Published online Mar 14, 2013. doi: 10.3748/wjg.v19.i10.1625
Revised: December 25, 2012
Accepted: January 11, 2013
Published online: March 14, 2013
AIM: To investigate the therapeutic efficacy and safety of continuous autotransfusion system (CATS) during liver transplantation of hepatocellular carcinoma patients.
METHODS: Eighty-three hepatocellular carcinoma (HCC) patients who underwent liver transplantation with intraoperative CATS (n = 24, CATS group) and without (n = 59, non-CATS group) between April 2006 and November 2011 at the Liver Transplant Institute of Inonu University were analyzed retrospectively. Postoperative HCC recurrence was monitored by measuring alpha-fetoprotein (AFP) levels at 3-mo intervals and performing imaging analysis by thoracoabdominal multidetector computed tomography at 6-month intervals. Inter-group differences in recurrence and correlations between demographic, clinical, and pathological data were assessed by ANOVA and χ2 tests. Overall and disease-free survivals were calculated by the univariate Kaplan-Meier method.
RESULTS: Of the 83 liver transplanted HCC patients, 89.2% were male and the overall mean age was 51.3 ± 8.9 years (range: 18-69 years). The CATS and non-CATS groups showed no statistically significant differences in age, sex ratio, body mass index, underlying disease, donor type, graft-to-recipient weight ratio, Child-Pugh and Model for End-Stage Liver Disease scores, number of tumors, tumor size, AFP level, Milan and University of California San Francisco selection criteria, tumor differentiation, macrovascular invasion, median hospital stay, recurrence rate, recurrence site, or mortality rate. The mean follow-up time of the non-CATS group was 17.9 ± 12.8 mo, during which systemic metastasis and/or locoregional recurrence developed in 25.4% of the patients. The mean follow-up time for the CATS group was 25.8 ± 15.1 mo, during which systemic metastasis and/or locoregional recurrence was detected in 29.2% of the patients. There was no significant difference between the CATS and non-CATS groups in recurrence rate or site. Additionally, no significant differences existed between the groups in overall or disease-free survival.
CONCLUSION: CATS is a safe procedure and may decrease the risk of tumor recurrence in HCC patients.
- Citation: Akbulut S, Kayaalp C, Yilmaz M, Ince V, Ozgor D, Karabulut K, Eris C, Toprak HI, Aydin C, Yilmaz S. Effect of autotransfusion system on tumor recurrence and survival in hepatocellular carcinoma patients. World J Gastroenterol 2013; 19(10): 1625-1631
- URL: https://www.wjgnet.com/1007-9327/full/v19/i10/1625.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i10.1625
Since the first report of a successful human liver transplantation (LT) in 1963, this procedure has become the gold standard treatment for end-stage liver diseases, including acute liver failure, some metabolic liver diseases, primary liver cancers [such as hepatocellular carcinoma (HCC)], and some metastatic liver tumors[1-3]. Despite its widespread application, intraoperative hemorrhaging remains a significant limitation of the procedure[1,4]. The liver is directly connected to three different vascular systems, all of which may be weakened by the underlying disease’s pathogenic processes. Serious collateral circulation damage can accompany chronic liver disease and parallel severity of the disease status, increasing the risk of hemorrhage during LT surgery.
Clinical studies have identified the most important factors influencing development of major hemorrhage during surgery as adhesions between cirrhotic liver and surrounding structures, impaired clotting factor synthesis, inherent hemorrhage tendency of some liver diseases, retransplantation, portal vein thrombosis, and the experience level of the involved hepatobiliary surgical team[3,5-7]. Moreover, the extent of any hemorrhage that develops during surgery has been defined as one of the most significant factors affecting patient morbidity and mortality[6,8]. Suitable and balanced blood replacement is vital for patients undergoing major surgery. Unfortunately, the exogenous blood transfusion methods remain inadequate for resolving major blood loss that occurs during surgery. Various intraoperative blood salvage autotransfusion systems have been developed to overcome this clinical challenge, and are based upon re-use of the patient’s own blood that has accumulated at the surgical site[4,9].
Intraoperative blood salvage autotransfusion (IBSA) systems have emerged as cost-effective intraoperative tools that reduce the blood requirement by up to 60%[5]. However, some studies have indicated that the IBSA systems may cause cancer cell dissemination when used during cancer surgery[5], thereby increasing a patient’s risk for metastasis or recurrence. These results have not been upheld by other studies[4] and the therapeutic efficacy and safety of IBSA systems remain to be definitely established. The IBSA system employed in our healthcare institute is the continuous autotransfusion system (CATS) manufactured by Fresenius Kabi AG (Bad Homburg, Germany). To gain a better understanding of the therapeutic efficacy and safety of CATS during LT, this research study was designed to retrospectively analyze groups of HCC patients who underwent LT with and without CATS to determine postoperative rates of tumor recurrence and survival.
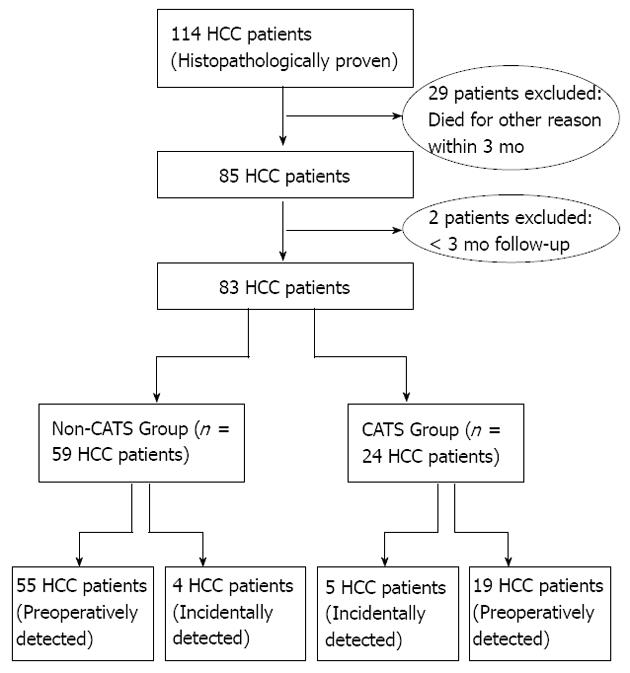
Between April 2006 and November 2011, a total of 702 LTs were performed for 664 patients at the Liver Transplant Institute of Inonu University. Histopathological evaluation of the resected hepatectomy specimens revealed HCC foci of varying sizes and numbers for 17.1% (114/702) of the LT recipients. Of those, 28.1% (32/114) were considered ineligible for this study based upon insufficient follow-up data (< 3 mo; n = 2) and other-cause mortality within 3 mo after the LT (n = 29). From the remaining cohort of LT HCC patients, only those receiving primary liver transplants were selected for study inclusion. These 83 patients were then divided into two groups according to use of CATS during the LT surgery: CATS group, n = 24; non-CATS group, n = 59. The study participant selection process is outlined in Figure 1.
The following data were recorded for comparative analysis between the CATS and non-CATS groups: age, sex, body mass index (BMI; in kg/m2), underlying disease [hepatitis B virus (HBV) infection, hepatitis C virus (HCV) infection, or primary HCC], donor type (living/deceased), graft-to-recipient weight ratio (GRWR), Child-Pugh score, model for end-stage liver disease (MELD) score, number of tumors (< 10 or > 10), mean tumor size (in cm), Milan selection criteria (within/beyond), University of California San Francisco (UCSF) selection criteria (within/beyond), preoperative alpha-fetoprotein (AFP) levels (< 200 or > 200 ng/mL)[10], tumor differentiation (good/average/poor), macrovascular invasion (positive/negative), systemic metastasis and locoregional recurrence (positive/negative), recurrence site (hepatic and/or extrahepatic), current status (alive/died), and duration of follow-up (in mo).
The LT patients had been classified according to the Milan and UCSF criteria, both of which consider the diameter and number of HCC lesions observed by pathological examination to identify individuals likely to benefit from and survive the LT procedure. Specifically, patients with a single lesion of ≤ 5 cm diameter or with three or less lesions for which the largest was ≤ 3 cm were defined as ‘within’ Milan criteria, whereas patients with a single lesion of ≤ 6.5 cm or with three or less nodules for which the largest was ≤ 4.5 cm and the total tumor diameter was ≤ 8 cm were defined as ‘within’ UCSF criteria[2,11]. Donor type (deceased or living) was based on recommendations of the Milan group and the criteria accepted by the United Network of Organ Sharing. HCC cases within the Milan criteria (n = 30) were first given the chance of deceased-donor liver transplantation (DDLT; n = 3), whereas HCC cases beyond the Milan criteria were given living-donor liver transplantation (LDLT; n = 80).
In the LDLT procedure, venous drainage was facilitated by creating a wide-orifice venous drainage model using a saphenous vein graft followed by a wide-aperture anastomosis created between the wide-orifice hepatic vein and inferior vena cava. In the DDLT procedure, a hepato-caval anastomosis was created by using the standard piggyback technique. Anastomoses of other vascular structures and the biliary tract were created by standard techniques.
In the early postoperative period, all known HCC cases were given the immunosuppressive regimen of low-dose sirolimus, steroids, and mycophenolate mofetil. The postoperative pharmaceutical regimen for non-HCC patients who received LT for end-stage liver disease included the calcineurin inhibitor; however, nine of those patients were subsequently diagnosed with HCC by pathology and were immediately switched to sirolimus treatment.
All patients were followed-up with measurement of AFP levels at 3-mo intervals and thoracoabdominal multidetector computed tomography (MDCT) imaging at 6-mo intervals. Suspicion of tumor recurrence led to shorter interval monitoring. Sixty-nine patients with chronic liver disease showed enhanced AFP values during follow-up and were monitored by dynamic MDCT. Five additional cases of primary HCC showed enhanced AFP levels by other tests carried out for symptoms unrelated to clinical liver disease and were then monitored by dynamic MDCT. HCC recurrence was indicated when dynamic MDCT scan images showed heterogeneous contrast with the arterial phase, evidenced by hypodense or isodense regions in the normal liver parenchyma of the portal phase, and contrast enhancement extending to the capsule in the late phase. In cases of suspected HCC, ultrasonography or CT-guided biopsy was performed, depending on mass location. A total of 74 patients were diagnosed with HCC during follow-up.
HCC recurrence was diagnosed by enhanced blood AFP levels, MDCT tumor detection, and/or biopsy-detected cancer of the same cell type as the originally resected HCC. Recurrence was categorized as either systemic metastasis (in a different organ system with no other identifiable cause) or locoregional (within the transplanted liver and/or perihepatic lymph node chain). Overall survival was defined as the time interval from LT to death from any cause, or to the last outpatient clinical follow-up for censored patients. Disease-free survival was defined as the time from LT to HCC recurrence or death from any cause.
Statistical analyses were performed by the SPSS software package (version 13.0; SPSS, Chicago, IL, United States). Categorical data were analyzed by the χ2 test. Continuous data were analyzed by the ANOVA test. Overall and disease-free survivals were calculated by the univariate Kaplan-Meier method. Statistical significance was indicated by P < 0.05. Data are expressed as mean ± SD errors of the mean (SEM).
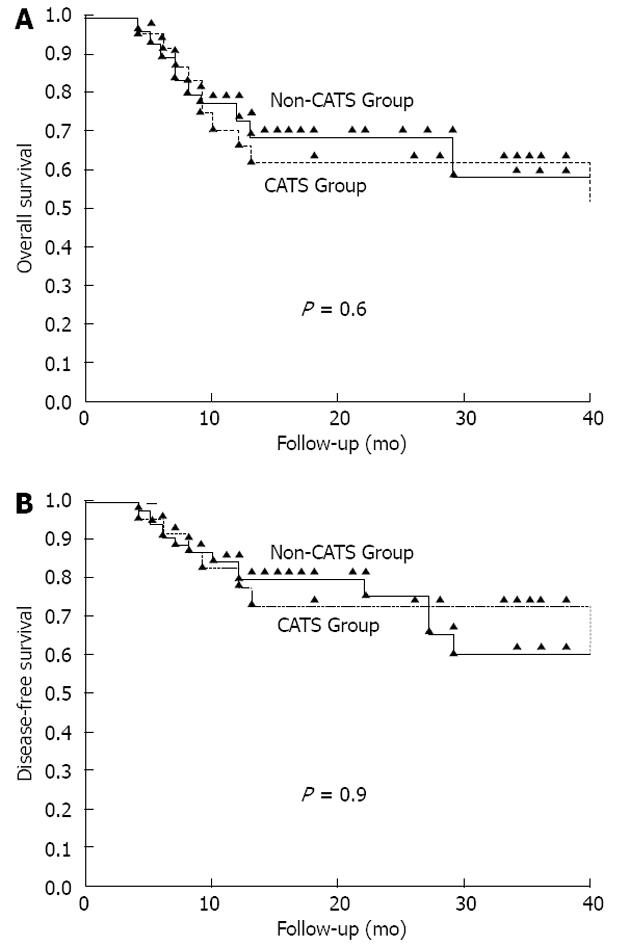
Eighty-three HCC patients who underwent LT were analyzed. The male:female ratio was strongly biased (74: 9). The mean age was 51.3 ± 8.9 years (range: 18-69). The CATS procedure was used in only 28.9% of the LT patients (CATS group, n = 4; non-CATS group, n = 59). No significant differences were identified between the two groups in terms of age, sex, BMI, underlying disease, donor type, GRWR, Child-Pugh score, MELD score, tumor count, tumor size, AFP levels, Milan criteria, UCSF criteria, tumor differentiation, macrovascular invasion, presence of recurrence, recurrence site, mortality rate, or duration of hospital stay. Clinical and demographic data of both groups are shown in Table 1. The tumor characteristics of all patients are shown in Table 2. There was no difference between the groups for disease-free (P = 0.9) or overall (P = 0.06) survival (Figure 2).
| Patient Characteristics | CATS group (n = 24) | Non-CATS group (n = 59) | P |
| Sex | |||
| Male | 22 (91.7) | 52 (88.1) | 0.6 |
| Female | 2 (8.3) | 7 (11.9) | |
| Age, yr | |||
| mean ± SE | 52.0 ± 1.8 | 51.0 ± 1.2 | 0.7 |
| Median (range) | 54 (37-67) | 53 (18-69) | |
| BMI, kg/m2 | 25.5 ± 0.8 | 25.1 ± 0.6 | 0.8 |
| Underlying disease | |||
| HBV | 18 (75) | 39 (66) | 0.2 |
| HBV + HCV | 0 | 1 (1.7) | |
| HBV + HDV | 5 (20.8) | 9 (15.3) | |
| HCV | 0 | 5 (8.5) | |
| Primary HCC | 0 | 5 (8.5) | |
| Wilson | 1 (4.2) | 0 | |
| Donor type | |||
| Living | 24 (100) | 56 (95) | 0.1 |
| Deceased | 0 | 3 (5) | |
| GRWR | 1.10 ± 0.06 | 1.20 ± 0.05 | 0.9 |
| Child score | |||
| A | 6 (25.0) | 18 (30.5) | 0.7 |
| B | 11 (45.8) | 29 (49.2) | |
| C | 7 (29.2) | 12 (20.3) | |
| MELD score | 14.5 ± 0.9 | 13.6 ± 0.8 | |
| Current status | |||
| Alive | 13 (54.2) | 41 (69.5) | 0.2 |
| Mortality | 11 (45.8) | 18 (30.5) | |
| Hospital stay, d (range) | 35.0 ± 13.8 (14-69) | 33.0 ± 38.2 (7-274) | 0.6 |
| Follow-up, mo (range) | 25.8 ± 15.1 (4-53) | 17.9 ± 12.8 (4-56) |
| Tumor characteristics | CATS group (n = 24) | Non-CATS group (n = 59) | P value |
| Tumor count | |||
| < 10 | 17 (70.8) | 47 (79.6) | 0.4 |
| > 10 | 7 (29.2) | 12 (20.3) | |
| Mean tumor size, cm | 5.8 ± 0.9 | 5.2 ± 0.5 | 0.7 |
| Milan criteria | |||
| Within | 8 (33.3) | 22 (37.3) | 0.7 |
| Beyond | 16 (66.6) | 37 (62.7) | |
| UCSF criteria | |||
| Within | 9 (37.5) | 29 (49.2) | 0.3 |
| Beyond | 15 (62.5) | 30 (50.8) | |
| AFP levels | |||
| < 200 | 17 (70.8) | 43 (73) | 0.7 |
| > 200 | 7 (29.2) | 16 (27) | |
| Tumor differentiation | |||
| Good | 12 (50.0) | 24 (41.0) | 0.6 |
| Average | 9 (37.5) | 23 (39.0) | |
| Poor | 3 (12.5) | 12 (20.0) | |
| Macrovascular invasion | |||
| Present | 2 (8.4) | 3 (5.0) | 0.6 |
| Absent | 22 (91.6) | 56 (95.0) | |
| HCC recurrence | |||
| Present | 7 (29.2) | 15 (25.4) | 0.7 |
| Absent | 17 (70.8) | 44 (74.6) | |
| Recurrence and/or metastasis | |||
| Hepatic | 2 | 3 | 0.8 |
| Extrahepatic | 1 | 2 | |
| Both | 4 | 10 | |
| Recurrence site | |||
| Liver | 2 | 3 | |
| Lung | 0 | 2 | |
| Liver + bone | 1 | 0 | |
| Liver + lung | 1 | 1 | |
| Liver + stomach | 1 | 0 | |
| Liver + bone + lung | 1 | 0 | |
| Lung + surrenal gland | 1 | 0 | |
| Liver + surrenal gland | 0 | 4 | |
| Liver + surrenal gland + lung | 0 | 2 | 0 |
| Liver + esophagus | 0 | 1 | |
| Liver + lung + brain | 0 | 1 | |
| Liver + surrenal gland + peritoneum | 0 | 1 |
The mean follow-up time for the CATS group was 25.8 ± 15.1 mo (range: 4-53), during which systemic metastasis and/or locoregional recurrence was detected in 29.2% (7/24) of the patients. Four of these patients experienced locoregional recurrence together with distant organ metastasis. One patient developed distant organ metastasis only, and the remaining two patients developed locoregional recurrence only. The mean follow-up time of the non-CATS group was 17.9 ± 12.8 mo (range: 4-56 mo), during which systemic metastasis and/or locoregional recurrence developed in 25.4% (15/59) of the patients. Ten patients experienced locoregional recurrence together with distant organ metastasis. Two patients developed distant organ metastasis only, and the remaining three patients developed locoregional recurrence only. There was no significant difference between the CATS and non-CATS groups in recurrence rate (P < 0.7) or site (P < 0.8) (Table 2).
IBSA collects a patient’s own blood accumulated at a surgical site for re-use after aspiration, washing, filtration, and reinfusion[9,12-15]. Following the first description of an IBSA system, the Bentley autotransfusion device, the Haemonetics Co. manufactured an IBSA-based device for washing and concentrating of erythrocytes; known as the Cell Saver (Haemonitics, Braintree, MA, United States), this system became commercially available in 1974. Nearly two decades later, the CATS was produced by the Fresenius Kabi Co. and has since been successfully applied in clinical practice.
Correlations of IBSA with postoperative complications, tumor recurrence, and mortality of patients receiving either homologous (allogenic) or autologous blood transfusion have been extensively studied, but the results have been inconsistent[16]. Some studies have shown increased infection rates, delayed wound healing, and increased mortality, depending on the amount of blood used following homologous transfusion. Similarly, increased rates of tumor recurrence and mortality were reported for patients who underwent homologous transfusion. Further study has suggested this situation is associated with suppression of natural killer cells and cytotoxic T-cells, and activation of T-suppressor cells[7,13,16-18]. Some studies have also demonstrated that the infectious and non-infectious complications associated with homologous blood transfusion occur less frequently with autologous blood transfusion[13,15,17]. The autologous transfusion strategy boasts other advantages as well, including: lower volume requirement for exogenous blood, which increases cost-effectiveness; higher oxygen-carrying capacity, which accelerates wound healing; an immunostimulant effect, which reduces tumor recurrence and increases patient survival rates[5,7,13-15,18,19].
Although positive results have been obtained with autologous transfusion in patients undergoing tumor surgery, the possibility that tumor dissemination may be caused by the IBSA transfusion method remains a significant concern[17,20]. This potential risk was first reported by Yaw et al[15], who demonstrated the ability of tumor cells to pass through the filter of the Bentley autotransfusion device. Subsequently, the American Medical Association designated IBSA systems as unsuitable for use in cancer surgery[13,17]. However, this restriction was later modified by the National Institute of Clinical Excellence, which indicated that IBSA systems could be used in cancer surgery when combined with leukocyte depletion filters (LDFs)[13,19]. Since then, the Association of Anaesthetists of Great Britain and Ireland, Obstetric Anaesthetists Association, American College of Obstetricians and Gynecologists, and British Confidential Enquiry of Maternal and Child Health, have confirmed the clinical utility and safety of various IBSA systems alone or integrated with LDF in cancer surgery.
The most comprehensive study of IBSA systems in cancer surgery was conducted as a meta-analysis of 10 published studies of 2326 prostate, liver, cervical, and gastrointestinal cancer patients[12]. The results showed that IBSA did not constitute a risk in terms of tumor recurrence and metastasis. Yet, it has been shown in many studies that 91%-100% of tumor cells passing through the IBSA system filter reach the reinfusion bag[1,9,13,14,16,17,19-21]; therefore, LDF should be integrated into the IBSA systems.
Catling et al[17] showed that the Cell Saver filter failed to remove 91.2% of tumor cells but that integration of LDF resolved this completely. Another study of patients undergoing LT for HCC showed that the Cell Saver filter system alone removed only 25% of tumor cells, but 93.3% of tumor cells when integrated with an LDF[1]. However, the IBSA CATS system used in our study was not integrated with LDF and was not associated with increased tumor recurrence. This difference may reflect the pore diameters of the different IBSA systems’ filters; for example, the pore diameter range of the Cell Saver’s filter is 20-150 μm, whereas the CATS machine’s filter is 40-120 μm. The different cancer cells types examined in the different studies may also influence the finding. Cervical cancer cells generally range from 25-65 μm diameter, whereas HCC cells range from 41-55 μm. Thus, certain cancers may require different filters and IBSA systems, integrated or unintegrated, should be applied accordingly.
Most studies of IBSA systems in gastrointestinal surgery have involved the liver and emergency situations[17]. The complicated vascular structure of the liver and the frequency of severely cirrhotic conditions necessitate highly expert surgeons to lessen the high risk of hemorrhage. Nonetheless, massive blood loss remains a common occurrence in liver tumor surgeries involving major resection, and particularly in LT[7]. Two studies have investigated the survival rates associated with IBSA systems in patients undergoing liver resection for HCC. Fujimato et al[22] found equal cumulative overall and disease-free survival rates for patients treated with and without IBSA, and showed that the IBSA system was superior in its requirement for a lower volume of transfused blood. Hirano et al[7] reported better 10-year overall and disease-free survival rates for patients treated with IBSA. These authors also showed that the overall and disease-free survival rates were better for early-stage HCC cases treated with IBSA, but no significant advantage was found when used in advanced-stage HCC cases.
A few studies have reported on IBSA systems in LT. One study by Philips et al[23], in which HCC and sepsis cases had been excluded, use of the Cell Saver during LT reduced the requirement for homologous blood transfusion and was cost-effective. Similarly, Sankarankutty et al[8] showed that use of the Cell Saver during LT reduced the requirement for homologous blood transfusion and lowered the risk of infection. In a study of CATS during LT performed by Massicote et al[24], in which patients with preoperative infection had been excluded, the procedure reduced the requirement for homologous blood transfusion. Finally, Liang et al[3] showed that the Cell Saver integrated with LDF reduced bacterial contamination rates by 90.3%. Thus, IBSA systems exhibit a reduced requirement for homologous blood transfusion, are cost-effective, and have less risk of microbiological contamination.
According to our knowledge, only three studies have investigated the effect on tumor recurrence of IBSA systems used during LC in HCC patients[1,4,5]. Foltys et al[5] reported use of the Cell Saver in 40 of 136 HCC patients undergoing LT showed no influence on recurrence rate, overall survival, or disease-free survival during a mean follow-up period of 1015 d. In another study of the Cell Saver autotransfusion system in 31 of 47 HCC patients undergoing LT, Muscari et al[4] reported no influence on recurrence rates during a mean 34-month follow-up period. Liang et al[1] reported that 15 samples of blood collected in the re-infusion bag by the Cell Saver from the surgical sites of 20 HCC patients undergoing LT contained tumor cells; however, when a two-stage LDF system was used with the Cell Saver process, 14 of those samples were tumor-cell negative. In these studies, cases that were beyond the Milan and UCSF criteria and developed tumor perforation due to manipulation during surgery, the Cell Saver system was shown to be insufficient. It is, thus, presumed that the cell-holding capacity of the filters decreases due to the combined tumor load in the surgical area and that resulting from the IBSA device.
Studies in our laboratory have attempted to determine whether the IBSA system-displaced tumor cells enter systemic circulation and, if so, to what extent and whether their presence promotes metastasis. It is well established that the surgical procedure itself can passage tumor cells into the patient’s systemic circulation. However, once in circulation, these tumor cells have a 0.000 001%-0.01% chance of causing metastasis[9,17,19,20]. Therefore, cells passing into the circulation via the IBSA system are expected to have a less-than-absolute potential to cause metastases. In the current study, there was no difference in postoperative metastasis between the CATS group and the non-CATS group. This finding may reflect the fact that > 50% of the CATS group was beyond the Milan and UCSF criteria. Thus, our results indicate that cancer cells passing into the circulation did not cause metastasis.
This study has some important limitations that may have influenced the results. First, some patients’ records were missing blood transfusion data, making it impossible to determine how much CATS reduced the need for homologous blood transfusion. Second, the retrospective design of the study restricted the data available for analysis. A future study of prospective design may allow for quantitative detection of tumor cells or AFP using a genetic-based procedure, such as polymerase chain reaction; in this way, blood collected from surgical sites and in the re-infusion bag can be compared to a preoperative peripheral blood sample to more directly determine whether the CATS procedure contributed to recurrence, as opposed to preexisting tumor cells in circulation.
In conclusion, our findings suggest that the use of IBSA systems during LT of HCC patients has no effect on tumor recurrence and survival.
Intraoperative continuous autotransfusion system (CATS), a system that involves collecting and washing blood from surgical sites and returning washed blood products to the same patient, has been proven effective for saving blood and reducing hemorrhagic complications during many surgical procedures, such as liver transplantation. However, tumor cell dissemination may be caused by autotransfusion systems when used during cancer surgery.
The authors examined whether the use of CATS during liver transplantation to treat hepatocellular carcinoma (HCC) affected tumor recurrence and mortality rates by retrospectively analyzing tumor development and overall and disease-free survival in 83 HCC patients who underwent liver transplantation with (n = 24) and without (n = 59) CATS.
Kaplan-Meier analysis was used to compare disease-free and overall survival associated with the CATS procedure during liver transplantation to treat HCC. There was no difference observed between the CATS and non-CATS groups in terms of disease-free or overall survival.
Systemic metastasis was defined as metastatic cancer of the same cell type in a different organ system with no other identifiable cause. Locoregional recurrence was defined as recurrent cancer within the transplanted liver or perihepatic lymph node chain, or both. Overall survival was defined as the time interval from liver transplantation to death from any cause or to the last outpatient clinic follow-up visit in censored patients. Disease-free survival was defined as the time from liver transplantation until recurrence of tumor or death from any cause
This article has retrogradely investigated the effects of Intraoperative blood salvage autotransfusion (IBSA) on the survival, recurrence and metastasis of hepatocellular carcinoma patients who underwent liver transplantation. Generally, the study is of interest and useful for evaluating IBSA on liver transplantation of HCC patients.
P- Reviewers Sun X, Uygun K S- Editor Huang XZ L- Editor A E- Editor Lu YJ
| 1. | Liang TB, Li DL, Liang L, Li JJ, Bai XL, Yu W, Wang WL, Shen Y, Zhang M, Zheng SS. Intraoperative blood salvage during liver transplantation in patients with hepatocellular carcinoma: efficiency of leukocyte depletion filters in the removal of tumor cells. Transplantation. 2008;85:863-869. [PubMed] [Cited in This Article: ] |
| 2. | Alqahtani SA. Update in liver transplantation. Curr Opin Gastroenterol. 2012;28:230-238. [PubMed] [Cited in This Article: ] |
| 3. | Liang TB, Li JJ, Li DL, Liang L, Bai XL, Zheng SS. Intraoperative blood salvage and leukocyte depletion during liver transplantation with bacterial contamination. Clin Transplant. 2010;24:265-272. [PubMed] [Cited in This Article: ] |
| 4. | Muscari F, Suc B, Vigouroux D, Duffas JP, Migueres I, Mathieu A, Lavayssiere L, Rostaing L, Fourtanier G. Blood salvage autotransfusion during transplantation for hepatocarcinoma: does it increase the risk of neoplastic recurrence? Transpl Int. 2005;18:1236-1239. [PubMed] [Cited in This Article: ] |
| 5. | Foltys D, Zimmermann T, Heise M, Kaths M, Lautem A, Wisser G, Weiler N, Hoppe-Lotichius M, Hansen T, Otto G. Liver transplantation for hepatocellular carcinoma--is there a risk of recurrence caused by intraoperative blood salvage autotransfusion? Eur Surg Res. 2011;47:182-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Hendriks HG, van der Meer J, Klompmaker IJ, Choudhury N, Hagenaars JA, Porte RJ, de Kam PJ, Slooff MJ, de Wolf JT. Blood loss in orthotopic liver transplantation: a retrospective analysis of transfusion requirements and the effects of autotransfusion of cell saver blood in 164 consecutive patients. Blood Coagul Fibrinolysis. 2000;11 Suppl 1:S87-S93. [PubMed] [Cited in This Article: ] |
| 7. | Hirano T, Yamanaka J, Iimuro Y, Fujimoto J. Long-term safety of autotransfusion during hepatectomy for hepatocellular carcinoma. Surg Today. 2005;35:1042-1046. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Sankarankutty AK, Teixeira AC, Souza FF, Mente ED, Oliveira GR, Almeida RC, Andrade CM, Origuella EA, Silva Ode C. Impact of blood salvage during liver transplantation on reduction in transfusion requirements. Acta Cir Bras. 2006;21 Suppl 1:44-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Gwak MS, Lee KW, Kim SY, Lee J, Joh JW, Kim SJ, Lee HH, Park JW, Kim GS, Lee SK. Can a leukocyte depletion filter (LDF) reduce the risk of reintroduction of hepatocellular carcinoma cells? Liver Transpl. 2005;11:331-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Sanai FM, Sobki S, Bzeizi KI, Shaikh SA, Alswat K, Al-Hamoudi W, Almadi M, Al Saif F, Abdo AA. Assessment of alpha-fetoprotein in the diagnosis of hepatocellular carcinoma in Middle Eastern patients. Dig Dis Sci. 2010;55:3568-3575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Yao FY, Ferrell L, Bass NM, Bacchetti P, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: comparison of the proposed UCSF criteria with the Milan criteria and the Pittsburgh modified TNM criteria. Liver Transpl. 2002;8:765-774. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 319] [Cited by in F6Publishing: 341] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 12. | Waters JH, Yazer M, Chen YF, Kloke J. Blood salvage and cancer surgery: a meta-analysis of available studies. Transfusion. 2012;52:2167-2173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 13. | Ashworth A, Klein AA. Cell salvage as part of a blood conservation strategy in anaesthesia. Br J Anaesth. 2010;105:401-416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 184] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 14. | Raval JS, Nelson JB, Woldemichael E, Triulzi DJ. Intraoperative cell salvage in radical prostatectomy does not appear to increase long-term biochemical recurrence, metastases, or mortality. Transfusion. 2012;52:2590-2593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Dionigi G, Boni L, Rovera F, Rausei S, Cuffari S, Cantone G, Bacuzzi A, Dionigi R. Effect of perioperative blood transfusion on clinical outcomes in hepatic surgery for cancer. World J Gastroenterol. 2009;15:3976-3983. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Engle DB, Connor JP, Morris PC, Bender DP, De Geest K, Ahmed A, Goodheart MJ. Intraoperative autologous blood transfusion use during radical hysterectomy for cervical cancer: long-term follow-up of a prospective trial. Arch Gynecol Obstet. 2012;286:717-721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Catling S, Williams S, Freites O, Rees M, Davies C, Hopkins L. Use of a leucocyte filter to remove tumour cells from intra-operative cell salvage blood. Anaesthesia. 2008;63:1332-1338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Takemura M, Osugi H, Higashino M, Takada N, Lee S, Kinoshita H. Effect of substituting allogenic blood transfusion with autologous blood transfusion on outcomes after radical oesophagectomy for cancer. Ann Thorac Cardiovasc Surg. 2005;11:293-300. [PubMed] [Cited in This Article: ] |
| 19. | Esper SA, Waters JH. Intra-operative cell salvage: a fresh look at the indications and contraindications. Blood Transfus. 2011;9:139-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 35] [Reference Citation Analysis (0)] |
| 20. | Ubee S, Kumar M, Athmanathan N, Singh G, Vesey S. Intraoperative red blood cell salvage and autologous transfusion during open radical retropubic prostatectomy: a cost-benefit analysis. Ann R Coll Surg Engl. 2011;93:157-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Gorin MA, Eldefrawy A, Manoharan M, Soloway MS. Oncologic outcomes following radical prostatectomy with intraoperative cell salvage. World J Urol. 2012;30:379-383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Fujimoto J, Okamoto E, Yamanaka N, Oriyama T, Furukawa K, Kawamura E, Tanaka T, Tomoda F. Efficacy of autotransfusion in hepatectomy for hepatocellular carcinoma. Arch Surg. 1993;128:1065-1069. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Phillips SD, Maguire D, Deshpande R, Muiesan P, Bowles MJ, Rela M, Heaton ND. A prospective study investigating the cost effectiveness of intraoperative blood salvage during liver transplantation. Transplantation. 2006;81:536-540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Massicotte L, Thibeault L, Beaulieu D, Roy JD, Roy A. Evaluation of cell salvage autotransfusion utility during liver transplantation. HPB (Oxford). 2007;9:52-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |