Published online May 7, 2013. doi: 10.3748/wjg.v19.i17.2668
Revised: February 14, 2013
Accepted: March 6, 2013
Published online: May 7, 2013
AIM: To investigate the predictive value of narrow-band imaging with magnifying endoscopy (NBI-ME) for identifying gastric intestinal metaplasia (GIM) in unselected patients.
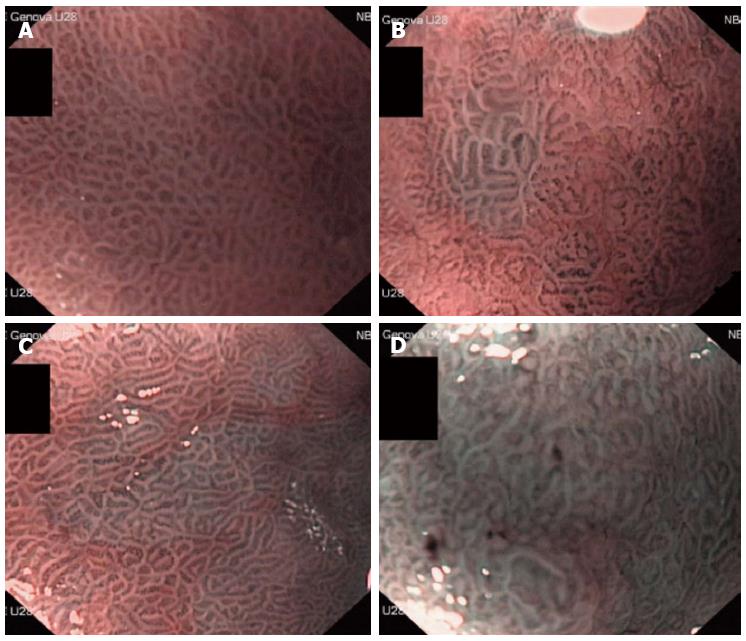
METHODS: We prospectively evaluated consecutive patients undergoing upper endoscopy for various indications, such as epigastric discomfort/pain, anaemia, gastro-oesophageal reflux disease, suspicion of peptic ulcer disease, or chronic liver diseases. Patients underwent NBI-ME, which was performed by three blinded, experienced endoscopists. In addition, five biopsies (2 antrum, 1 angulus, and 2 corpus) were taken and examined by two pathologists unaware of the endoscopic findings to determine the presence or absence of GIM. The correlation between light blue crest (LBC) appearance and histology was measured. Moreover, we quantified the degree of LBC appearance as less than 20% (+), 20%-80% (++) and more than 80% (+++) of an image field, and the semiquantitative evaluation of LBC appearance was correlated with IM percentage from the histological findings.
RESULTS: We enrolled 100 (58 F/42 M) patients who were mainly referred for gastro-esophageal reflux disease/dyspepsia (46%), cancer screening/anaemia (34%), chronic liver disease (9%), and suspected celiac disease (6%); the remaining patients were referred for other indications. The prevalence of Helicobacter pylori (H. pylori) infection detected from the biopsies was 31%, while 67% of the patients used proton pump inhibitors. LBCs were found in the antrum of 33 patients (33%); 20 of the cases were classified as LBC+, 9 as LBC++, and 4 as LBC+++. LBCs were found in the gastric body of 6 patients (6%), with 5 of them also having LBCs in the antrum. The correlation between the appearance of LBCs and histological GIM was good, with a sensitivity of 80% (95%CI: 67-92), a specificity of 96% (95%CI: 93-99), a positive predictive value of 84% (95%CI: 73-96), a negative predictive value of 95% (95%CI: 92-98), and an accuracy of 93% (95%CI: 90-97). The NBI-ME examination overlooked GIM in 8 cases, but the GIM was less than 5% in 7 of the cases. Moreover, in the 6 false positive cases, the histological examination showed the presence of reactive gastropathy (4 cases) or H. pylori active chronic gastritis (2 cases). The semiquantitative correlation between the rate of LBC appearance and the percentage of GIM was 79% (P < 0.01).
CONCLUSION: NBI-ME achieved good sensitivity and specificity in recognising GIM in an unselected population. In routine clinical practice, this technique can reliably target gastric biopsies.
