remains over 70%. Medical progress over the past 40 years in the field of organ preservation, surgical techniques, immunosuppressive drugs, treatment of post-transplant complications and organ allocation has permitted DCD to become reality in the modern era. Although there are concerns about the quality of such organs, with evidence that a prolonged WIT causes a raised incidence of PNF and biliary complications as well as suboptimal graft and patient survival when compared to DBD livers, DCD livers may be life-saving for those who would die waiting for a DBD liver[68] and do increase the number of organs available for LT. With careful donor/recipient selection and matching, minimization of ischemia and good post-operative care, acceptable results can be achieved. Essential results of most important publications in the last decade in DCD-LT are presented in Tables 2, 3 and 4.
| Authors study period | Transplant center | Publication year | Patient number and Maastricht category | WIT (min) | CIT (min) | Mean follow-up | PNF % | Major biliary complications % | ITBL % | HAT % | HAS % | Rejection % | Retransplan-tation % | Graft survival % | Patient survival % | ||||||
| 1 yr | 3 yr | 5 yr | 10 yr | 1 yr | 3 yr | 5 yr | 10 yr | ||||||||||||||
| Casavilla et al[85] 1989-1993 | Pittsburgh, United States | 1995 | 6 DCD4 | 37 | 10.6 h | - | 501 | - | - | 16.6 | - | - | 83.3 | 17 | - | - | - | 67 | - | - | - |
| 6 DCDc | 23.8 | 11 h | - | 01 | - | - | 33.3 | - | - | 33.3 | 50 | - | - | - | 50 | - | - | - | |||
| Otero et al[87] 1995-2000 | Madrid, Spain | 2003 | 20 DCD2 | 10 | 647 | > 2 yr | 251 | 301 | - | 0 | - | 27 | 25 | 80 | - | - | - | 80 | - | - | - |
| 40 DBD | 8 | 405 | - | 31 | 81 | - | - | - | 34 | 5 | 55 | - | - | - | 83 | - | - | - | |||
| Quintela et al[86] 1995-2004 | Spain | 2005 | 9 DCD2 + 1 DCD4 | 80 | 561 | - | 10 | - | - | - | - | - | 10 | 100 | - | - | - | 100 | - | - | - |
| Suárez et al[89] 1994-2005 | Spain | 2008 | 27 DCD2 | 13 | 635 | > 3 mo | 181 | 41.71 | 25.01 | 3.6 | - | 17.4 | - | - | - | 491 | - | - | - | 62 | - |
| 471 DBD | 7 | - | - | 31 | 16.81 | 2.31 | 3.1 | - | 28.6 | - | - | - | 681 | - | - | - | 74 | - | |||
| Fondevila et al[29] 2002-2006 | Barcelona, Spain | 2007 | 10 DCD1 | - | 399 | - | 10 | 10 | - | 10 | - | - | 20 | 50 | - | - | - | 70 | - | - | - |
| 20 DBD | - | - | - | 0 | 0 | - | 5 | - | - | 5 | 75 | - | - | - | 80 | - | - | - | |||
| Jiménez-Galanes et al[71] 2006-2008 | Madrid, Spain | 2009 | 20 DCD2 | 12 | 432 | 360 d | 10 | 5 | 50 | 0 | - | - | 11 | 80 | - | - | - | 85.5 | - | - | - |
| 40 DBD | 6 | 409 | - | 2.5 | - | - | - | - | - | 501 | 87.5 | - | - | - | 87.5 | - | - | - | |||
| Pine et al[72] 2002-2008 | St. James, London, United Kingdom | 2009 | 39 DCDc | 13.4 | 352 | 2.5 yr | 5.1 | 33.31 | 20.51 | 2.6 | 12.81 | 20.5 | 7.61 | 79.51 | 63.61 | - | - | 801 | 68.21 | - | - |
| 39 DBD | - | 593 | 6.6 yr | - | 10.21 | - | 5.1 | - | 23.1 | 2.51 | 97.41 | 97.41 | - | - | 1001 | 11 | - | - | |||
| de Vera et al[34] 1993-2007 | Pittsburgh, United States | 2009 | 141 DCDc | 19.8 | 657 | - | 121 | 251 | 16.31 | 66 | - | - | 181 | 691 | - | 561 | 441 | 79 | - | 70 | 57 |
| 282 DBD | - | 636 | - | 31 | 131 | < 11 | - | - | - | 71 | 821 | - | 731 | 631 | 85 | - | 76 | 64 | |||
| Yamamoto et al[90] 1984-1988 | Stockholm, Sweden | 2010 | 24 DCDc | 6 | 7 h | > 20 yr | 8.3 | 37.51 | - | 33.31 | - | 70.8 | - | 54.2 | - | 37.5 | 37.5 | 61.9 | - | 42.9 | 42.9 |
| 16 DBD | - | 6.8 h | > 20 yr | 18.7 | 6.31 | - | - | - | 56.2 | - | 43.8 | - | 37.5 | 37.5 | 63.6 | - | - | 54.5 | |||
| Fujita et al[105] 1990-2006 | Gainesville, Floria, United States | 2007 | 24 DCDc | 12.8 | 7.6 h | - | 2.8 | 25 | 12.5 | 8.3 | - | 39.1 | 20.8 | 69.1 | 58.6 | - | - | 86.8 | 81.7 | - | - |
| 1209 DBD | - | 8.1 h | - | - | 20.5 | - | 4.1 | - | - | 9.4 | 78.7 | 70.2 | - | - | 84 | 76 | - | - | |||
| Foley et al[91] 1993-2002 | Wisconsin, United States | 2005 | 36 DCDc | 17.8 | 8.2 h | 3 yr | 5.5 | 331 | 13.8 | 5.5 | 16.61 | 61 | 19.41 | 671 | 561 | - | - | 801 | 681 | - | - |
| 553 DBD | - | 8.3 h | 4.6 yr | 1.3 | 101 | 8 | 11.8 | 5.41 | 56 | 71 | 861 | 801 | - | - | 911 | 841 | - | - | |||
| Manzarbeitia et al[106] 1995-2002 | Philadelphia, United States | 2004 | 19 DCDc | 19.6 | 574 | 1000 d | 5.2 | 10.5 | - | - | - | - | 10.5 | - | - | - | - | 89.5 | - | - | - |
| 311 DBD | - | 557 | - | - | 13.8 | - | - | - | - | 8.7 | - | - | - | - | 84.2 | - | - | - | |||
| Abt et al[104] 1996-2001 | Pennsylvania, United States | 2003 | 15 DCDc | 20.4 | 366 | 819 d | 6.7 | 33.31 | 26.71 | 3.2 | - | 20 | 6.61 | 71.8 | 71.8 | - | - | 79 | 79 | - | - |
| 221 DBD | - | 464 | 690 d | 3.6 | 9.51 | 2.31 | - | - | 21.3 | 3.61 | 85.4 | 73.9 | - | - | 90.9 | 77.7 | - | - | |||
| Nguyen et al[45] 1998-2001 | Mayo Clinic, Floria, United States | 2009 | 19 DCDc | 16 | 6.7 h | > 4.5 yr | 5.3 | 26.3 | 10.5 | 0 | 5.3 | 5.31 | 15.8 | 73.7 | 68.4 | 63.2 | - | 89.5 | 89.5 | 89.5 | - |
| 234 ECD | - | 7.1 h | - | 4.7 | 22.6 | - | - | - | 33.31 | 8.51 | - | - | - | - | 85 | 78.6 | 72.3 | - | |||
| 214 SCD | - | 7.5 h | - | 1.7 | 15.9 | - | - | - | 33.21 | 19.61 | - | - | - | - | 84.3 | 80.7 | 76.5 | - | |||
| Authors study period | Transplant center | Publication year | Patient number and Maastricht category | WIT (min) | CIT (min) | Mean follow-up | PNF % | Major biliary complications % | ITBL % | HAT % | HAS % | Rejection % | Retransplantation % | Graft survival % | Patient survival % | ||||
| 1 yr | 3 yr | 5 yr | 1 yr | 3 yr | 5 yr | ||||||||||||||
| Grewal et al[20] 1998-2006 | Mayo Clinic, Floria, United States | 2009 | 108 DCDc | 22.3 - | 6.3 h | - - | 3.7 | - - | 8.31 | 0.9 | - - | - - | 14.8 | 79.3 | 74.5 | 71 | 91.5 | 88.1 | 88.1 77.2 |
| 1328 DBD | 7.1 h | 1.4 | 1.91 | 1.7 | 9.3 | 81.6 | 74.7 | 69.1 | 87.3 | 81.1 | |||||||||
| Kaczmarek et al[41] 1999-2006 | 2007 | 11 DCDc | 34.6 - | 7.6 h | > 14 mo - | 0 | 45.41 | 11 | 0 | - - | - - | 9.11 01 | - - | - - | - - | - - | - - | - - | |
| 164 DBD | - | - | 16.41 | 8.21 | - | ||||||||||||||
| Dubbeld et al[51] 2001-2006 | 2010 | 55 DCDc | 16.5 - | 456 | - - | 2 | 281 | 241 | 74.7 - | - - | - - | 18 | 74 | 68 | - - | 85 | 80 | - - | |
| 471 DBD | 515 | 1.5 | 8.31 | 7.91 | 10.4 | 80.4 | 74.5 | 86.3 | 80.8 | ||||||||||
| Chan et al[43] 2003-2006 | Seattle, United States | 2008 | 51 DCDc | - - | - | 3 yr | 0 3.3 | 23.51 | 13.71 | 4.8 - | - - | - - | 9.8 | 79 | 79 | - - | 83 | 83 | - - |
| 334 DBD | - | 8.91 | 1.21 | - | 85 | 77 | 88 | 78 | |||||||||||
| Skaro et al[36] 2003-2008 | Chicago, United States | 2009 | 32 DCDc | 15.8 - | 5.5 h | - - | 3 | 531 | 381 | 93 - | - - | - - | 21 | 611 | 531 | - - | 74 | 74 | - - |
| 237 DBD | 5.2 h | 1 | 221 | 21 | 271 | 851 | 741 | 90 | 81 | ||||||||||
| Jay et al[107] 2004-2008 | Chicago, United States | 2010 | 28 DCDc | 16.5 - | 5.7 h | 1.8 yr | 3.6 | 57.71 | 441 | 10.7 | 7.1 | - - | 21.41 | 601 | 501 | - - | 701 | 701 | - - |
| 198 DBD | 5.3 h | - | 0.5 | 211 | 1.61 | 3 | 6.1 | 7.11 | 891 | 781 | 961 | 931 | |||||||
| Dezza et al[92] 2003 -2006 | Ghent, Belgium | 2007 | 13 DCDc | 10 - | 6.16 h | 163 d | 81 | - - | 23.11 | - - | - - | - - | 311 | 541 | - - | - - | 621 | - - | - - |
| 98 DBD | 9.14 h | 603 d | 11 | - | 121 | 791 | 861 | ||||||||||||
| Maheshwari et al[95] 1997-2006 | Johns Hopkins Baltimore, United States | 20 DCDc | 33 | 8.7 h | - | 5 | 60 | 50 | 5 | - | - | 20 | 62 | 62 | 30 | 78 | 78 | 40 | |
| Muiesan et al[70] 2001-2004 | London, United Kingdom | 2005 | 31 DCDc | 14.7 | 8.6 h | - | 3.1 | 9.4 | 0 | 3.1 | - | 28.1 | 3.1 | 86.5 | - | - | 89.6 | - | - |
| Abou Abbass et al[97] 2004-2008 | United States | 2010 | 26 DCDc | 39 | 5.3 h | - | 0 | 46 | 15.4 | 11.5 | 7.7 | 26.9 | 23 | 77 | - | - | 92 | - | - |
| Detry et al[94] 2003-2007 | 2010 | 58 DCDc | 25 | 451 | - | 3.4 | 38 | 32.7 | 3.4 | 3.4 | - | 13.8 | 72.4 | 48.8 | - | 83.3 | 66.9 | - | |
| Hernandez-Alejandro et al[42] 2006-2007 | London, United Kingdom | 2010 | 10 DCDc | 54.7 | 5.8 h | - | 10 | 10 | 0 | 0 | 0 | - | 10 | - | - | - | - | - | - |
| Hashimoto et al[96] 2005-2009 | United States | 2010 | 22 DCDc | 21 | 422 | - | 4.5 | 27 | 9 | 0 | 0 | - | 9 | 81 | 81 | - | - | - | - |
| Authors and study period | Publication year | Patient number and Maastricht category | WIT (min) | CIT (h) | PNF (%) | Retransplantation (%) | Graft survival % | Patient survival % | ||||
| 1 yr | 3 yr | 5 yr | 1 yr | 3 yr | 5 yr | |||||||
| Abt et al[67] 1993-2001 | 2004 | 144 DCD | 12.7 | 8.1 | 11.81 | 13.91 | 70.21 | 63.31 | - | 79.7 | 72.1 | - |
| 26 856 DBD | 8.9 | 6.41 | 8.31 | 80.41 | 72.11 | 85 | 77.4 | |||||
| Mateo et al[74] 1996-2003 | 2006 | 367 DCD | 15.6 | 8.3 | - | - | 711 | 601 | 531 | - | - | - |
| 33 111 DBD | 8.4 | 801 | 721 | 651 | ||||||||
| Lee et al[32] 1996-2006 | 2006 | 874 DCD | 15.4 | 7.9 | - | - | 72.11 | 61.81 | 38.81 | 82.31 | 75.91 | 65.31 |
| 43 734 DBD | 8.2 | 80.71 | 71.91 | 65.61 | 85.41 | 77.51 | 71.51 | |||||
| Doshi et al[68] 1998-2004 | 2007 | 345 DCD | - | 8.2 | 6.41 | 13.01 | 75 | 65 | - | 83 | 77 | - |
| 20 289 young-DBD | 8.1 | 3.91 | 5.61 | 831 | 751 | 881 | 801 | |||||
| 3604 old-DBD | 8.2 | 5.31 | 76 | 64 | 83 | 73 | ||||||
| Merion et al[125] 2000-2004 | 2006 | 472 DCD | - | 7.9 | - | - | 70.11 | 60.51 | - | - | - | - |
| 23 598 DBD | 8.1 | 831 | 751 | |||||||||
| Selck et al[69] 2002-2007 | 2008 | 855 DCD | - | - | - | 21.61 | 73.81 | 57.61 | - | - | - | - |
| 21 089 DBD | 8.81 | 84.41 | 74.41 | |||||||||
| Mathur et al[127] 2001-2009 | 2010 | 1567 DCD | 16.1 | 7.5 | - | 13.6 | - | - | - | 78 | 64.9 | - |
PNF is usually defined as unrecoverable hepato-cellular dysfunction leading to patient death or re-transplantation within the first week post-transplant after excluding other causes of graft failure such as vascular thrombosis, biliary complications, rejection or recurrent disease[81-84]. Initial studies using uncontrolled DCD donors reported a rate of PNF as high as 50%[85]. Currently only a few transplant centers in the world (like Spain, France) used this kind of donors because of aforementioned reasons. By using different in vivo organ preservation methods to maintain DCD donors and by strictly applying donor selection criteria, authors in Madrid[71], Barcelona[29] and La Coruña[86-89] could obtain promising results from Maastricht category I and II donors with a PNF rate of 10%-25%. The discard rate nevertheless was high up to 50%-75%[29,71]. In controlled DCD donors, the PNF rates are 0% to 12%. Matched analysis[34,72] and registry data[67,68] showed a higher rate of PNF in controlled DCD than DBD donors, although no difference was found in most comparative studies[20,43,90,91] except one[92]. The increased risk of PNF in DCD-LT recipients was also confirmed in a recent meta-analysis (odds ratio = 3.6, 95% CI: 2.1-6.4)[93]. Case-series reports of controlled DCD-LT also had a rate of PNF between 0% and 10%[42,70,94-97].
PNF is the consequence of severe IRI with the initial period of warm ischemia playing a crucial role. Experimental evidence supported that donor WIT should be less than 30 min to minimize PNF[98]. This warm ischemia (WI) period increases graft susceptibility to damage during cold preservation and CIT was a main contributing factor to PNF[34,67]; therefore, both periods of ischemia must be kept to a minimum. Many laboratory tests have been developed both in animal models and in human to predict the probability of occurrence of PNF post-transplant, but none is yet clinically efficient[99]. Recently Dahaba et al[100] proposed bispectral index monitoring as an early intra-operative indicator of early graft dysfunction.
Since the introduction of LT up to now, biliary complications are always regarded as the “Achilles heel” and a major cause of morbidity and graft failure in patients after LT[101]. The most common biliary complications are bile leakage and bile duct stricture[102,103]. Strictures involving the donor bile duct (> 1 cm above the biliary anastomosis) and requiring endoscopic or radiological dilatation/stenting or surgery in the face of a patent, non-stenotic hepatic artery was referred to as ischemic-type biliary lesions (ITBL), based on the radiologic resemblance of those occurring after hepatic artery thrombosis (HAT)[51,91,103].
Abt et al[104] first mentioned the significantly higher incidences of overall biliary complications as well as ITBL in DCD-LT recipients, the finding which was later confirmed in both matched[34,72] and comparative[20,43,51,89,91,104] studies except series of Fujita et al[105] and Manzarbeitia et al[106]. The rates of overall biliary complications and ITBL were 10.5%- 53% and 8.3%-38%, respectively in DCD-LT compared to 8.3%-22% and 0%-8%, respectively in DBD-LT. Especially Jiménez-Galanes et al[71] reported only a 5% incidence of ITBL in their patients receiving livers from uncontrolled DCD donors under normothermic ECMO. A recent meta-analysis revealed that DCD recipients had a 2.4 times increased odds of biliary complications (95% CI: 1.8-3.4) and a 10.8 times increased odds of ITBL (95% CI: 4.8-24.2) vs DBD recipients. In average, biliary complications were present in 29% of DCD compared with 17% of DBD recipients and ITBL in 16% of DCD vs 3% of DBD recipients[93].
Furthermore DCD recipients who developed ITBL experienced a fairly rapid clinical deterioration, characterized by a relatively short mean time from transplant to first endoscopic retrograde percutaneous cholangio-pancreatography (ERCP), from first ERCP to relisting and from relisting to re-transplantation (within 180 d)[36,69]. ITBL results in re-operation, multiple endoscopic and percutaneous biliary interventions, re-transplantation and even patient death with markedly increased medical care costs[107]. The relative risk (RR) of developing graft loss with ITBL formation was 3.02 (95% CI: 1.9-5.3) and graft survival was significantly decreased in patients with non-anastomotic strictures, compared to patients without it[89]. Up to 50% of all occurrences of ITBL lead to death and/or re-transplantation[108].
ITBL is usually a reflection of severe IRI in relation to various factors. In animal models, irreversible biliary tract damage has been observed after 40 min of cardiac arrest although hepato-cellular function could be preserved[109]. Clinical observations showed that total WIT > 30 min and chaotic donor physiology before asystole may increase the risk of post-transplant biliary stricture[33,110]. The mechanism could come from the stasis of blood and clot formation in the peri-biliary micro-circulation whose blood is solely supplied by the hepatic artery[96]. Many multivariate analysis recognized DCD liver grafts as an independent risk factor for the appearance of ITBL (RR = 47.1)[51,89]. Biliary epithelium is also known to be sensitive to cold preservation-reperfusion injury and the correlation between the incidence of ITBL and the duration of cold ischemia has been well documented. Li et al[111] demonstrated that the rate of ITBL is significantly increased in livers with increased preservation injury, as reflected by post-transplant peaks in serum transaminases. Other variables implicating in the mechanisms of ITBL may include injury of the peri-biliary vascular plexus, bile salt toxicity and potential immunological etiologies (ABO incompatibility, liver diseases with autoimmune component like autoimmune hepatitis and primary sclerosing cholangitis)[102]. Chan et al[43] found donor age > 50 years, donor weight ≥ 100 kg and total ischemia time ≥ 9 h were predictive for the development of ITBL. Patients who underwent LT from DCD donors > 60 years had a markedly high rate of biliary complications (67%), with a RR of 5.6 (95% CI: 0.98-32.2)[34].
Due to serious consequences of ITBL on the patient’s quality of life and healthcare cost, preventive measures seem to play a pivotal role in the safe expansion of DCD liver use. Attempts to minimize biliary duct damage may include the use of normothermic ECMO for donor maintenance[29,71,112] and machine perfusion for liver grafts, choice of preservation solutions [histidine-tryptophan-ketoglutarate (HTK) vs University of Wisconsin][113-117], use of anticoagulation and thrombolytic agents[96], extensive irrigation of the donor bile duct and pressure perfusion of the hepatic artery during organ retrieval and/or at back table[113,118,119], early porto-caval shunt to reduce portal hypertension in the recipient, choice of reperfusion techniques (concomitant vs sequential reperfusion of portal vein and hepatic artery)[120] and certainly the most important thing is always minimizing warm and cold ischemia period[121].
HAT is a thrombo-embolic occlusion of the hepatic artery that can occur early or late after LT. Most authors used the first 30 d post-transplant as a time point to distinguish between early and late HAT[122]. Early HAT results in fulminant hepatic failure, bile duct necrosis and leaks, relapsing bacteremia and ultimately graft loss and recipient death. The frequencies of early HAT after DCD-LT varied from 0% to 16.6% and did not seem significantly higher than those after DBD-LT in most studies[20,29,34,36,43,51,71,72,89,91,104,105] except Yamamoto et al[90] (33.3% vs 0%). Risk factors for early HAT have been well analyzed in a recent systemic review[123]. Few detailed studies discussed late HAT.
The incidence of hepatic artery stenosis (HAS) was not consistently found higher in DCD than DBD grafts (12.8%-16.6% vs 0%-5.4%)[72,91]. It is possible that hepatic arteries are susceptible to WI during DCD organ retrieval, resulting in subsequent scar and stenosis. Moreover the increased susceptibility of DCD livers to post-operative arterial ischemia might be responsible for more biliary strictures in DCD than DBD recipients with HAS (83% vs 37%) as well as shorter time to the development of biliary strictures after HAS in the DCD group[91]. Inadequate surgical technique, vascular trauma by clamps, graft rejection, recurrent hepatic disease might also play a role in the mechanisms for HAS[72,124].
Graft survival is defined as the time from transplantation to either re-transplantation or patient death, with “early” and “late” graft failure occurring within and beyond 1 year post-transplant, respectively[34]. Few studies reported experience with LT from uncontrolled DCD donors. Early results were poor with a PNF rate of 50% and one-year graft survival rate of only 17%[85] leading to a scarce usage of this donor category in the United States. Subsequent series in Spain using advanced in vivo organ preservation methods showed promising outcomes with one- and five-year graft survival rates of 50%-80% and 49%, and one- and five-year patient survival rates of 70%-85.5% and 62%, respectively[29,71,89]. LT from controlled DCD donors offered better results although they still appeared inferior to DBD-LT in matched studies[34,72], registry data analysis[32,67-69,74,125] and in some comparative studies[36,91,92]. One-, three-, five- and ten-year graft survival rates were 54%-79.5%, 53%-74.5%, 37.5%-71% and 37.5%-44%, respectively. Patient survival rates at corresponding time points were 61.9%-91.5%, 62.8%-89.5%, 42.9%-89.5% and 42.9%-57%, respectively. Transplant outcomes comparable to those obtained from DBD-LT have been sporadically reported in select centers through careful donor selection and optimization of CIT or through invasive techniques designed to optimize recovery before declaration of death[20,43,51,104].
Significant risk factors for DCD liver graft loss have been identified by multivariate Cox regression technique in both single center studies and large data registry analysis[32,67-69,74,126,127]. Among donor risk factors, age > 50 years, total WIT > 30-35 min, CIT > 6 h, body weight > 100 kg and regional or national liver distribution had deleterious effects on graft survival[32,74,127]. There is a stepwise increase in the relative risk of graft failure among donor age, WIT and CIT[32,127]. Strong recipient determinants of graft failure include age > 55 years, history of previous transplantation, medical status at transplantation [intensive care unit (ICU) or non-ICU hospitalization, life support, dialysis, renal insufficiency], high MELD score (> 30) and positive HCV serology[32,74,127]. In the DBD-LT model, it has been shown that a single risk factor lessened outcome marginally, however, the additive effect of multiple risk factors in a given donor-recipient pair were disastrous[83]. Grafts with ≥ 3 donor risk factors had significantly lower 1-year post-transplant survival than no or only 1 or 2 risk factors (58.3% vs 72.6%, 69.2% and 73.9%, respectively). No grafts with 4 risk factors survived within 1 year[128]. The relative risk of allograft failure from LT utilizing DCD donors was 31%-87% higher than LT utilizing DBD donors[67-69,125,126]. Causes of early graft failure included PNF, biliary complications, HAT and deaths from sepsis/multi-organ failure. Late graft failure was often secondary to chronic rejection and recipient death with a functioning graft.
Although DCD livers may not be as good as DBD ones with potential inferior transplant outcomes, there are subgroups of grafts and recipients that could give favorable results through appropriate graft and recipient matching. Low-risk DCD grafts which are transplanted in low-risk patients lead to comparable graft survival rates with DBD livers. Livers from DCD donors transplanted into high-risk recipients fared poorly independent of the allograft quality[74]. Doshi et al[68] showed DCD liver grafts were not inferior to DBD livers from older donors (≥ 60 years). Given the ever increasing demand for LT, DCD livers appear to be a reasonable alternative to increasing use of older or split livers and are a reasonable option when death is imminent[68]. Even if graft or/and patient survival is lower with a DCD liver, it is still better than dying because of turning down a DCD offer and continuing to wait for a DBD liver on these d as the patient’s choice is frequently not between marginal livers (including DCD) and standard livers but between marginal livers and no livers[105]. The benefit of earlier access to LT provided by a DCD graft could outweigh the risks of prolonged waiting for a standard graft[77].
DCD recipients more often require re-transplantation. Respectively, 21.6%-42% vs 8.8%-16% of DCD and DBD recipients were listed for re-transplantation[36,69]. The re-transplantation rate ranged from 7.6% to 31% in DCD-LT compared to 2.5%-12% in DBD-LT[20,29,34,36,51,67-69,71,72,91,92,106]. DCD livers exhibited a 2.1 times greater risk of graft failure, a 2.5 times greater risk of re-listing, and a 3.2 times greater risk of re-transplantation compared with DBD livers[36]. The majority of re-listing and re-transplantation in the DCD group were a consequence of biliary complications, especially ischemic cholangiography, but not due to an increased incidence of PNF, HAT or technical complications[36,69]. Particularly DCD livers had a temporally different failure pattern within the first year post-transplant that limited access to re-transplantation[36,69]: graft failure was more likely to occur within the first 180 d (18.1% vs 11.7%[67], 10.2% vs 2.5%[72] and 20.5% vs 11.5%[69] of DCD and DBD grafts failed within 60, 90 and 180 d, respectively); at re-transplantation, DCD recipients waited longer and received higher risk allografts; and more DCD recipients remained waiting for re-transplantation with fewer removed for death, clinical deterioration, or improvement. Re-transplantation arouses controversy on medical, economic, and ethical grounds: patient and graft survival rates after a second LT are inferior to those after initial grafting, the procedure is more expensive and in the context of organ shortage, re-transplantation inevitably denies organs to first-time recipients[129].
Utilization of DCD allografts for re-transplantation was rare (2.5% of initial DCD vs 3.1% of initial DBD) and outcomes from each group were comparable[69]. The general practice is to avoid re-transplantation with a DCD graft[36]. The use of DCD donors in the setting of re-transplantation resulted in an increased risk of recipient death (hazard ratio = 2.1, 95% CI: 1.2-3.6)[129].
The acute rejection rate did not differ significantly between DCD- and DBD-LT in most studies (1.9%-29% vs 0.6%-34%)[20,72,87,89,104]. Foley et al[91] reported a one-year rejection rate of 61% in the DCD group similar to that in the DBD group (56%). There were little data looking at the impact of DCD source on the risk of acute rejection.
The progressively increased DCD liver procurement to solve the shortage of DBD organs and to alleviate the waiting-list mortality has raised many challenges to the transplant community and transplant policy makers[110]. A lot of experimental researches have been performed over the past decade, intervening in both donors and recipients at different phases of the transplantation process, at the aim of tackling some of these challenges and providing a deep insight into IRI mechanisms.
Various cyto-protective substances have been successfully administered into the donor prior to cardiac arrest for prevention of liver microcirculatory disturbance. Microcirculatory disturbance was the main obstacle to successful DCD-LT, which was due to four major mechanisms: deterioration of sinusoidal endothelial cells (SEC) caused by activated Kupffer cells, sinusoidal narrowing caused by some vasoconstrictors and swollen hepatocytes, leukocyte and platelet adhesion, and hyper-coagulability[130]. Up to now, only Heparin and phentolamin (an anticoagulative substance and alpha-adrenergic antagonist) are allowed in clinical DCD organ procurement[131], other substances remain in animal models. Tacrolimus, besides its powerful immunosuppression, enabled to prevent liver normothermic IRI by multiple mechanisms[132]. Milrinone, a type 3 phosphodiesterase inhibitor, attenuated graft injury caused by warm and cold ischemia via an increase in intracellular cAMP levels, protection of SEC, relaxation of hepatic stellate cells, inhibition of platelet aggregation and anti-inflammatory effect[133]. Lazaroids, an antioxidant designed to inhibit iron-dependent lipid peroxidation, ameliorated SEC viability via antioxidant effects and membrane stabilization[134]. N-acetylcystein has a direct effect on oxygen free radicals, but its usage had no effect in both graft viability and lipid peroxidation[135].
Animal studies clearly showed the concept of pharmacological modulation of organ donors before procurement is feasible to improve the viability of marginal grafts. Nevertheless there are no definitive recommendations for the use of these drugs. Application of this method to clinical LT would require management of some practical problems and possible ethical conflicts[136].
Preservation of DCD livers by hypothermic machine perfusion (HMP) was shown superior to static cold storage (SCS) in many experimental studies[137,138].
Nonetheless a putative drawback of HMP for livers is to induce alterations at the vascular endothelial site, especially if HMP was performed for a long time or under suboptimal conditions[139]. Endoplasmic stress activation promoted cellular apoptosis via activation of caspase-12[140,141]. The efficiency of HMP was markedly increased by oxygenation of the perfusate[142]. The concern that high oxygenation might favor the generation of oxygen free radicals, which in turn could impair tissue integrity, was not justified. Several investigators could demonstrate the beneficial effect of oxygenated HMP in reducing the liver expression of pro-inflammatory cytokines (TNF-α, IL-8), adhesion molecules (ICAM-1) and major histocompatibility complex class II antigens[143-145]. This benefit will likely be more pronounced in marginal grafts such as elderly, steatotic and DCD livers[144]. Cyto-protective agents can be added into the machine perfusion (MP) solution to ameliorate the efficiency of HMP organ preservation[146].
The positive effects of HMP on warm-ischemically pre-damaged livers were observed even after a brief period of MP, before (pre-conditioning) or after SCS (post-conditioning)[143,147] and therefore it was not necessary to require MP over a full preservation period and helped avoid side-effects of HMP on vascular endothelium[141]. The use of HMP as the initial method for organ preservation followed by secondary SCS during transportation combined the advantage of aerobic resuscitation (i.e., restitution of cellular homeostasis) with an ease of SCS for later surveillance and transportation[141]. Manekeller et al[148] showed a post-conditioning of 1 h after SCS can ameliorate the viability of marginal livers. The extension or abbreviation of post-conditioning time seems to have no further beneficial effects[148].
Schön et al[66] reported advantages of normothermic machine perfusion (NMP) over SCS in pig DCD-LT models. Livers subjected to 1 h of WI and then cold-stored for 4-24 h were rendered completely nonviable while such livers under 4-24 h of oxygenated NMP recovered function to a viable level[149]. Due to the complexity of the logistics of clinical multi-organ recovery and of the NMP device, a period of cold preservation prior to warm perfusion of the liver is unescapable. A brief period of cold preservation (1 h) prior to NMP could maintain the synthetic and metabolic function but resulted in significant hepatocellular damage, sinusoidal endothelial cell dysfunction and Kupffer cell injury[150]. Once this duration was prolonged to 4 h, NMP completely failed to resuscitate porcine livers[151]. Normothermically perfusing DCD livers throughout the preservation period not only replenished cellular substrate, ameliorating the ischemic injury, but also provided a clear assessment of liver function and therefore could permit the use of severely injured organs with reassurance of function[149,152].
Despite the aforementioned benefits of MP over SCS in liver preservation, only SCS is clinically approved up to now, MP is still in the pre-clinical stage and early clinical studies[153]. Tojimbara et al[154] showed the impact of viscosity and temperature of initial flushing solutions on graft function. A low viscosity flushing solution was associated with lower vascular resistance, whereas a warm flush solution prevented cold-induced vasospasm and therefore improved the washout effect of the microcirculation[154]. HTK solution possessing a low viscosity and low potassium is more preferable in the DCD setting. The role of aeration of the cold-stored liver was also clarified. Oxygen provided either by surface diffusion (surface oxygenation) or intravascular diffusion (oxygen persufflation) helps improve the energy status of organs thus leading to earlier recovery. Surface oxygenation was not in use any more due to complicated technique, limited efficiency and risk of oxygen intoxication[155]. Venous systemic oxygen persufflation (VSOP) was shown to improve organ viability during hypothermic storage of the grafts and to be a feasible means for reconditioning of warm-ischemically pre-injured livers from DCD donors[155-158]. Experimentally even a short period of VSOP prior to long-term preservation of the liver by SCS may be sufficient for a relevant improvement of liver integrity upon reperfusion[159]. Gaseous persufflation with carbon monoxide was also tested in a DCD-LT rat model with enhanced liver graft viability[160]. However no additive or synergistic effect was noted when livers were persufflated with a mixture of gaseous oxygen and carbon monoxide[161].
Pharmaceutical interventions during SCS aimed at conditioning marginal organs also increasingly gained attention. Different cyto-protective drugs have been added into the flush and/or preservation solution, like vasodilators (phentolamin, epoprosterol, dopamine)[162,163], anti-coagulants (heparin), fibrinolytic agents (streptokinase)[164], antioxydants (superoxide dismutase, edaravone)[165,166], antibiotics, hormones (glucagon, growth factors)[167]. In the DCD setting, vasodilators, anti-coagulants, thrombolytic agents and antibiotics seem particularly necessary because the organs tend to develop vasospasm, thrombus formation in the microcirculation and the risk of colonic bacterial contamination secondary to translocation of organisms during the WI period[168,169].
Due to serious consequences of transplanting a DCD liver with potentially severe IRI (PNF, re-transplantation or even recipient death), it would be ideal if the viability of such livers could be predicted prior to rather than after transplantation. WIT is not always exactly known and thus cannot be a reliable parameter. Light microscopic examination of biopsy specimens was unable to uniformly predict liver function after transplantation[170]. Monbaliu et al[171] showed the extent of parenchyma vacuolation predicted pig liver graft viability before LT. Muiesan et al[70] applied the mechanical digestion of liver biopsies with collagenase and assessed the viability of hepatocytes by trypan blue exclusion method. However, the test was not helpful and the decision as to whether to use the liver was generally made on gross appearance, ease of perfusion, degree of steatosis and donor characteristics[172].
Another approach is to evaluate the vascular resistance and enzyme release in the perfusate of HMP livers. Resistance index of the portal vein and hepatic artery showed no utility[173]. Biomarkers of liver cell damage, like transaminases, lactate dehydrogenase and liver fatty acid binding protein, correlated well with WI duration and concomitant hepatocyte damage in pig DCD-LT models[174]. Possible other parameters are the ATP content and redox active iron status of the liver during HMP[175]. During NMP, the assessment of liver viability may be easier because the liver is in a normal metabolic state. Bile production was a good viability indicator besides the measurement of other liver functions (detoxification, metabolism or synthesis)[176]. Recently Liu et al[177] has tested the utility of magnetic resonance imaging and proton magnetic resonance spectroscopy to evaluate WI livers without success.
Pharmaceutical strategies aimed at modulating IRI mechanisms were also applied successfully in animal recipients and generally did not impose ethical problems as donor pre-treatment. Such protocols without donor pretreatment will be favorable in clinical application. Most studies tested a single agent for a specific target of the IRI process. A multi-factorial approach acting on different pathways of the IRI process have been advocated and remarkably ameliorated transplant outcomes[162].
The future of DCD-LT is promising. Concerted efforts should concentrate on the identification of suitable donors (probably Maastricht category III DCD donors), better donor and recipient matching (high risk donors to low risk recipients), use of advanced organ preservation techniques (oxygenated HMP and NMP, VSOP), and pharmacological modulation (probably a multi-factorial biologic modulation strategy) so that liver procurement and transplantation from DCD donors could be widely expanded and attain equivalent results as DBD-LT.
Peer reviewers: Bijan Eghtesad, Associate Professor, Depart-ment of General Surgery, Cleveland Clinic Foundation, 9500 Euclid Avenue, Cleveland, OH 44195, United States; Tokihiko Sawada, Associate Professor, Second Department of Surgery, Dokkyo University School of Medicine, Kitakobayashi 880, Mibu, Shimotsuga, Tochigi 321-0293, Japan; Philip Rosenthal, Professor, Pediatrics, UCSF, 500 parnassus Avenue, San Francisco, CA 94143-0136, United States
S- Editor Gou SX L- Editor A E- Editor Li JY
| 1. | Starzl TE, Marchioro TL, Vonkaulla KN, Hermann G, Brittain RS, Waddell WR. Homotransplantation of the liver in humans. Surg Gynecol Obstet. 1963;117:659-676. [PubMed] [Cited in This Article: ] |
| 2. | Starzl TE, Groth CG, Brettschneider L, Penn I, Fulginiti VA, Moon JB, Blanchard H, Martin AJ, Porter KA. Orthotopic homotransplantation of the human liver. Ann Surg. 1968;168:392-415. [PubMed] [Cited in This Article: ] |
| 3. | Calne RY, Williams R. Liver transplantation in man. I. Observations on technique and organization in five cases. Br Med J. 1968;4:535-540. [PubMed] [Cited in This Article: ] |
| 4. | Calne RY. Early days of liver transplantation. Am J Transplant. 2008;8:1775-1778. [PubMed] [Cited in This Article: ] |
| 5. | Ascher HL. Liver transplantation--the first 25 years. West J Med. 1988;149:316-321. [PubMed] [Cited in This Article: ] |
| 6. | Ledinh H, Bonvoisin C, Weekers L, de Roover A, Honoré P, Squifflet JP, Meurisse M, Detry O. Results of kidney transplantation from donors after cardiac death. Transplant Proc. 2010;42:2407-2414. [PubMed] [Cited in This Article: ] |
| 7. | Thuluvath PJ, Guidinger MK, Fung JJ, Johnson LB, Rayhill SC, Pelletier SJ. Liver transplantation in the United States, 1999-2008. Am J Transplant. 2010;10:1003-1019. [PubMed] [Cited in This Article: ] |
| 8. | Klein AS, Messersmith EE, Ratner LE, Kochik R, Baliga PK, Ojo AO. Organ donation and utilization in the United States, 1999-2008. Am J Transplant. 2010;10:973-986. [PubMed] [Cited in This Article: ] |
| 9. | Kauffman HM, Rosendale JD, Taranto SE, McBride MA, Marks WH. Non–heart-beating donors (then) and donation after cardiac death (now). Transplant Rev. 2007;21:237-248. [Cited in This Article: ] |
| 10. | Wynn JJ, Alexander CE. Increasing organ donation and transplantation: the U.S. experience over the past decade. Transpl Int. 2011;24:324-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Available from: http: //www.uktransplant.org.uk/ukt/statistics/transplant_activity_report/archive_activity_reports/pdf/ukt/transplant_activity_uk_2008-2009.pdf. Access date: 15/1/2011. [Cited in This Article: ] |
| 12. | Devey L, Wigmore SJ. Non-heart-beating organ donation. Br J Surg. 2009;96:833-835. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Roberts KJ, Bramhall S, Mayer D, Muiesan P. Uncontrolled organ donation following prehospital cardiac arrest: a potential solution to the shortage of organ donors in the United Kingdom? Transpl Int. 2011;24:477-481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Available from: http: //www.edqm.eu/medias/fichiers/Newsletter_Transplant_Vol_14_No_1_Sept_2009.pdf. Access date: 15/1/2011. [Cited in This Article: ] |
| 15. | Available from: http: //www.eurotransplant.org/files/annual_report/ar_2008.pdf. Access date: 15/1/2011. [Cited in This Article: ] |
| 16. | Squifflet JP. Why did it take so long to start a non-heart-beating donor program in Belgium? Acta Chir Belg. 2006;106:485-488. [PubMed] [Cited in This Article: ] |
| 17. | Available from: http: //www.agence-biomedecine.fr/uploads/document/RA_Biomed_2009-B.pdf. Access date: 15/1/2011. [Cited in This Article: ] |
| 18. | Noguchi H, Hatanaka N, Matsumoto S. Renal and islet transplantation from non-heart-beating donors in Japan. Organ donation and transplantation after cardiac death. Oxford, New York: Oxford University Press 2009; 289-305. [Cited in This Article: ] |
| 19. | Chaib E, Massad E. The potential impact of using donations after cardiac death on the liver transplantation program and waiting list in the state of Sao Paulo, Brazil. Liver Transpl. 2008;14:1732-1736. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Grewal HP, Willingham DL, Nguyen J, Hewitt WR, Taner BC, Cornell D, Rosser BG, Keaveny AP, Aranda-Michel J, Satyanarayana R. Liver transplantation using controlled donation after cardiac death donors: an analysis of a large single-center experience. Liver Transpl. 2009;15:1028-1035. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 21. | Reddy S, Zilvetti M, Brockmann J, McLaren A, Friend P. Liver transplantation from non-heart-beating donors: current status and future prospects. Liver Transpl. 2004;10:1223-1232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Domínguez-Gil B, Haase-Kromwijk B, Van Leiden H, Neuberger J, Coene L, Morel P, Corinne A, Muehlbacher F, Brezovsky P, Costa AN. Current situation of donation after circulatory death in European countries. Transpl Int. 2011;24:676-686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 181] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 23. | Matesanz R, Domínguez-Gil B, Coll E, de la Rosa G, Marazuela R. Spanish experience as a leading country: what kind of measures were taken? Transpl Int. 2011;24:333-343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 24. | Roels L, Rahmel A. The European experience. Transpl Int. 2011;24:350-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | Bruzzone P. Ethical and legal issues in donation after cardiac death in Italy. Transplant Proc. 2010;42:1046-1047. [PubMed] [Cited in This Article: ] |
| 26. | Massip-Salcedo M, Roselló-Catafau J, Prieto J, Avíla MA, Peralta C. The response of the hepatocyte to ischemia. Liver Int. 2007;27:6-16. [PubMed] [Cited in This Article: ] |
| 27. | Kootstra G, Daemen JH, Oomen AP. Categories of non-heart-beating donors. Transplant Proc. 1995;27:2893-2894. [PubMed] [Cited in This Article: ] |
| 28. | Sánchez-Fructuoso AI, Prats D, Torrente J, Pérez-Contín MJ, Fernández C, Alvarez J, Barrientos A. Renal transplantation from non-heart beating donors: a promising alternative to enlarge the donor pool. J Am Soc Nephrol. 2000;11:350-358. [PubMed] [Cited in This Article: ] |
| 29. | Fondevila C, Hessheimer AJ, Ruiz A, Calatayud D, Ferrer J, Charco R, Fuster J, Navasa M, Rimola A, Taurá P. Liver transplant using donors after unexpected cardiac death: novel preservation protocol and acceptance criteria. Am J Transplant. 2007;7:1849-1855. [PubMed] [Cited in This Article: ] |
| 30. | Geraci PM, Sepe V. Non-heart-beating organ donation in Italy. Minerva Anestesiol. 2011;77:613-623. [PubMed] [Cited in This Article: ] |
| 31. | Ysebaert D, Van Beeumen G, De Greef K, Squifflet JP, Detry O, De Roover A, Delbouille MH, Van Donink W, Roeyen G, Chapelle T. Organ procurement after euthanasia: Belgian experience. Transplant Proc. 2009;41:585-586. [PubMed] [Cited in This Article: ] |
| 32. | Lee KW, Simpkins CE, Montgomery RA, Locke JE, Segev DL, Maley WR. Factors affecting graft survival after liver transplantation from donation after cardiac death donors. Transplantation. 2006;82:1683-1688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 33. | Bernat JL, D'Alessandro AM, Port FK, Bleck TP, Heard SO, Medina J, Rosenbaum SH, Devita MA, Gaston RS, Merion RM. Report of a National Conference on Donation after cardiac death. Am J Transplant. 2006;6:281-291. [PubMed] [Cited in This Article: ] |
| 34. | de Vera ME, Lopez-Solis R, Dvorchik I, Campos S, Morris W, Demetris AJ, Fontes P, Marsh JW. Liver transplantation using donation after cardiac death donors: long-term follow-up from a single center. Am J Transplant. 2009;9:773-781. [PubMed] [Cited in This Article: ] |
| 35. | Fukumori T, Kato T, Levi D, Olson L, Nishida S, Ganz S, Nakamura N, Madariaga J, Ohkohchi N, Satomi S. Use of older controlled non-heart-beating donors for liver transplantation. Transplantation. 2003;75:1171-1174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Skaro AI, Jay CL, Baker TB, Wang E, Pasricha S, Lyuksemburg V, Martin JA, Feinglass JM, Preczewski LB, Abecassis MM. The impact of ischemic cholangiopathy in liver transplantation using donors after cardiac death: the untold story. Surgery. 2009;146:543-552; discussion 552-553. [PubMed] [Cited in This Article: ] |
| 37. | Olson L, Kisthard J, Cravero L, Fung J, Eghtesad B, Savo A, Levy A, Polissar N, Marks W. Livers transplanted from donors after cardiac death occurring in the ICU or the operating room have excellent outcomes. Transplant Proc. 2005;37:1188-1193. [PubMed] [Cited in This Article: ] |
| 38. | Attia M, Silva MA, Mirza DF. The marginal liver donor--an update. Transpl Int. 2008;21:713-724. [PubMed] [Cited in This Article: ] |
| 39. | Reich DJ, Mulligan DC, Abt PL, Pruett TL, Abecassis MM, D'Alessandro A, Pomfret EA, Freeman RB, Markmann JF, Hanto DW. ASTS recommended practice guidelines for controlled donation after cardiac death organ procurement and transplantation. Am J Transplant. 2009;9:2004-2011. [PubMed] [Cited in This Article: ] |
| 40. | Reich DJ, Munoz SJ, Rothstein KD, Nathan HM, Edwards JM, Hasz RD, Manzarbeitia CY. Controlled non-heart-beating donor liver transplantation: a successful single center experience, with topic update. Transplantation. 2000;70:1159-1166. [PubMed] [Cited in This Article: ] |
| 41. | Kaczmarek B, Manas MD, Jaques BC, Talbot D. Ischemic cholangiopathy after liver transplantation from controlled non-heart-beating donors-a single-center experience. Transplant Proc. 2007;39:2793-2795. [PubMed] [Cited in This Article: ] |
| 42. | Hernandez-Alejandro R, Caumartin Y, Chent C, Levstik MA, Quan D, Muirhead N, House AA, McAlister V, Jevnikar AM, Luke PP. Kidney and liver transplants from donors after cardiac death: initial experience at the London Health Sciences Centre. Can J Surg. 2010;53:93-102. [PubMed] [Cited in This Article: ] |
| 43. | Chan EY, Olson LC, Kisthard JA, Perkins JD, Bakthavatsalam R, Halldorson JB, Reyes JD, Larson AM, Levy AE. Ischemic cholangiopathy following liver transplantation from donation after cardiac death donors. Liver Transpl. 2008;14:604-610. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 177] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 44. | Kootstra G. Statement on non-heart-beating donor programs. Transplant Proc. 1995;27:2965. [PubMed] [Cited in This Article: ] |
| 45. | Nguyen JH, Bonatti H, Dickson RC, Hewitt WR, Grewal HP, Willingham DL, Harnois DM, Schmitt TM, Machicao VI, Ghabril MS. Long-term outcomes of donation after cardiac death liver allografts from a single center. Clin Transplant. 2009;23:168-173. [PubMed] [Cited in This Article: ] |
| 46. | Wells A, Watson C, Jamieson N, Bradley JA. Which time is it? A suggestion for unambiguous nomenclature in transplantation. Am J Transplant. 2007;7:1315-1316. [PubMed] [Cited in This Article: ] |
| 47. | Halazun KJ, Al-Mukhtar A, Aldouri A, Willis S, Ahmad N. Warm ischemia in transplantation: search for a consensus definition. Transplant Proc. 2007;39:1329-1331. [PubMed] [Cited in This Article: ] |
| 48. | Pine JK, Goldsmith PJ, Ridgway DM, Pollard SG, Menon KV, Attia M, Ahmad N. Predicting donor asystole following withdrawal of treatment in donation after cardiac death. Transplant Proc. 2010;42:3949-3950. [PubMed] [Cited in This Article: ] |
| 49. | Domínguez-Gil B, Delmonico FL, Shaheen FA, Matesanz R, O'Connor K, Minina M, Muller E, Young K, Manyalich M, Chapman J. The critical pathway for deceased donation: reportable uniformity in the approach to deceased donation. Transpl Int. 2011;24:373-378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 50. | D'Alessandro AM. The process of donation after cardiac death: a United States perspective. Transplant Rev. 2007;21:230-236. [Cited in This Article: ] |
| 51. | Dubbeld J, Hoekstra H, Farid W, Ringers J, Porte RJ, Metselaar HJ, Baranski AG, Kazemier G, van den Berg AP, van Hoek B. Similar liver transplantation survival with selected cardiac death donors and brain death donors. Br J Surg. 2010;97:744-753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 164] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 52. | Manara AR, Murphy PG, O'Callaghan G. Donation after circulatory death. Br J Anaesth. 2012;108 Suppl 1:i108-i121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 181] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 53. | Lewis J, Peltier J, Nelson H, Snyder W, Schneider K, Steinberger D, Anderson M, Krichevsky A, Anderson J, Ellefson J. Development of the University of Wisconsin donation After Cardiac Death Evaluation Tool. Prog Transplant. 2003;13:265-273. [PubMed] [Cited in This Article: ] |
| 54. | DeVita MA, Brooks MM, Zawistowski C, Rudich S, Daly B, Chaitin E. Donors after cardiac death: validation of identification criteria (DVIC) study for predictors of rapid death. Am J Transplant. 2008;8:432-441. [PubMed] [Cited in This Article: ] |
| 55. | Suntharalingam C, Sharples L, Dudley C, Bradley JA, Watson CJ. Time to cardiac death after withdrawal of life-sustaining treatment in potential organ donors. Am J Transplant. 2009;9:2157-2165. [PubMed] [Cited in This Article: ] |
| 56. | Catania A, Lonati C, Sordi A, Gatti S. Detrimental consequences of brain injury on peripheral cells. Brain Behav Immun. 2009;23:877-884. [PubMed] [Cited in This Article: ] |
| 57. | Pratschke J, Weiss S, Neuhaus P, Pascher A. Review of nonimmunological causes for deteriorated graft function and graft loss after transplantation. Transpl Int. 2008;21:512-522. [PubMed] [Cited in This Article: ] |
| 58. | Nijboer WN, Schuurs TA, van der Hoeven JAB, Ploeg RJ. Effect of brain death and donor treatment on organ inflammatory response and donor organ viability. Curr Opin Organ Transplant. 2004;9:110-115. [Cited in This Article: ] |
| 59. | Gasser M, Waaga AM, Laskowski IA, Tilney NL. The influence of donor brain death on short and long-term outcome of solid organ allografts. Ann Transplant. 2000;5:61-67. [PubMed] [Cited in This Article: ] |
| 60. | Zhang SJ, Wang T. The influence of brain death on donor liver and the potential mechanisms of protective intervention. Front Med. 2011;5:8-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 61. | Pratschke J, Neuhaus P, Tullius SG. What can be learned from brain-death models? Transpl Int. 2005;18:15-21. [PubMed] [Cited in This Article: ] |
| 62. | Jassem W, Koo DD, Cerundolo L, Rela M, Heaton ND, Fuggle SV. Leukocyte infiltration and inflammatory antigen expression in cadaveric and living-donor livers before transplant. Transplantation. 2003;75:2001-2007. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 63. | Weiss S, Kotsch K, Francuski M, Reutzel-Selke A, Mantouvalou L, Klemz R, Kuecuek O, Jonas S, Wesslau C, Ulrich F. Brain death activates donor organs and is associated with a worse I/R injury after liver transplantation. Am J Transplant. 2007;7:1584-1593. [PubMed] [Cited in This Article: ] |
| 64. | Singhal AK, Sheng X, Drakos SG, Stehlik J. Impact of donor cause of death on transplant outcomes: UNOS registry analysis. Transplant Proc. 2009;41:3539-3544. [PubMed] [Cited in This Article: ] |
| 65. | Moers C, Leuvenink HG, Ploeg RJ. Donation after cardiac death: evaluation of revisiting an important donor source. Nephrol Dial Transplant. 2010;25:666-673. [PubMed] [Cited in This Article: ] |
| 66. | Schön MR, Kollmar O, Wolf S, Schrem H, Matthes M, Akkoc N, Schnoy NC, Neuhaus P. Liver transplantation after organ preservation with normothermic extracorporeal perfusion. Ann Surg. 2001;233:114-123. [PubMed] [Cited in This Article: ] |
| 67. | Abt PL, Desai NM, Crawford MD, Forman LM, Markmann JW, Olthoff KM, Markmann JF. Survival following liver transplantation from non-heart-beating donors. Ann Surg. 2004;239:87-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 288] [Cited by in F6Publishing: 300] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 68. | Doshi MD, Hunsicker LG. Short- and long-term outcomes with the use of kidneys and livers donated after cardiac death. Am J Transplant. 2007;7:122-129. [PubMed] [Cited in This Article: ] |
| 69. | Selck FW, Grossman EB, Ratner LE, Renz JF. Utilization, outcomes, and retransplantation of liver allografts from donation after cardiac death: implications for further expansion of the deceased-donor pool. Ann Surg. 2008;248:599-607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 70. | Muiesan P, Girlanda R, Jassem W, Melendez HV, O'Grady J, Bowles M, Rela M, Heaton N. Single-center experience with liver transplantation from controlled non-heartbeating donors: a viable source of grafts. Ann Surg. 2005;242:732-738. [PubMed] [Cited in This Article: ] |
| 71. | Jiménez-Galanes S, Meneu-Diaz MJ, Elola-Olaso AM, Pérez-Saborido B, Yiliam FS, Calvo AG, Usera MA, González MC, González JC, González EM. Liver transplantation using uncontrolled non-heart-beating donors under normothermic extracorporeal membrane oxygenation. Liver Transpl. 2009;15:1110-1118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 72. | Pine JK, Aldouri A, Young AL, Davies MH, Attia M, Toogood GJ, Pollard SG, Lodge JP, Prasad KR. Liver transplantation following donation after cardiac death: an analysis using matched pairs. Liver Transpl. 2009;15:1072-1082. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 73. | LaMattina JC, Mezrich JD, Fernandez LA, D'Alessandro AM, Bellingham JM, Musat AI, Foley DP. Simultaneous liver and kidney transplantation using donation after cardiac death donors: a brief report. Liver Transpl. 2011;17:591-595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 74. | Mateo R, Cho Y, Singh G, Stapfer M, Donovan J, Kahn J, Fong TL, Sher L, Jabbour N, Aswad S. Risk factors for graft survival after liver transplantation from donation after cardiac death donors: an analysis of OPTN/UNOS data. Am J Transplant. 2006;6:791-796. [PubMed] [Cited in This Article: ] |
| 75. | Hernandez-Alejandro R, Croome KP, Quan D, Mawardi M, Chandok N, Dale C, McAlister V, Levstik MA, Wall W, Marotta P. Increased risk of severe recurrence of hepatitis C virus in liver transplant recipients of donation after cardiac death allografts. Transplantation. 2011;92:686-689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 76. | Tao R, Ruppert K, Cruz RJ, Malik SM, Shaikh O, Ahmad J, DiMartini A, Humar A, Fontes PA, de Vera ME. Hepatitis C recurrence is not adversely affected by the use of donation after cardiac death liver allografts. Liver Transpl. 2010;16:1288-1295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 77. | Taner CB, Bulatao IG, Keaveny AP, Willingham DL, Pungpapong S, Perry DK, Rosser BG, Harnois DM, Aranda-Michel J, Nguyen JH. Use of liver grafts from donation after cardiac death donors for recipients with hepatitis C virus. Liver Transpl. 2011;17:641-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 78. | Uemura T, Ramprasad V, Hollenbeak CS, Bezinover D, Kadry Z. Liver transplantation for hepatitis C from donation after cardiac death donors: an analysis of OPTN/UNOS data. Am J Transplant. 2012;12:984-991. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 79. | Muiesan P, Girlanda R, Baker A, Rela M, Heaton N. Successful segmental auxiliary liver transplantation from a non-heart-beating donor: implications for split-liver transplantation. Transplantation. 2003;75:1443-1445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 80. | Muiesan P, Jassem W, Girlanda R, Steinberg R, Vilca-Melendez H, Mieli-Vergani G, Dhawan A, Rela M, Heaton N. Segmental liver transplantation from non-heart beating donors--an early experience with implications for the future. Am J Transplant. 2006;6:1012-1016. [PubMed] [Cited in This Article: ] |
| 81. | Ploeg RJ, D'Alessandro AM, Knechtle SJ, Stegall MD, Pirsch JD, Hoffmann RM, Sasaki T, Sollinger HW, Belzer FO, Kalayoglu M. Risk factors for primary dysfunction after liver transplantation--a multivariate analysis. Transplantation. 1993;55:807-813. [PubMed] [Cited in This Article: ] |
| 82. | Nissen NN, Colquhoun SD. Graft failure: etiology, recognition and treatment. Transplantation of the liver. Philadelphia: Elsevier Saunders 2005; 915-928. [Cited in This Article: ] |
| 83. | Strasberg SM, Howard TK, Molmenti EP, Hertl M. Selecting the donor liver: risk factors for poor function after orthotopic liver transplantation. Hepatology. 1994;20:829-838. [PubMed] [Cited in This Article: ] |
| 84. | Briceño J, Ciria R, de la Mata M, Rufián S, López-Cillero P. Prediction of graft dysfunction based on extended criteria donors in the model for end-stage liver disease score era. Transplantation. 2010;90:530-539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 85. | Casavilla A, Ramirez C, Shapiro R, Nghiem D, Miracle K, Bronsther O, Randhawa P, Broznick B, Fung JJ, Starzl T. Experience with liver and kidney allografts from non-heart-beating donors. Transplantation. 1995;59:197-203. [PubMed] [Cited in This Article: ] |
| 86. | Quintela J, Gala B, Baamonde I, Fernández C, Aguirrezabalaga J, Otero A, Suárez F, Fernández A, Gomez M. Long-term results for liver transplantation from non-heart-beating donors maintained with chest and abdominal compression-decompression. Transplant Proc. 2005;37:3857-3858. [PubMed] [Cited in This Article: ] |
| 87. | Otero A, Gómez-Gutiérrez M, Suárez F, Arnal F, Fernández-García A, Aguirrezabalaga J, García-Buitrón J, Alvarez J, Máñez R. Liver transplantation from maastricht category 2 non-heart-beating donors: a source to increase the donor pool? Transplant Proc. 2004;36:747-750. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 88. | Gómez M, Garcia-Buitrón JM, Fernandez-Garcia A, Vilela D, Fernández-Selles C, Corbal R, Fraguela J, Suárez F, Otero A, Alvarez J. Liver transplantation with organs from non-heart-beating donors. Transplant Proc. 1997;29:3478-3479. [PubMed] [Cited in This Article: ] |
| 89. | Suárez F, Otero A, Solla M, Arnal F, Lorenzo MJ, Marini M, Vázquez-Iglesias JL, Gómez M. Biliary complications after liver transplantation from maastricht category-2 non-heart-beating donors. Transplantation. 2008;85:9-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 90. | Yamamoto S, Wilczek HE, Duraj FF, Groth CG, Ericzon BG. Liver transplantation with grafts from controlled donors after cardiac death: a 20-year follow-up at a single center. Am J Transplant. 2010;10:602-611. [PubMed] [Cited in This Article: ] |
| 91. | Foley DP, Fernandez LA, Leverson G, Chin LT, Krieger N, Cooper JT, Shames BD, Becker YT, Odorico JS, Knechtle SJ. Donation after cardiac death: the University of Wisconsin experience with liver transplantation. Ann Surg. 2005;242:724-731. [PubMed] [Cited in This Article: ] |
| 92. | Dezza MC, Berrevoet F, Sainz-Barriga M, Rossetto A, Colenbie L, Haentjens I, Van Vlierberghe H, Colle I, Van Huysse J, Praet M. The choice of recipient does not have a bearing on early outcome in liver transplant patients receiving grafts from non-heart-beating donors: a reappraisal? Transplant Proc. 2007;39:2675-2677. [PubMed] [Cited in This Article: ] |
| 93. | Jay CL, Lyuksemburg V, Ladner DP, Wang E, Caicedo JC, Holl JL, Abecassis MM, Skaro AI. Ischemic cholangiopathy after controlled donation after cardiac death liver transplantation: a meta-analysis. Ann Surg. 2011;253:259-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 94. | Detry O, Donckier V, Lucidi V, Ysebaert D, Chapelle T, Lerut J, Ciccarelli O, Pirenne J, Monbaliu D, De Roover A. Liver transplantation from donation after cardiac death donors: initial Belgian experience 2003-2007. Transpl Int. 2010;23:611-618. [PubMed] [Cited in This Article: ] |
| 95. | Maheshwari A, Maley W, Li Z, Thuluvath PJ. Biliary complications and outcomes of liver transplantation from donors after cardiac death. Liver Transpl. 2007;13:1645-1653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 140] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 96. | Hashimoto K, Eghtesad B, Gunasekaran G, Fujiki M, Uso TD, Quintini C, Aucejo FN, Kelly DM, Winans CG, Vogt DP. Use of tissue plasminogen activator in liver transplantation from donation after cardiac death donors. Am J Transplant. 2010;10:2665-2672. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 97. | Abou Abbass A, Abouljoud M, Yoshida A, Kim DY, Slater R, Hundley J, Kazimi M, Moonka D. Biliary complications after orthotopic liver transplantation from donors after cardiac death: broad spectrum of disease. Transplant Proc. 2010;42:3392-3398. [PubMed] [Cited in This Article: ] |
| 98. | Monbaliu D, Crabbé T, Roskams T, Fevery J, Verwaest C, Pirenne J. Livers from non-heart-beating donors tolerate short periods of warm ischemia. Transplantation. 2005;79:1226-1230. [PubMed] [Cited in This Article: ] |
| 99. | Vilca Melendez H, Rela M, Murphy G, Heaton N. Assessment of graft function before liver transplantation: quest for the lost ark? Transplantation. 2000;70:560-565. [PubMed] [Cited in This Article: ] |
| 100. | Dahaba AA, Feng ZY, Zhu SM, Bornemann H, Rehak PH, Metzler H. The utility of using bispectral index monitoring as an early intraoperative indicator of initial poor graft function after orthotopic or split-graft liver transplantation. Gut. 2009;58:605-606. [PubMed] [Cited in This Article: ] |
| 101. | Fung JJ, Eghtesad B, Patel-Tom K. Using livers from donation after cardiac death donors--a proposal to protect the true Achilles heel. Liver Transpl. 2007;13:1633-1636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 102. | Buis CI, Hoekstra H, Verdonk RC, Porte RJ. Causes and consequences of ischemic-type biliary lesions after liver transplantation. J Hepatobiliary Pancreat Surg. 2006;13:517-524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 157] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 103. | Verdonk RC, Buis CI, Porte RJ, Haagsma EB. Biliary complications after liver transplantation: a review. Scand J Gastroenterol Suppl. 2006;89-101. [PubMed] [Cited in This Article: ] |
| 104. | Abt P, Crawford M, Desai N, Markmann J, Olthoff K, Shaked A. Liver transplantation from controlled non-heart-beating donors: an increased incidence of biliary complications. Transplantation. 2003;75:1659-1663. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 232] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 105. | Fujita S, Mizuno S, Fujikawa T, Reed AI, Kim RD, Howard RJ, Hemming AW. Liver transplantation from donation after cardiac death: a single center experience. Transplantation. 2007;84:46-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 106. | Manzarbeitia CY, Ortiz JA, Jeon H, Rothstein KD, Martinez O, Araya VR, Munoz SJ, Reich DJ. Long-term outcome of controlled, non-heart-beating donor liver transplantation. Transplantation. 2004;78:211-215. [PubMed] [Cited in This Article: ] |
| 107. | Jay CL, Lyuksemburg V, Kang R, Preczewski L, Stroupe K, Holl JL, Abecassis MM, Skaro AI. The increased costs of donation after cardiac death liver transplantation: caveat emptor. Ann Surg. 2010;251:743-748. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 108. | Perkins JD. Risk factors for developing ischemic-type biliary lesions after liver transplantation. Liver Transpl. 2009;15:1882-1887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 109. | García-Valdecasas JC, Tabet J, Valero R, Deulofeu R, Taurá P, Rull R, Capdevila L, Cifuentes A, González FX, Net M. Evaluation of ischemic injury during liver procurement from non-heart-beating donors. Eur Surg Res. 1999;31:447-456. [PubMed] [Cited in This Article: ] |
| 110. | Wigmore SJ. Current challenges in liver transplantation following donation after cardiac death. Transplantation. 2010;90:1048-1049. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 111. | Li S, Stratta RJ, Langnas AN, Wood RP, Marujo W, Shaw BW. Diffuse biliary tract injury after orthotopic liver transplantation. Am J Surg. 1992;164:536-540. [PubMed] [Cited in This Article: ] |
| 112. | Net M, Valero R, Almenara R, Barros P, Capdevila L, López-Boado MA, Ruiz A, Sánchez-Crivaro F, Miquel R, Deulofeu R. The effect of normothermic recirculation is mediated by ischemic preconditioning in NHBD liver transplantation. Am J Transplant. 2005;5:2385-2392. [PubMed] [Cited in This Article: ] |
| 113. | Heidenhain C, Pratschke J, Puhl G, Neumann U, Pascher A, Veltzke-Schlieker W, Neuhaus P. Incidence of and risk factors for ischemic-type biliary lesions following orthotopic liver transplantation. Transpl Int. 2010;23:14-22. [PubMed] [Cited in This Article: ] |
| 114. | Pirenne J, Van Gelder F, Coosemans W, Aerts R, Gunson B, Koshiba T, Fourneau I, Mirza D, Van Steenbergen W, Fevery J. Type of donor aortic preservation solution and not cold ischemia time is a major determinant of biliary strictures after liver transplantation. Liver Transpl. 2001;7:540-545. [PubMed] [Cited in This Article: ] |
| 115. | Buis CI, Verdonk RC, Van der Jagt EJ, van der Hilst CS, Slooff MJ, Haagsma EB, Porte RJ. Nonanastomotic biliary strictures after liver transplantation, part 1: Radiological features and risk factors for early vs. late presentation. Liver Transpl. 2007;13:708-718. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 156] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 116. | Welling TH, Heidt DG, Englesbe MJ, Magee JC, Sung RS, Campbell DA, Punch JD, Pelletier SJ. Biliary complications following liver transplantation in the model for end-stage liver disease era: effect of donor, recipient, and technical factors. Liver Transpl. 2008;14:73-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 117. | Gong J, Lao XJ, Wang XM, Long G, Jiang T, Chen S. Preservation of non-heart-beating donor livers in extracorporeal liver perfusion and histidine-trytophan-ketoglutarate solution. World J Gastroenterol. 2008;14:2338-2342. [PubMed] [Cited in This Article: ] |
| 118. | Moench C, Moench K, Lohse AW, Thies J, Otto G. Prevention of ischemic-type biliary lesions by arterial back-table pressure perfusion. Liver Transpl. 2003;9:285-289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 119. | Moench C, Heimann A, Foltys D, Schneider B, Minouchehr S, Schwandt E, Knaak M, Kempski O, Otto G. Flow and pressure during liver preservation under ex situ and in situ perfusion with University of Wisconsin solution and histidine-tryptophan-ketoglutarate solution. Eur Surg Res. 2007;39:175-181. [PubMed] [Cited in This Article: ] |
| 120. | Sankary HN, McChesney L, Frye E, Cohn S, Foster P, Williams J. A simple modification in operative technique can reduce the incidence of nonanastomotic biliary strictures after orthotopic liver transplantation. Hepatology. 1995;21:63-69. [PubMed] [Cited in This Article: ] |
| 121. | Demetris AJ, Fontes P, Lunz JG, Specht S, Murase N, Marcos A. Wound healing in the biliary tree of liver allografts. Cell Transplant. 2006;15 Suppl 1:S57-S65. [PubMed] [Cited in This Article: ] |
| 122. | Pastacaldi S, Teixeira R, Montalto P, Rolles K, Burroughs AK. Hepatic artery thrombosis after orthotopic liver transplantation: a review of nonsurgical causes. Liver Transpl. 2001;7:75-81. [PubMed] [Cited in This Article: ] |
| 123. | Bekker J, Ploem S, de Jong KP. Early hepatic artery thrombosis after liver transplantation: a systematic review of the incidence, outcome and risk factors. Am J Transplant. 2009;9:746-757. [PubMed] [Cited in This Article: ] |
| 124. | da Silva RF, Raphe R, Felício HC, Rocha MF, Duca WJ, Arroyo PC, Palini GL, Vasquez AM, Miquelin DG, Reis LF. Prevalence, treatment, and outcomes of the hepatic artery stenosis after liver transplantation. Transplant Proc. 2008;40:805-807. [PubMed] [Cited in This Article: ] |
| 125. | Merion RM, Pelletier SJ, Goodrich N, Englesbe MJ, Delmonico FL. Donation after cardiac death as a strategy to increase deceased donor liver availability. Ann Surg. 2006;244:555-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 126. | Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, Greenstein SM, Merion RM. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783-790. [PubMed] [Cited in This Article: ] |
| 127. | Mathur AK, Heimbach J, Steffick DE, Sonnenday CJ, Goodrich NP, Merion RM. Donation after cardiac death liver transplantation: predictors of outcome. Am J Transplant. 2010;10:2512-2519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 175] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 128. | Nickkholgh A, Weitz J, Encke J, Sauer P, Mehrabi A, Büchler MW, Schmidt J, Schemmer P. Utilization of extended donor criteria in liver transplantation: a comprehensive review of the literature. Nephrol Dial Transplant. 2007;22 Suppl 8:viii29-viii36. [PubMed] [Cited in This Article: ] |
| 129. | Northup PG, Pruett TL, Kashmer DM, Argo CK, Berg CL, Schmitt TM. Donor factors predicting recipient survival after liver retransplantation: the retransplant donor risk index. Am J Transplant. 2007;7:1984-1988. [PubMed] [Cited in This Article: ] |
| 130. | Tsukamoto S, Ohkohchi N, Fukumori T, Satomi S. Microcirculatory disturbance is an obstacle to liver transplantation from agonal NHBD. Transplant Proc. 1999;31:1091-1093. [PubMed] [Cited in This Article: ] |
| 131. | Richter S, Yamauchi J, Minor T, Menger MD, Vollmar B. Heparin and phentolamine combined, rather than heparin alone, improves hepatic microvascular procurement in a non-heart-beating donor rat-model. Transpl Int. 2000;13:225-229. [PubMed] [Cited in This Article: ] |
| 132. | St Peter SD, Moss AA, Mulligan DC. Effects of tacrolimus on ischemia-reperfusion injury. Liver Transpl. 2003;9:105-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 133. | Ikegami T, Nishizaki T, Hiroshige S, Ohta R, Yanaga K, Sugimachi K. Experimental study of a type 3 phosphodiesterase inhibitor on liver graft function. Br J Surg. 2001;88:59-64. [PubMed] [Cited in This Article: ] |
| 134. | Xu HS, Stevenson WC, Pruett TL, Jones RS. Donor lazaroid pretreatment improves viability of livers harvested from non-heart-beating rats. Am J Surg. 1996;171:113-116; discussion 116-117. [PubMed] [Cited in This Article: ] |
| 135. | Manika A, Trinh T, Lagacé G, Dugas MA, Proulx F, Lepage G, Champagne J, Lavoie JC, Cousineau J, Russo P. N-acetylcysteine in pig liver transplantation from non-heart-beating donors. Transplantation. 1999;68:327-330. [PubMed] [Cited in This Article: ] |
| 136. | Takada Y, Taniguchi H, Fukunaga K, Yuzawa K, Otsuka M, Todoroki T, Iijima T, Fukao K. Prolonged hepatic warm ischemia in non-heart-beating donors: protective effects of FK506 and a platelet activating factor antagonist in porcine liver transplantation. Surgery. 1998;123:692-698. [PubMed] [Cited in This Article: ] |
| 137. | Lee CY, Jain S, Duncan HM, Zhang JX, Jones JW, Southard JH, Clemens MG. Survival transplantation of preserved non-heart-beating donor rat livers: preservation by hypothermic machine perfusion. Transplantation. 2003;76:1432-1436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 138. | Matsuno N, Uchiyama M, Iwamoto H, Hama K, Narumi Y, Kikuchi K, Degawa H, Kozaki K, Nagao T. Machine perfusion preservation for liver transplantation from non-heart-beating donors with agonal stage. Transplant Proc. 2002;34:2610-2611. [PubMed] [Cited in This Article: ] |
| 139. | Xu H, Lee CY, Clemens MG, Zhang JX. Pronlonged hypothermic machine perfusion preserves hepatocellular function but potentiates endothelial cell dysfunction in rat livers. Transplantation. 2004;77:1676-1682. [PubMed] [Cited in This Article: ] |
| 140. | Manekeller S, Schuppius A, Stegemann J, Hirner A, Minor T. Role of perfusion medium, oxygen and rheology for endoplasmic reticulum stress-induced cell death after hypothermic machine preservation of the liver. Transpl Int. 2008;21:169-177. [PubMed] [Cited in This Article: ] |
| 141. | Minor T, Manekeller S, Sioutis M, Dombrowski F. Endoplasmic and vascular surface activation during organ preservation: refining upon the benefits of machine perfusion. Am J Transplant. 2006;6:1355-1366. [PubMed] [Cited in This Article: ] |
| 142. | Lüer B, Koetting M, Efferz P, Minor T. Role of oxygen during hypothermic machine perfusion preservation of the liver. Transpl Int. 2010;23:944-950. [PubMed] [Cited in This Article: ] |
| 143. | Saad S, Minor T. Short-term resuscitation of predamaged donor livers by brief machine perfusion: the influence of temperature. Transplant Proc. 2008;40:3321-3326. [PubMed] [Cited in This Article: ] |
| 144. | Guarrera JV, Henry SD, Chen SW, Brown T, Nachber E, Arrington B, Boykin J, Samstein B, Brown RS, Emond JC. Hypothermic machine preservation attenuates ischemia/reperfusion markers after liver transplantation: preliminary results. J Surg Res. 2011;167:e365-e373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 145. | Lauschke H, Olschewski P, Tolba R, Schulz S, Minor T. Oxygenated machine perfusion mitigates surface antigen expression and improves preservation of predamaged donor livers. Cryobiology. 2003;46:53-60. [PubMed] [Cited in This Article: ] |
| 146. | Iwamoto H, Matsuno N, Narumi Y, Uchiyama M, Kozaki K, Degawa H, Hama K, Kikuchi K, Takeuchi H, Kozaki M. Beneficial effect of machine perfusion preservation on liver transplantation from non-heart-beating donors. Transplant Proc. 2000;32:1645-1646. [PubMed] [Cited in This Article: ] |
| 147. | Manekeller S, Minor T. Possibility of conditioning predamaged grafts after cold storage: influences of oxygen and nutritive stimulation. Transpl Int. 2006;19:667-674. [PubMed] [Cited in This Article: ] |
| 148. | Manekeller S, Seinsche A, Stegemann J, Hirner A. Optimising post-conditioning time of marginal donor livers. Langenbecks Arch Surg. 2008;393:311-316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 149. | St Peter SD, Imber CJ, Lopez I, Hughes D, Friend PJ. Extended preservation of non-heart-beating donor livers with normothermic machine perfusion. Br J Surg. 2002;89:609-616. [PubMed] [Cited in This Article: ] |
| 150. | Reddy S, Greenwood J, Maniakin N, Bhattacharjya S, Zilvetti M, Brockmann J, James T, Pigott D, Friend P. Non-heart-beating donor porcine livers: the adverse effect of cooling. Liver Transpl. 2005;11:35-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 151. | Reddy SP, Bhattacharjya S, Maniakin N, Greenwood J, Guerreiro D, Hughes D, Imber CJ, Pigott DW, Fuggle S, Taylor R. Preservation of porcine non-heart-beating donor livers by sequential cold storage and warm perfusion. Transplantation. 2004;77:1328-1332. [PubMed] [Cited in This Article: ] |
| 152. | Vogel T, Brockmann JG, Friend PJ. Ex-vivo normothermic liver perfusion: an update. Curr Opin Organ Transplant. 2010;15:167-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 153. | Guarrera JV, Henry SD, Samstein B, Odeh-Ramadan R, Kinkhabwala M, Goldstein MJ, Ratner LE, Renz JF, Lee HT, Brown RS. Hypothermic machine preservation in human liver transplantation: the first clinical series. Am J Transplant. 2010;10:372-381. [PubMed] [Cited in This Article: ] |
| 154. | Tojimbara T, Wicomb WN, Garcia-Kennedy R, Burns W, Hayashi M, Collins G, Esquivel CO. Liver transplantation from non-heart beating donors in rats: influence of viscosity and temperature of initial flushing solutions on graft function. Liver Transpl Surg. 1997;3:39-45. [PubMed] [Cited in This Article: ] |
| 155. | Minor T. Gaseous oxygen to improve viability of marginal or pre-damaged organ grafts during hypothermic storage. Organ donation and transplantion after cardiac death. Oxford, New York: Oxford University Press 2009; 131-151. [Cited in This Article: ] |
| 156. | Minor T, Saad S, Nagelschmidt M, Kötting M, Fu Z, Paul A, Isselhard W. Successful transplantation of porcine livers after warm ischemic insult in situ and cold preservation including postconditioning with gaseous oxygen. Transplantation. 1998;65:1262-1264. [PubMed] [Cited in This Article: ] |
| 157. | Tolba RH, Akbar S, Puetz U, Minor T. Use and limitations of reconditioning ischemically damaged livers from non-heart-beating donors by venous oxygen persufflation. Transplant Proc. 2000;32:2118-2119. [PubMed] [Cited in This Article: ] |
| 158. | Treckmann J, Minor T, Saad S, Ozcelik A, Malagó M, Broelsch CE, Paul A. Retrograde oxygen persufflation preservation of human livers: a pilot study. Liver Transpl. 2008;14:358-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 159. | Minor T, Saad S, Kötting M, Nagelschmidt M, Isselhard W. Short-term restoration by gaseous oxygen for long-term preservation of predamaged livers from non-heart-beating donors. Transplant Proc. 1997;29:3474-3475. [PubMed] [Cited in This Article: ] |
| 160. | Koetting M, Leuvenink H, Dombrowski F, Minor T. Gaseous persufflation with carbon monoxide during ischemia protects the isolated liver and enhances energetic recovery. Cryobiology. 2010;61:33-37. [PubMed] [Cited in This Article: ] |
| 161. | Koetting M, Dombrowski F, Minor T. No synergistic effect of carbon monoxide and oxygen during static gaseous persufflation preservation of DCD livers. J Surg Res. 2011;171:859-864. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 162. | Monbaliu D, Vekemans K, Hoekstra H, Vaahtera L, Libbrecht L, Derveaux K, Parkkinen J, Liu Q, Heedfeld V, Wylin T. Multifactorial biological modulation of warm ischemia reperfusion injury in liver transplantation from non-heart-beating donors eliminates primary nonfunction and reduces bile salt toxicity. Ann Surg. 2009;250:808-817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 163. | Koetting M, Stegemann J, Minor T. Dopamine as additive to cold preservation solution improves postischemic integrity of the liver. Transpl Int. 2010;23:951-958. [PubMed] [Cited in This Article: ] |
| 164. | Minor T, Hachenberg A, Tolba R, Pauleit D, Akbar S. Fibrinolytic preflush upon liver retrieval from non-heart beating donors to enhance postpreservation viability and energetic recovery upon reperfusion. Transplantation. 2001;71:1792-1796. [PubMed] [Cited in This Article: ] |
| 165. | Song SW, Tolba RH, Yonezawa K, Manekeller S, Minor T. Exogenous superoxide dismutase prevents peroxynitrite-induced apoptosis in non-heart-beating donor livers. Eur Surg Res. 2008;41:353-361. [PubMed] [Cited in This Article: ] |
| 166. | Kobayashi Y, Akamatsu Y, Iwane T, Nakamura A, Satomi S. Signaling pathway on the effect of oxygenated warm perfusion prior to cold preservation of the liver grafts from non-heart-beating donors, and the additive effect of edaravone. Transplant Proc. 2009;41:49-51. [PubMed] [Cited in This Article: ] |
| 167. | Minor T, Akbar S. Enhancement of endogenous cyclic AMP signal: a new approach to allow for cold preservation of rat livers from non-heart-beating donors? Transplantation. 1998;66:990-994. [PubMed] [Cited in This Article: ] |
| 168. | Kootstra G. The asystolic, or non-heartbeating, donor. Transplantation. 1997;63:917-921. [PubMed] [Cited in This Article: ] |
| 169. | Wilson C. Perfusate development for the NHBD. Organ donation and transplantation after cardiac death. Oxford, New York: Oxford University Press 2009; 67-102. [Cited in This Article: ] |
| 170. | López-Boado MA, García-Valdecasas JC, Ordi J, Tabet J, Net M, Cifuentes A, Rull R, González FX, Valero R, Deulofeu R. Histological changes during and after liver transplantation from non-heart-beating donor pig. Transplant Proc. 1997;29:3471. [PubMed] [Cited in This Article: ] |
| 171. | Monbaliu D, Libbrecht L, De Vos R, Vekemans K, Walter H, Liu Q, Heedfeld V, Goossens V, Pirenne J, Roskams T. The extent of vacuolation in non-heart-beating porcine donor liver grafts prior to transplantation predicts their viability. Liver Transpl. 2008;14:1256-1265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 172. | Muiesan P. Liver transplantation using non heart beating donors. Organ donation and transplantation after cardiac death. Oxford, New York: Oxford University Press 2009; 213-229. [Cited in This Article: ] |
| 173. | Derveaux K, Monbaliu D, Crabbé T, Schein D, Brassil J, Kravitz D, Fevery J, Jacobbi L, Roskams T, Pirenne J. Does ex vivo vascular resistance reflect viability of non-heart-beating donor livers? Transplant Proc. 2005;37:338-339. [PubMed] [Cited in This Article: ] |
| 174. | Monbaliu D, Vekemans K, De Vos R, Brassil J, Heedfeld V, Qiang L, D'hollander M, Roskams T, Pirenne J. Hemodynamic, biochemical, and morphological characteristics during preservation of normal porcine livers by hypothermic machine perfusion. Transplant Proc. 2007;39:2652-2658. [PubMed] [Cited in This Article: ] |
| 175. | Vekemans K, Liu Q, Pirenne J, Monbaliu D. Artificial circulation of the liver: machine perfusion as a preservation method in liver transplantation. Anat Rec (Hoboken). 2008;291:735-740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 176. | Monbaliu D, Brassil J. Machine perfusion of the liver: past, present and future. Curr Opin Organ Transplant. 2010;15:160-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 177. | Liu Q, Monbaliu D, Vekemans K, Peeters R, De Keyzer F, Dresselaers T, Ni Y, Van Hecke P, Komuta M, Brassil J. Can apparent diffusion coefficient discriminate ischemic from nonischemic livers? A pilot experimental study. Transplant Proc. 2007;39:2643-2646. [PubMed] [Cited in This Article: ] |