Core tip: Gastric cancer is one of the most common neoplastic diseases in the Western world and has a poor prognosis and inconsistent signs and symptoms in the early phases. Narrow-band imaging with magnifying endoscopy was shown to be a valid method for intestinal metaplasia (IM) detection, this technique can reliably target which patients should be biopsied to evaluate IM and those who do not need biopsies. Moreover, a semi-quantitative evaluation of light blue crest appearance was feasible as there was a good correlation with the histological assessment of IM percentage.
- Citation: Savarino E, Corbo M, Dulbecco P, Gemignani L, Giambruno E, Mastracci L, Grillo F, Savarino V. Narrow-band imaging with magnifying endoscopy is accurate for detecting gastric intestinal metaplasia. World J Gastroenterol 2013; 19(17): 2668-2675
- URL: https://www.wjgnet.com/1007-9327/full/v19/i17/2668.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i17.2668
Gastric cancer is the fourth most common cancer and the second leading cause of cancer-related death worldwide[1,2]. However, the mechanism of gastric carcinogenesis is unknown. The intestinal type tumour, according to the Lauren classification, is characterised by the presence of malignant cells forming glandular structures; in contrast to diffuse type carcinomas, these tumours are generally thought to be preceded by a sequence of precursor lesions. In this multistep model of gastric carcinogenesis, described several years ago by Correa et al[3], Helicobacter pylori (H. pylori) causes chronic inflammation of the gastric mucosa, which slowly progresses through the premalignant stages of atrophic gastritis, intestinal metaplasia and dysplasia, intramucosal carcinoma and invasive neoplasia[4].
Intestinal metaplasia (IM) is generally considered as the “field cancerization” in the gastric mucosa. At the cell level, intestinalized glands provide the cellular substrate that allows the gastric non-invasive neoplasia to develop. The reported progression rates of IM and dysplasia to cancer vary greatly from 0% to 10% per year for IM and from 0% to 73% per year for dysplasia[1]. The prevalence of this condition in Europe varies by country[5-9]; for instance, a Swedish group reported an IM prevalence of 23% in the general population[10], while the prevalence has been estimated to be up to 25% in the Italian population, mainly occurring in the gastric antrum[7,11-13].
In Western countries, premalignant gastric lesions and early gastric cancer are generally diagnosed upon histologic examination of random biopsies, whereas in Asian countries, especially in Japan, the presence and extension of these lesions are frequently established during an endoscopy[14-16]. These remarkable differences are explained by different training and attitudes towards the inspection of the stomach in countries with a high gastric cancer incidence.
The narrow-band imaging system (NBI) is based on the modification of optical filter spectral characteristics in the light source, which improves the visibility of mucosal structures. NBI may be combined with magnification endoscopy to obtain a clear visualisation of surface and vascular patterns[1,2]. Earlier studies using the NBI system with magnification endoscopy (NBI-ME) in the gastric mucosa showed that the appearance of a light blue crest (LBC) in the mucosa is a distinctive endoscopic finding that suggests an increased likelihood of detecting IM in the stomach. More precisely, blue-whitish patchy areas are often observed in NBI images of the antrum in patients with gastric IM. Uedo et al[16] tested NBI-ME on 34 patients with atrophic gastritis and found that the LBC appearance was frequently observed in gastric antrum as blue-white lines visible on the epithelial surface. These authors demonstrated that the appearance of LBCs correlated with histological evidence of IM, with a sensitivity of 89% (95%CI: 83-96), a specificity of 93% (95%CI: 88-97), a positive predictive value of 91% (95%CI: 85-96), a negative predictive value of 92% (95%CI: 87-97) and an accuracy of 91% (95%CI: 88-95).
Despite these promising findings, the added value of NBI-ME in the stomach, and more precisely in detecting premalignant and malignant gastric lesions, requires confirmation from controlled longitudinal trials. Furthermore, a uniform and validated classification system is needed. Thus, the aim of our study was to define the value of NBI-ME for identifying gastric IM in an unselected population through the visualisation of LBC appearance. A secondary aim was to provide a semiquantitative evaluation of LBC appearance and to verify a correlation with the histological measurement of IM percentage.
This prospective, blinded study included unselected consecutive patients presenting to the endoscopy unit of the University Hospital of Genoa, Italy. Patients underwent an upper endoscopy for various indications, such as epigastric discomfort/pain, anaemia, gastro-oesophageal reflux disease, suspicion of peptic ulcer disease, or chronic liver diseases. Exclusion criteria included a previous gastrectomy or partial gastric resection; treatment with antiplatelet medication, anticoagulant medication or non-steroidal anti-inflammatory drugs; and the presence of haemorrhagic diseases. The study protocol was approved by the local Ethics Committee and was performed according to the Declaration of Helsinki. All patients provided written informed consent before the start of the study.
All subjects who agreed to participate in our investigation underwent a thorough physical and clinical examination, a careful collection of their medical history (including current medications, tobacco use, and alcohol and coffee consumption) and an upper gastric intestinal (GI) endoscopy with the NBI-ME system to determine the presence or absence of gastric IM. Five biopsies (2 antrum, 1 angulus and 2 corpus) were collected from all patients according to the updated Sydney classification[17].
The endoscopic examinations were performed by experienced endoscopists (Corbo M, Savarino E, and Dulbecco P) who were blinded to the conditions and medical histories of the patients. Before starting the study, each endoscopist underwent training to obtain good expertise in the detection of IM using NBI-ME. Each endoscopist performed > 300 NBI-ME gastroscopies in patients selected independently of a previous diagnosis of IM and compared their NBI-ME findings with those obtained after a histologic evaluation. At the end of this training, the overall ability to detect histologic IM using NBI-ME was very high (92%). The procedure was conducted with a magnifying endoscope (GIF-Q160Z; Olympus Medical Systems, Tokyo, Japan; 10.9 mm outer diameter insertion tube with a 2.8 mm channel diameter; optical magnification × 115). A disposable attachment was fitted to the scope tip to maintain focal distance from the mucosa for magnifying observation (approximately 2 mm). A conscious sedation was performed at the request of the patient. Before the procedure, all patients ingested 66.6 mg of simethicone diluted in 40 mL of water (Istituto Biochimico Italiano Giovanni Lorenzini S.p.A., Aprilia, Italy). If poor visualisation persisted, the gastric mucosal surface was rinsed with an additional 30-60 mL of the simethicone solution. After white light examination, the gastric antrum and body were examined by NBI for blue-whitish patchy areas and then by NBI-ME observation at the maximum magnification, as LBC was frequently observed in these areas. If no blue-whitish areas were observed, NBI-ME was conducted at the proximal and distal portions of the following areas: the anterior wall, the lesser curvature, the posterior wall, and the greater curvature, as previously described[16]. During NBI-ME observation, we assessed for the presence or absence of LBCs, defined as a fine, blue-white lines on the crests of the epithelial surface that are similar in appearance to the light reflected from a mirror. The patient was defined as LBC-negative if LBCs were not observed in any of the image fields and as LBC-positive if LBCs were observed in any of the image fields. Moreover, we quantified the degree of LBC appearance as less than 20% (+), 20%-80% (++) and more than 80% (+++) of an image field (Figure 1). In each examination, six pictures were taken and stored (one during NBI and two during NBI-ME observation, both in the antrum and in the corpus). A five biopsy set (2 antrum, 1 angulus, and 2 corpus) of LBC-positive areas was collected in all LBC-positive examinations; biopsy samples were taken from the non-LBC mucosa in the LBC-negative patients.
All biopsy specimens were immersed in formalin and then embedded in paraffin. Sections cut from the paraffin blocks were stained with haematoxylin and eosin, Alcian blue PAS and Giemsa. The histological findings were evaluated by two expert pathologists who specialised in GI pathology; the pathologists were unaware of the medical history of the patients and of the endoscopic findings regarding LBC appearance. Inflammation, atrophy, metaplasia and dysplasia were classified according to the updated Sydney system[17] and the revised Vienna classification[18]. Intestinal metaplasia was also expressed as the percentage of metaplastic glands on the entire antral or oxyntic mucosa specimens. Moreover, each sample was evaluated for the presence of H. pylori.
Based on prior studies reporting a sensitivity of more than 80% and a specificity of more than 90%, we determined that a cohort of 100 patients was needed to detect an effect with a confidence interval of 95%, a sensitivity of 80% (interval width of 17%), and a specificity of 90% (interval width of 13%) (calculated with the Clopper-Pearson method using PASS 11; NCSS, LLC, Kaysville, Utah, United States; http://www.ncss.com). The sensitivity, specificity, positive and negative predictive values and accuracy were calculated and expressed as percentages and 95%CI. Pearson correlation analysis was used to compare the LBC grading with the percentage of histologically assessed metaplasia.
The study included 100 consecutive patients (58 F/42 M, mean age 67 ± 12 years) who presented at our endoscopy unit between December 2010 and February 2012. The demographic and clinical characteristics of the patients are shown in Table 1. The patients were referred to our endoscopy unit mainly because of gastro-oesophageal reflux disease and/or dyspepsia symptoms (46%); 34% underwent the endoscopic procedure for cancer screening, anaemia, a positive faecal occult blood test or suspicion of a peptic ulcer; 9% for chronic liver disease; 6% for suspected celiac disease and the remaining patients for other indications. The prevalence of H. pylori infection on the biopsies was 31%; in addition, 67% of the patients used proton pump inhibitors, 16% were current smokers, and 25% of the patients were alcohol consumers.
| Demographic and clinical features | Patients |
| Age, yr (mean ± SD) | 67 ± 12 |
| Sex | |
| Male | 42 (42) |
| Female | 58 (58) |
| H. pylori infection | |
| Positive | 31(31) |
| Negative | 69 (69) |
| Smoking | |
| Non-smoker | 68 (68) |
| Current smoker | 16 (16) |
| Former smoker | 16 (16) |
| Alcohol consuming | |
| Non-drinker | 75 (75) |
| Current drinker | 25 (25) |
| Medication | |
| PPI users | 67 (67) |
| Light blue crests | |
| Present | 33 (33) |
| Absent | 67 (67) |
During the upper NBI-ME procedure, we observed LBC appearance in the gastric antrum of 33 patients (33%); 20 of the LBCs were classified as LBC+, 9 as LBC++, and 4 as LBC+++. Moreover, we detected LBC appearance in the gastric body in only 6 patients (6%), and 5 of the patients also had LBC appearance in the antrum; of these, 3 cases were classified as LBC+, 2 as LBC++, and 1 as LBC+++.
Intestinal metaplasia in the biopsy specimens (2 antrum, 1 angulus and 2 corpus) obtained during the endoscopy from both LBC-positive and -negative patients was classified as complete and incomplete based on special staining. Gastric IM was histologically detected in 35 (35%) patients, and 27 cases were LBC-positive. In five patients, histological IM was found in both the antrum and body, and this finding correlated with LBC appearance at both sites. A percentage of IM greater than 20% was observed in 13 (13%) of the cases.
For the diagnosis of IM, compared to histological assessment, the NBI-ME findings had an accuracy of 93% (95%CI: 90-97), a sensitivity of 80% (95%CI: 67-92), a specificity of 96% (95%CI: 93-99), a positive predictive value of 84% (95%CI: 73-96), and a negative predictive value of 95% (95%CI: 92-98).
Interestingly, in our study, the NBI-ME examination overlooked the presence of gastric IM in 8 cases (false negative cases), and 7 out of the 8 cases had an IM that was less than 5%. Moreover, in the 6 false positive cases, the histological examination showed the presence of reactive gastropathy (4 cases) or H. pylori active chronic gastritis (2 cases).
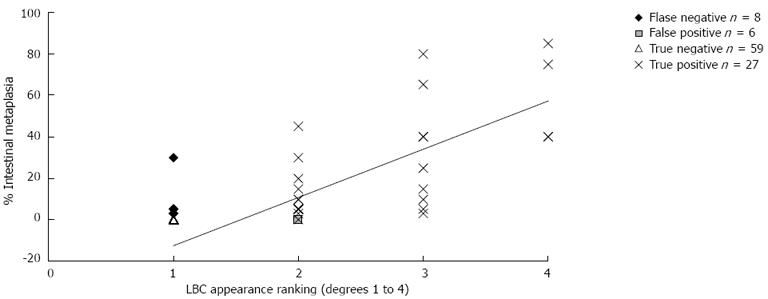
The semiquantitative correlation between the grades of LBC appearance and the percentage of histological IM is shown in Table 2 and Figure 2; the correlation index was 79% (P < 0.01).
| Intestinal metaplasia | LBC (n) | Total (n) | |||
| Negative | + | ++ | +++ | ||
| 0% | 59 | 6 | 0 | 0 | 65 |
| ≤ 5% | 7 | 8 | 2 | 0 | 17 |
| 5%-45% | 1 | 6 | 5 | 2 | 14 |
| 45%-80% | 0 | 0 | 2 | 1 | 3 |
| ≥ 80% | 0 | 0 | 0 | 1 | 1 |
Intestinal metaplasia has been widely studied as the main mucosal background for the development of oesophageal and gastric adenocarcinomas. However, to date, no endoscopic patterns that clearly define IM or dysplasia in the stomach have been found. Kaminishi et al[19] evaluated the accuracy of endoscopic findings for diagnosing chronic gastritis and found that ash-coloured nodular changes were highly specific (98% ± 99%) but had a very poor sensitivity (6% ± 12%) for detecting histological IM; the authors concluded that conventional endoscopy is not really useful for diagnosing gastric IM. Later, Dinis-Ribeiro et al[20] observed that magnifying chromoendoscopy with methylene blue staining had good sensitivity (76%) and specificity (87%) for detecting gastric IM. Moreover, methylene blue chromoendoscopy has been found to be useful for assessing the extent of IM[21]. However, this latter technique is time consuming and has some limitations, including the need for preparation with mucolytic agents, dye spraying and vigorous irrigation of the mucosal surface; furthermore, a recent report suggests that methylene blue chromoendoscopy induced questionable oxidative DNA damage in a patient with Barrett’s mucosa[22]. Therefore, collecting biopsies for subsequent histological evaluation remains the current gold standard for diagnosing IM. However, the fact that premalignant gastric lesions may occur multifocally represents a limitation of random biopsy sampling.
Several new imaging techniques have been developed recently to improve the diagnostic yield of endoscopy. The NBI-ME system has the practical advantage of not requiring complex preparation procedures, the use of any staining technique or a long duration. The additional value of NBI-ME in the detection of gastric pre-malignant lesions is still unclear, especially in countries with a low gastric cancer incidence[7].
Recently, Capelle et al[23] compared the yield of NBI alone to that of white light endoscopy (WLE) in the surveillance of 43 patients with IM and dysplasia. The authors concluded that NBI alone considerably increased the detection of premalignant gastric lesions compared to routine WLE and appeared to be superior to WLE for the surveillance of patients with these advanced gastric lesions. However, a combination of NBI and magnification is likely to provide the best alternative to current endoscopic practice. Previous studies have already demonstrated a high correlation between the microvascular patterns found with NBI-ME and the diagnosis of gastric cancer[24,25]. Moreover, the combined optical technology of NBI with ME has been shown to identify with great accuracy the presence of gastric IM through the visualisation of LBC appearance on the epithelial surface[16]. However, the main limitations of the above-mentioned studies are that they were all conducted in patients with already known diagnoses of intestinal metaplasia/dysplasia/gastric cancer or in a population at a high risk for developing gastric cancer. Other limitations included the small sample sizes and the limited assessment of the gastric antrum and angulus with the exclusion of the gastric body.
Therefore, no prospective data are available on the diagnostic utility and accuracy of NBI-ME for detecting IM in an unselected population presenting at an endoscopy unit in routine clinical practice. Our study showed that NBI-ME allowed us to detect gastric IM areas with an accuracy of 93%, a sensitivity of 80%, a specificity of 96%, a positive predictive value of 84% and a negative predictive value of 95%. These results are similar to those recently published by Pimentel-Nunes et al[26], who found a sensitivity of 68% and a specificity of 87% using the same method and a simplified classification system for NBI in the diagnosis of gastric lesions. In our investigation, NBI-ME underestimated the presence of IM in 8 patients; for 7 of these patients, metaplasia was histologically estimated to be less than 5%. The fact that NBI-ME has its major limitation in identifying gastric IM lower than 5% when performed by experienced endoscopists represents a new finding about the diagnostic yield of this technique; at this stage, we can assume that this value constitutes the sensitivity limit of this technique. The false positive cases (6 patients) presented with a histological diagnosis of reactive gastropathy (4 cases) or H. pylori active chronic gastritis (2 cases). Both of these conditions may sometimes represent a confounding factor because they show endoscopic features similar to those of gastric IM.
The prevalence of histologically observed IM in our cohort (35%) was slightly higher than the prevalence estimated in the general Western population (25%)[5-9] and in other studies on the Italian population (20%-30%)[5,8,11-13]. A possible explanation for our findings may be related to the higher mean age and prevalence of H. pylori infection in our patients, as it is well known that H. pylori infection, increased age and smoking (> 20 cigarettes/daily) are the main risk factors for the development of premalignant gastric lesions[27]. However, the prevalence of IM in the general population is reported to be highly variable. Additionally, the majority of data come from studies on patients with dyspeptic symptoms and/or suspected H. pylori infections, while information derived from unselected patients (i.e., consecutive patients enrolled independently of GI symptoms or clinical status) is limited.
The semiquantitative evaluation of LBC appearance in our study and its correlation with the histological measurement of IM percentage shows that the agreement was high (79%). Previously, Uedo et al[16] also found a significant correlation between the diffuse positivity of LBC (+++) mucosa and the presence of histological markers of IM, such as immunohistochemical staining for CD10 and Alcian blue/high iron diamine. However, these authors did not assess the correlation between LBC appearance and the percentage of histologic IM. Therefore, to the best of our knowledge, our study is the first to show a good correlation between the degree of IM detected by NBI-ME and the severity of IM identified by histological measurement in a series of consecutive patients without specific indications to perform an upper GI endoscopy.
In contrast to Uedo et al[16] who used NBI-ME and Capelle et al[23] who performed NBI alone, we opted to evaluate not only the antrum and the angulus of the stomach but also the body; including the body was recently emphasised in the ESGE guidelines[28], despite the significantly longer examination time. This measurement was included because our intention was to demonstrate the potential application of NBI-ME in a population-based screening and surveillance setting, and we thought that gastric assessment of only one region of the stomach was not adequate and fully informative. However, IM is predominantly found in the antrum, which was confirmed in our study. Therefore, limiting the endoscopic evaluation to the antrum permits an accurate observation and a shorter duration of the examination. Moreover, it has been shown that three biopsies (two antral and one angular) appear to be the best compromise between acceptable accuracy and ease of use in the detection of IM by endoscopy[29].
Our study had some limitations. First, the study included only a single centre. Further research in larger multicentre prospective studies is necessary to evaluate whether NBI-ME yields adequate results in the detection and grading of gastric IM. Second, the endoscopic procedure of WLE and NBI-ME was performed by the same endoscopist; thus, detection of IM by NBI-ME could possibly be biased by the previous WLE observations. Third, as a learning curve must still be defined, regular training is mandatory to improve our findings. Indeed, despite our prolonged training, we obtained a value of sensitivity that was lower than the value obtained by Uedo et al[16]. This difference was likely the result of differences in training between Japanese and Western gastroenterologists. Due to the higher gastric cancer incidence, Japanese endoscopists are trained to scrutinise gastric mucosal areas that are compatible with atrophy and early cancer and spend more time on a thorough mucosal examination than in Western countries. In addition, uniform criteria for this technique must be adopted in large controlled trials in Western or Eastern countries.
In conclusion, NBI-ME was shown to be a valid method for IM detection, except in those cases with IM lower than 5%, which may not be clinically relevant. In routine clinical practice, this technique can reliably target which patients should be biopsied to evaluate IM and those who do not need biopsies. Moreover, a semi-quantitative evaluation of LBC appearance was feasible as there was a good correlation with the histological assessment of IM percentage.
Gastric cancer is one of the most common neoplastic diseases in the Western world and has a poor prognosis and inconsistent signs and symptoms in the early phases. Therefore, adequate screening and prevention are needed to improve the diagnostic process and to identify this condition and its risk factors earlier. Recently, upper gastric intestinal endoscopy has been enhanced with the addition of the narrow band imaging with magnification endoscopy (NBI-ME) technique, which was found to identify precancerous lesions, such as gastric intestinal metaplasia (GIM).
Light blue crests (LBCs) are light-blue patches or lines visible on gastric mucosa by NBI endoscopy. Several studies have demonstrated that LBCs are a sign of underlying intestinal metaplasia, with a good correlation between endoscopic findings and histological assessments. Therefore, authors have assessed the predictive values of the NBI-ME technique to identify and estimate the extension of GIM to biopsies of the antrum, angulus and corpus in the stomach. Authors also studied the correlation between the appearance of LBCs and the histological detection of GIM in prospectively recruited consecutive unselected patients.
One hundred consecutive patients were studied. NBI-ME-aimed gastric bioptic samples were collected (2 in the antrum, 1 in the angulus, and 2 in the corpus) from each patient and compared with the presence or absence of LBCs as stated by skilled endoscopists; the biopsies were assessed according to the pathologists’ expertise in the upper digestive tract. They determined a correlation of 79% between the appearance of LBCs and histologically assessed GIM. Moreover, authors observed that the appearance of LBCs correlated with the histological evidence of intestinal metaplasia, with a sensitivity of 80%, a specificity of 96%, a positive predictive value of 84%, a negative predictive value of 95% and an overall accuracy of 93%. Their results are similar, if not superior, to those of previously published studies in selected populations.
NBI-ME is a good technique to identify areas suggestive of GIM in the stomach and can be used to take adequate biopsies from the stomach to provide the pathologist with a useful sample. This technique is easy to learn and provides an accuracy of greater than 90% for diagnosing GIM.
NBI-ME is an endoscopic technique that uses narrow band imaging to produce superior image contrast combined with a magnification of the image up to × 115; this technique enables a more accurate observation of the gastric epithelium. Gastric intestinal metaplasia is a condition characterised by the replacement of gastric epithelial cells with cells of intestinal morphology. GIM is a pre-cancerous condition that can progress towards gastric cancer, especially the intestinal-type according to the Lauren classification. This progress varies from 0% to 10% according to different studies, with a prevalence of more than 20% in Western countries, and is considered to be the “breaking point” in the Correa sequence of carcinogenesis. Identifying this condition could halt or reverse the neoplastic progression.
This research was performed by endoscopists who belong to single medical center in Italy. This article should be helpful for understanding the efficacy of NBI-magnifying endoscopy in detecting the presence of intestinal metaplasia of stomach.
P- Reviewer Tohda G S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | de Vries AC, Haringsma J, Kuipers EJ. The detection, surveillance and treatment of premalignant gastric lesions related to Helicobacter pylori infection. Helicobacter. 2007;12:1-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13286] [Cited by in F6Publishing: 13416] [Article Influence: 706.1] [Reference Citation Analysis (1)] |
| 3. | Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735-6740. [PubMed] [Cited in This Article: ] |
| 4. | Busuttil RA, Boussioutas A. Intestinal metaplasia: a premalignant lesion involved in gastric carcinogenesis. J Gastroenterol Hepatol. 2009;24:193-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Inghelmann R, Grande E, Francisci S, Verdecchia A, Micheli A, Baili P, Capocaccia R, De Angelis R. Regional estimates of stomach cancer burden in Italy. Tumori. 2007;93:367-373. [PubMed] [Cited in This Article: ] |
| 6. | Peleteiro B, Bastos J, Barros H, Lunet N. Systematic review of the prevalence of gastric intestinal metaplasia and its area-level association with smoking. Gac Sanit. 2008;22:236-247; discussion 246-247. [PubMed] [Cited in This Article: ] |
| 7. | Craanen ME, Dekker W, Blok P, Ferwerda J, Tytgat GN. Intestinal metaplasia and Helicobacter pylori: an endoscopic bioptic study of the gastric antrum. Gut. 1992;33:16-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 136] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Zullo A, Hassan C, Romiti A, Giusto M, Guerriero C, Lorenzetti R, Campo SM, Tomao S. Follow-up of intestinal metaplasia in the stomach: When, how and why. World J Gastrointest Oncol. 2012;4:30-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 67] [Cited by in F6Publishing: 61] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 9. | Tulassay Z, Stolte M, Engstrand L, Butruk E, Malfertheiner P, Dítê P, Tchernev K, Wong BC, Gottlow M, Eklund S. Twelve-month endoscopic and histological analysis following proton-pump inhibitor-based triple therapy in Helicobacter pylori-positive patients with gastric ulcers. Scand J Gastroenterol. 2010;45:1048-1058. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Petersson F, Borch K, Franzén LE. Prevalence of subtypes of intestinal metaplasia in the general population and in patients with autoimmune chronic atrophic gastritis. Scand J Gastroenterol. 2002;37:262-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Rugge M, Cassaro M, Leandro G, Baffa R, Avellini C, Bufo P, Stracca V, Battaglia G, Fabiano A, Guerini A. Helicobacter pylori in promotion of gastric carcinogenesis. Dig Dis Sci. 1996;41:950-955. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Romano M, Cuomo A, Gravina AG, Miranda A, Iovene MR, Tiso A, Sica M, Rocco A, Salerno R, Marmo R. Empirical levofloxacin-containing versus clarithromycin-containing sequential therapy for Helicobacter pylori eradication: a randomised trial. Gut. 2010;59:1465-1470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 13. | Cassaro M, Rugge M, Gutierrez O, Leandro G, Graham DY, Genta RM. Topographic patterns of intestinal metaplasia and gastric cancer. Am J Gastroenterol. 2000;95:1431-1438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 192] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 14. | Sauerbruch T, Schreiber MA, Schüssler P, Permanetter W. Endoscopy in the diagnosis of gastritis. Diagnostic value of endoscopic criteria in relation to histological diagnosis. Endoscopy. 1984;16:101-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 58] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Endoscopic Classification Review Group. Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy. 2005;37:570-578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 559] [Cited by in F6Publishing: 567] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 16. | Uedo N, Ishihara R, Iishi H, Yamamoto S, Yamamoto S, Yamada T, Imanaka K, Takeuchi Y, Higashino K, Ishiguro S. A new method of diagnosing gastric intestinal metaplasia: narrow-band imaging with magnifying endoscopy. Endoscopy. 2006;38:819-824. [PubMed] [Cited in This Article: ] |
| 17. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3221] [Cited by in F6Publishing: 3366] [Article Influence: 120.2] [Reference Citation Analysis (2)] |
| 18. | Dixon MF. Gastrointestinal epithelial neoplasia: Vienna revisited. Gut. 2002;51:130-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 445] [Cited by in F6Publishing: 462] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 19. | Kaminishi M, Yamaguchi H, Nomura S, Oohara T, Sakai S, Fukutomi H, Nakahara A, Kashimura H, Oda M, Kitahora T. Endoscopic classification of chronic gastritis based on a pilot study by the Research Society for Gastritis. Dig Endosc. 2002;14:138-151. [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Dinis-Ribeiro M, da Costa-Pereira A, Lopes C, Lara-Santos L, Guilherme M, Moreira-Dias L, Lomba-Viana H, Ribeiro A, Santos C, Soares J. Magnification chromoendoscopy for the diagnosis of gastric intestinal metaplasia and dysplasia. Gastrointest Endosc. 2003;57:498-504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 115] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Tatsuta M, Iishi H, Ichii M, Noguchi S, Okuda S, Taniguchi H. Chromoendoscopic observations on extension and development of fundal gastritis and intestinal metaplasia. Gastroenterology. 1985;88:70-74. [PubMed] [Cited in This Article: ] |
| 22. | Olliver JR, Wild CP, Sahay P, Dexter S, Hardie LJ. Chromoendoscopy with methylene blue and associated DNA damage in Barrett’s oesophagus. Lancet. 2003;362:373-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 216] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 23. | Capelle LG, Haringsma J, de Vries AC, Steyerberg EW, Biermann K, van Dekken H, Kuipers EJ. Narrow band imaging for the detection of gastric intestinal metaplasia and dysplasia during surveillance endoscopy. Dig Dis Sci. 2010;55:3442-3448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Nakayoshi T, Tajiri H, Matsuda K, Kaise M, Ikegami M, Sasaki H. Magnifying endoscopy combined with narrow band imaging system for early gastric cancer: correlation of vascular pattern with histopathology (including video). Endoscopy. 2004;36:1080-1084. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 328] [Cited by in F6Publishing: 321] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 25. | Kaise M, Kato M, Urashima M, Arai Y, Kaneyama H, Kanzazawa Y, Yonezawa J, Yoshida Y, Yoshimura N, Yamasaki T. Magnifying endoscopy combined with narrow-band imaging for differential diagnosis of superficial depressed gastric lesions. Endoscopy. 2009;41:310-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 26. | Pimentel-Nunes P, Dinis-Ribeiro M, Soares JB, Marcos-Pinto R, Santos C, Rolanda C, Bastos RP, Areia M, Afonso L, Bergman J. A multicenter validation of an endoscopic classification with narrow band imaging for gastric precancerous and cancerous lesions. Endoscopy. 2012;44:236-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 27. | de Vries AC, van Grieken NC, Looman CW, Casparie MK, de Vries E, Meijer GA, Kuipers EJ. Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology. 2008;134:945-952. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 483] [Cited by in F6Publishing: 505] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 28. | Dinis-Ribeiro M, Areia M, de Vries AC, Marcos-Pinto R, Monteiro-Soares M, O’Connor A, Pereira C, Pimentel-Nunes P, Correia R, Ensari A. Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). Endoscopy. 2012;44:74-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 442] [Cited by in F6Publishing: 451] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 29. | Mastracci L, Bruno S, Spaggiari P, Ceppa P, Fiocca R. The impact of biopsy number and site on the accuracy of intestinal metaplasia detection in the stomach A morphometric study based on virtual biopsies. Dig Liver Dis. 2008;40:632-640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |