Published online Jul 7, 2013. doi: 10.3748/wjg.v19.i25.4015
Revised: May 1, 2013
Accepted: May 9, 2013
Published online: July 7, 2013
AIM: To investigate the prevalence and implications of unusual histopathological findings in appendectomy specimens from patients with suspected acute appendicitis.
METHODS: The demographic and histopathological data of 1621 patients (≥ 16 years-old) who underwent appendectomy to treat an initial diagnosis of acute appendicitis between January 1999 and November 2011 were retrospectively assessed. Microscopic findings were used to classify the patients under six categories: appendix vermiformis, phlegmonous appendicitis, gangrenous appendicitis, perforated appendicitis, supurative appendicitis, and unusual histopathologic findings. The demographic and clinicopathologic characteristics of patients with unusual histopathologic findings were evaluated in detail, and re-analysis of archived resected appendix specimens was carried out.
RESULTS: A total of 912 males and 709 females, from 16 to 94 years old, were included in the study and comprised 789 cases of suppurative appendicitis, 370 cases of appendix vermiformis, 243 cases of perforated gangrenous appendicitis, 53 cases of flegmaneous appendicitis, 32 cases of gangrenous appendicitis, and 134 (8.3%) cases of unusual histopathological findings. The unusual histopathological findings included fibrous obliteration (n = 62), enterobius vermicularis (n = 31), eosinophilic infiltration (n = 10), mucinous cystadenoma (n = 8), carcinoid tumor (n = 6), granulomatous inflammation (n = 5), adenocarcinoma (n = 4; one of them mucinous), and mucocele (n = 3), adenomatous polyp (n = 1), taenia sup (n = 1), ascaris lumbricoides (n = 1), appendiceal diverticula (n = 1), and B cell non-hodgkin lymphoma (n = 1). None of the 11 patients with subsequent diagnosis of tumor were suspected of cancer prior to the appendectomy.
CONCLUSION: Even when the macroscopic appearance of appendectomy specimens is normal, histopathological assessment will allow early diagnosis of many unusual diseases.
Core tip: Appendectomy is one of the most frequently performed surgical procedures worldwide. Although most of the resected appendectomy specimens show typical histopathologic findings, some (< 2%) show unusual histopathologic findings. The most common of these unusual features are primary or secondary appendiceal malignancies, mucocele, enterobisis, schistosomiasis, ascariasis, tuberculosis, amobiasis, and entometriosis. While some of the patients with unusual histopatholic findings require close follow-up and/or additional surgical treatment, others also necessitate antimicrobial therapy. Infectious appendicitis is responsible for a significant majority of the most commonly observed unusual features, especially in cases from developing nations in geographic regions with tropical and sub-tropical climates. Therefore, regardless of the underlying etiology, the results from histopathological examination of the resected appendectomy specimen may help guide the subsequent management of cases to prevent serious appendicular diseases.
- Citation: Yilmaz M, Akbulut S, Kutluturk K, Sahin N, Arabaci E, Ara C, Yilmaz S. Unusual histopathological findings in appendectomy specimens from patients with suspected acute appendicitis. World J Gastroenterol 2013; 19(25): 4015-4022
- URL: https://www.wjgnet.com/1007-9327/full/v19/i25/4015.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i25.4015
Appendicitis remains one of the most common acute conditions of the abdomen, and suspected cases are frequently treated with emergency appendectomy[1]. The complete organ excision not only allows for definitive diagnosis but also significantly reduces the risk of life-threatening complications, such as perforation, plastron and sepsis. However, the surgical procedure itself is very invasive, representing additional risks to the patient’s morbidity and mortality as well as remarkable costs to the healthcare providers. Epidemiologic studies have revealed that the incidence of acute appendicitis roughly parallels that of lymphoid development, with the peak incidence occurring between the ages of 10 and 30 years old. The most important causative factor of acute appendicitis appears to be development of luminal obstruction. In addition, several factors have been implicated as causative etiologies of this underlying feature, and show an age-related trend[1-5]. For example, lymphoid hyperplasia is the most common factor identified in patients under 20 years old, while fecalith plugs are the most common factor identified in the elderly. Apart from these usual factors, numerous other less frequent (and thus “unusual”) factors have been identified as having caused the clinical symptoms that indicated the suspicion of acute appendicitis with or without histopathologic evidence for acute appendicitis[1,3,4]. The primary objective of this study was to assess the incidence and implications of unusual histopathological findings detected in appendectomy specimens from patients who received surgery to address an initial diagnosis of acute appendicitis.
In this retrospective study, the electronic records of the Inonu University Medical Faculty Department of Surgery were searched to identify all patients who underwent appendectomy to treat an initial diagnosis of acute appendicitis between January 1999 and November 2011. The recorded demographic and histopathological data extracted for each patient included age, sex, appendectomy surgery date, and macroscopic and microscopic properties of appendix vermicularis. Patients who had received the appendectomy incidental to other surgeries, such as colorectal or gynecological cancer surgery or trauma surgery, were excluded from study enrollment. In addition, pediatric patients younger than 16 years old were also excluded from study enrollment. Four researchers working independently collected the demographic and pathologic data of all patients fitting the inclusion criteria in excel spreadsheets, which were then adjudicated and analyzed by the group.
Using the microscopic findings of each patient’s appendectomy specimen that were recorded in the pathology report, the patients were classified into one of six categories: (1) appendix vermiformis; (2) phlegmonous appendicitis; (3) gangrenous appendicitis; (4) perforated appendicitis; (5) suppurative appendicitis; and (6) unusual histopathologic findings. The archived appendectomy specimens (pathology blocks and microscopic slides) were retrieved for the 134 patients in group 6 and were re-evaluated by two experienced pathologists. The patient data for each demographic or histopathologic characteristic were summarized as mean ± SD, and incidence of a characteristic within a particular group was calculated as percentage of the entire study population.
A total of 1621 patients underwent appendectomy to treat an initial diagnosis of acute appendicitis. The mean age of these patients was 36.7 ± 17.4 years (range: 16-94 years) and the male-to-female ratio was nearly equal (912: 709) but with a slight male bias (56.3% males). According to the histopathological findings, 789 patients had suppurative appendicitis, 370 had appendix vermiformis, 243 had perforated gangrenous appendicitis, 134 had unusual histopathological findings, 53 had flegmaneous appendicitis, and 32 had gangrenous appendicitis. Overall, the majorities (67.3%) of the patients were ≤ 40 years old, and 13.5% were ≥ 61 years old. There was also an age bias towards patients ≤ 40 years old for those in the negative appendicitis group (64.6% of the 370 patients), with only 17.5% in that group being ≥ 61 years old. Clinicopathologic characteristics of the 1621 patients who underwent appendectomy for clinical signs of acute appendicitis are summarized in Table 1.
| Patient characteristics | Results |
| Patients | 1621 |
| Sex | |
| Male | 912 (56.3) |
| Female | 709 (43.7) |
| Age in years, mean (range) | |
| Overall | 36.7 ± 17.4 (16-94) |
| Male | 36.2 ± 17.5 (16-89) |
| Female | 37.3 ± 17.3 (16-94) |
| Distribution of patients according to age range (yr) | |
| 16-20 | 275 |
| 21-30 | 526 |
| 31-40 | 290 |
| 41-50 | 165 |
| 51-60 | 146 |
| 61-70 | 134 |
| ≥ 71 | 85 |
| Histopathologic findings | |
| Suppurative appendicitis | 789 (48.7) |
| Appendix vermiformis | 370 (22.8) |
| Gangrenous appendicitis-perforated | 243 (15.0) |
| Unusual histopathologic findings | 134 (8.3) |
| Phlegmonous appendicitis | 53 |
| Gangrenous appendicitis | 32 |
| Age distribution of the 370 patients with negative appendectomy (yr) | |
| 16-20 | 67 |
| 21-30 | 111 |
| 31-40 | 61 |
| 41-50 | 35 |
| 51-60 | 32 |
| 61-70 | 43 |
| ≥ 71 | 21 |
One-hundred-and-thirty-four (8.3%) of the patients who received appendectomy to treat the initial diagnosis of acute appendicitis had unusual histopathological findings in their appendectomy specimens. This group of patients had a mean age of 48.4 ± 19.5 years old, and the male-to-female ratio was relatively equal (60:74) but with a slight female bias (55.2% females). The mean age of the males (50.9 ± 19.3 years old; range: 16-87 years) was slightly higher than that of the females (46.4 ± 19.5 years old; range: 16-94 years). Unlike any of the five other pathology groups, the group with unusual histopathological findings had a majority (60.4%) of patients > 40 years old.
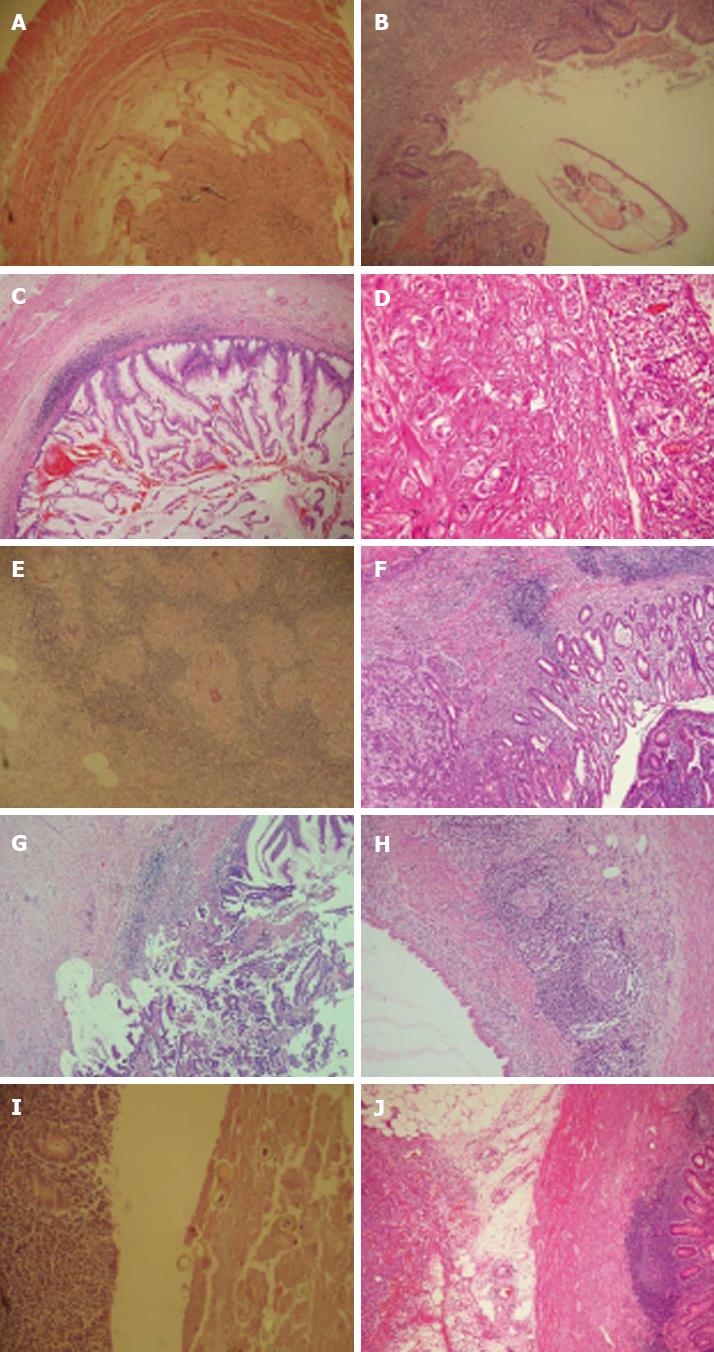
The histopathologic findings of these 134 patients with unusual histopathological findings included fibrous obliteration (n = 62; Figure 1A), enterobius vermicularis (n = 31; Figure 1B), eosinophilic infiltration (n = 10), mucinous cystadenoma (n = 8; Figure 1C), carcinoid tumor (n = 6; Figure 1D), granulomatous infiltration (n = 5; Figure 1E), adenocarcinoma (n = 4; Figure 1F-G), mucocele (n = 3; Figure 1H), adenomatous polyp (n = 1), taenia sup (n = 1; Figure 1I), ascaris lumbricoides (n = 1), appendiceal diverticula (n = 1), and B cell non-Hodgkins lymphoma (NHL) (n = 1; Figure 1J).
Ninety-six of the 134 total patients with unusual histopathologic findings showed no evidence of inflammatory cell infiltration. However, 21 of these 96 cases had additional inflammation-related findings, including lymphoid hyperplasia (n = 18) and ovarian cyst rupture (n = 3). The remaining 38 of the 134 total patients did show evidence of inflammatory cell infiltration to varying degrees. Among those patients, five were histologically confirmed as having perforated appendicitis and three as having gangrenous appendicitis. Five of 134 patients showed evidence of classical granulomas and multinucleated giant cells formed by epitheloid histiocytes. Staining with erlich ziehl-nielsen and periodic acid-schiff revealed a complete absence of microorganisms; thus all cases were reported as granulomatous appendicitis. Taenia sup eggs were detected in one specimen, although the adult form of the parasite was not detected and the case was not specified as Taenia saginata or Taenia solium. Clinicopathologic features of the 134 appendectomized patients with unusual histopathological findings are summarized in Table 2.
| Patient characteristic | Results |
| Patients | 134 |
| Sex, n (%) | |
| Male | 60 (44.8) |
| Female | 74 (55.2) |
| Age in years, mean ± SD (range) | |
| Overall | 48.4 ± 19.5 (16-94) |
| Male | 50.9 ± 19.3 (16-87) |
| Female | 46.4 ± 19.5 (16-94) |
| Histopathologic findings, n | |
| Fibrous obliteration | 62 |
| Enterobius vermicularis | 31 |
| Eosinophilic infiltration | 10 |
| Mucinous cystadenoma | 8 |
| Carcinoid tumor | 6 |
| Granulomatous inflammation | 5 |
| Adenocarcinoma | 3 |
| Mucinous adenocarcinoma | 1 |
| Mucocele | 3 |
| Adenomatous polyp | 1 |
| Taenia saginata | 1 |
| Ascaris lumbricoides | 1 |
| Non-Hodgkin’s lymphoma (B cell) | 1 |
| Appendicular diverticulitis | 1 |
| Age distribution of 134 patients with unusual findings (yr) | |
| 16-20 | 8 |
| 21-30 | 30 |
| 31-40 | 15 |
| 41-50 | 16 |
| 51-60 | 22 |
| 61-70 | 22 |
| ≥ 71 | 21 |
Malignancy was detected in the appendectomy specimens of 11 of the 134 patients of this group. The mean age of these patients was 49.1 ± 16.7 years old (range: 21-74 years), and the majority was female (4:7). There was no suspicion of cancer prior to the appendectomy surgery for any of these patients. Histopathological findings, however, indicated carcinoid tumor (n = 6), adenocarcinoma (n = 4) and B cell NHL (n = 1). Standard appendectomy was carried out in five of the six patients with carcinoid tumor, and the tumor diameters of these cases ranged from 5-25 mm; only the sixth case underwent subsequent right hemicolectomy procedure following the cancer diagnosis. Detailed tumor data could be retrieved for only one of the four patients with adenocarcinoma (age range: 51-74 years) but all of these patients underwent subsequent right hemicolectomy following the cancer diagnosis. The one patient diagnosed with B cell NHL was referred to the Medical Oncology Department following the appendectomy surgery, and medical records indicate that the patient was in remission at the 1-year follow-up. Detailed characteristics of the 11 appendectomized patients with histologically-diagnosed appendicular malignancy are summarized in Table 3.
| No. | Age (yr) | Sex | Primary tumor type | Tumor size (mm) | Pleomorphism | Mitosis (HPF) | Necrosis | Parietal spread | Surgical approach |
| 1 | 55 | M | B-NHL | CD20(+), CD79a(+) | Appendectomy | ||||
| 2 | 64 | F | Adenoca | 40 | Muscularis propria | Appendectomy-right hemicolectomy | |||
| 3 | 67 | M | Adenoca | 15 | Appendectomy-right hemicolectomy | ||||
| 4 | 74 | F | Adenoca | 50 | Serosa | Appendectomy-right hemicolectomy | |||
| 5 | 51 | M | Adenoca | 50 | Serosa | Appendectomy-right hemicolectomy | |||
| 6 | 41 | F | Carcinoid | 25 | Minimal | 1/10 | No | Mesoappendix | Appendectomy-right hemicolectomy |
| 7 | 28 | F | Carcinoid | 5 | Minimal | 1/10 | No | Submucosa | Appendectomy |
| 8 | 28 | F | Carcinoid | 8 | Minimal | 0/10 | No | Mesoappendix | Appendectomy |
| 9 | 60 | F | Carcinoid | 10 | Moderate | 2/10 | No | Mesoappendix | Appendectomy |
| 10 | 21 | M | Carcinoid | 12 | Moderate | 2/10 | No | Mesoappendix | Appendectomy |
| 11 | 52 | F | Carcinoid | 13 | Moderate | 1/10 | No | Muscularis propria | Appendectomy |
Acute appendicitis manifests upon inflammation of the inner lining of the appendix vermiformis, which can spread to other parts of the organ. This condition may be brought on by several different physiopathological processes, but luminal obstruction is considered the most important triggering factor of the underlying inflammation[1-5]. Although lymphoid hyperplasia and fecaliths are the most frequently observed etiologies of luminal obstruction, other, less frequent factors have been observed in patients with symptoms of acute appendicitis. According to the literature, the most common of these unusual factors are mucinous cystadenoma or mucocele[6-9], carcinoid tumor[9-12], granulomatous diseases[13-15], enterobiasis[1,5,16,17], taeniasis[3,18-20], ascariasis[4,21], diverticulitis[22-25], primary or secondary adenocarcinoma[26-30], lymphoma[26,31,32], and neurogenic appendicopathy[33,34]. In addition, the study by Akbulut et al[1] reported cases associated with eosinophilic granuloma, amebiasis, actinomycosis, schistosomiasis, balantidiasis, tuberculosis, adenovirus, melanosis, neurofibroma, endometriosis, adenomatous or hyperplastic polyps, villous or tubulovillous adenoma, gastrointestinal stromal tumor, leukemia, and foreign body reactions.
Appendiceal tumors, which have been reported in < 3% of all appendectomy specimens, are rarely associated with manifestation of clinical symptomology. Thus, this condition is most often recognized incidentally, either during an abdominal operation or general pathological examination of a resected appendix specimen. The most frequently diagnosed type of appendiceal primary malignant lesion is the carcinoid tumor. Although it accounts for about 60% of all appendiceal tumors, its incidence in patients undergoing appendectomy is only 0.30%-2.27%. Most of the carcinoid tumors are located at the tip of the appendix and are < 1 cm in diameter. Fortunately, malignancy and metastasis of these tumors are very rare, and usually only involve tumors that exceed 1 cm. Therefore, simple appendectomy is considered sufficient management for these tumors. The risk of metastasis jumps up to 85%, however, once the tumor size reaches 2 cm or larger, in which case a formal right hemicolectomy is recommended[10-12]. In our patient series, the incidence of appendiceal carcinoid (0.37%) was similar to that in the overall literature.
Primary adenocarcinoma of the appendix is an extraordinarily rare tumor, with overall incidence in the literature between 0.01% and 0.20%. However, this tumor is most likely to occur in persons between 50 and 55 years old. Adenocarcinomas generally show aggressive behavior, the pattern of which has been likened to colonic adenocarcinomas. Therefore, appendicular adenocarcinomas are often treated by oncologic resection with right hemicolectomy[10,26,29]. In our patient series, only four patients presented with this tumor type, giving an incidence of 0.25% that is similar to that in the overall literature. In addition, these patients were within the age range of 51 and 74 years old (mean ± SD, 64.0 ± 8.3 years).
Appendiceal mucinous adenocarcinoma, also known as mucinous cystadenocarcinoma, is another rare condition of the appendix. This tumor type, however, is most often associated with a second malignancy of the gastrointestinal tract and the most common manifestation is symptoms of acute appendicitis. Like the other appendix-related cancers, diagnosis of mucinous adenocarcinoma is usually only made upon the subsequent pathological evaluation of a resected appendiceal specimen[27,28].
Mucocele is a condition in which mucoid material accumulates in the intraluminal region of the appendix, eventually causing obstructive dilatation of the organ. However, the occlusion of the appendiceal lumen may also be caused by endometriosis or carcinoid tumors[8,9]. The overall incidence of this condition in the literature ranges from 0.2% to 0.7%. Currently, four histologic types of appendiceal mucocele are recognized, and these include (in order of incidence): Mucinous cystadenoma, mucosal hyperplasia, mucinous cystadenocarcinoma, and retention cyst[1,6,7]. Up to one-half of mucocele cases are asymptomatic and the condition is incidentally diagnosed by histological examination of tissues from appendectomy, or sometimes during a laparotomy surgery. Appendectomy is the standard of care for mucinous cystadenoma, whereas a cystadenocarcinoma requires a right hemicolectomy[1,6,7].
The gastrointestinal tract is the most common site for extranodal lymphomas, accounting for 30%-45% of all extranodal cases. The incidence of primary appendiceal lymphoma is extremely low, and has been estimated at between 0.015% and 0.05%[26,31,32]. Cases of appendiceal lymphoma most often occur in young adults, between the ages of 20 and 40 years old. The usual manifestation of symptoms of acute appendicitis explain its diagnosis most frequently occurring following appendectomy and upon histopathologic analysis of the resected organ. Unfortunately, the rarity of the disease has impeded establishment of evidence-based guidelines for treatment.
The incidence of neurogenic appendicopathy is estimated to be about 30%. Although this process is often described as fibrous obliteration, recent studies have demonstrated that the occlusive proliferation is predominantly neurogenic in some cases. As of yet, the pathogenesis of this condition remains to be fully elucidated, but some studies have indicated that it may actually be secondary to hyperplasia of neuroendocrine cells. Differential diagnosis between appendiceal neuroma and acute appendicitis is relatively subjective and depends upon a patient’s clinical history, symptomology, and findings from laboratory and physical examination. Accordingly, most appendiceal neuromas are incidentally indicated by histological evidence of fibrous obliteration in appendix specimens of otherwise asymptomatic patients[1,33,34]. In the current patient series, the incidence of fibrous obliteration was only 3.7%, which is lower than in the overall literature.
Enterobius vermicularis, commonly known as the pinworm, is a widespread parasitic infection that is estimated to affect up to 200 million people worldwide. The association of pinworm infection and appendicitis was first made in the late 19th century. While the reported incidence of pinworm infections in appendectomy specimens from patients with presumed appendicitis has ranged from 0.2% to 41.8%, inflammation is often associated with pinworm infection in the appendix[1,5,16,17]. In the current patient series, the incidence of pinworms in the appendectomy specimens was 1.9%, which is similar to the overall literature.
Taeniasis manifests upon intestinal infection with helminths. The first sign of infection is usually a segment of the parasite that appears in the stool. Taenia sup infection of the appendix, in particular, is so rare that the situation invites a case report. In general, cases of taeniasis do not necessitate identification of the specific species in order to initiate appropriate treatment, and a single dose of praziquantel can efficiently clear the infection[3,18-20].
Ascaris lumbricoides is one of the most common human helminthic pathogens infecting humans worldwide; however, epidemiologic studies have revealed that the highest prevalence of ascariasis occurs in tropical and semitropical countries. In the human host, the worm can establish residence in the gastrointestinal region from the stomach to the ileocecal valve, but up to 99% of the cases reported have worms localized to the jejunum and proximal ileum. Infections involving the appendix are only rarely seen. The ability of a roundworm to migrate to the appendix, thereby causing appendicitis, is controversial. The physical and physiological effects of such a migration may indeed simulate other physiopathogenic processes that promote appendicitis, but are believed less likely to be the direct cause of it[1,4,21].
Granulomatous appendicitis is another rare condition that may be discovered incidentally in a patient with a clinical presentation of acute appendicitis. The reported incidence in Western countries has ranged from 0.14% to 0.30%, and is higher (1.3%-2.3%) in underdeveloped countries[13,14]. The criteria for diagnosis are similar to those of other diseases of the gastrointestinal tract, and include granulomatous inflamation, transmural lymphoid aggregates, and fissuring-type ulcers. Various infectious agents (such as Yersinia spp., Mycobacterium tuberculosis, and Schistosoma spp.) and non-infectious factors (such as Crohn’s disease and sarcoidosis) have been implicated as causative factors of this condition[1,14-16]. In the current series of patients, granulomatous inflammation was observed in only 0.3%. As tuberculosis is endemic in the region where our hospital is located, all of the patients had been tested accordingly; yet, no findings related to tuberculosis were encountered.
Appendiceal diverticulum is another very rare clinical entity, and the incidence is reported between 0.004% and 2.1%. The diverticula may occur as singlets or in multiples, but generally involve the distal third of the appendix, on its mesenteric side, and their size is usually < 0.5 cm. Cases of appendiceal diverticula are routinely classified as either congenital or acquired. While the congenital form (considered a true diverticulum) is extremely rare, the acquired form (a pseudodiverticulum consisting of mucosa and submucosa herniated through vascular clefts in the muscular layer) are encountered much more often. Four clinical variations of either form of this condition have been described, and include the appendiceal diverticula without inflammation, acute appendicitis with diverticula, acute appendiceal diverticulitis with acute appendicitis, and isolated acute diverticulitis. However, all four forms are generally asymptomatic, with the related complications of perforation and inflammation causing the abdominal pain that mimics acute appendicitis[22-25].
Considering the overall case reports in the literature and the case series presented herein, it is clear that even when the macroscopic appearance of a resected appendix is normal, histopathological assessment of specimens will allow early diagnosis of malign and infectious appendiceal diseases.
Appendicitis is one of the most common acute surgical conditions of the abdominal cavity. While this clinicopathologic condition may manifest from several underlying etiologies, luminal obstruction is the essential triggering factor for development of the inflammatory process. Although lymphoid hyperplasia and fecaliths are the most common cause of luminal obstruction, other less commonly observed factors, such as infectious and malignant appendiceal diseases, may also underlie this pathogenic condition.
According to the literature, the most common of unusual histopathologic findings are mucinous cystadenoma or mucocele, carcinoid tumor, granulomatous diseases, enterobiasis, taeniasis, ascariasis, diverticulitis, primary or secondary adenocarcinoma, lymphoma, and neurogenic appendicopathy, eosinophilic granuloma, amebiasis, actinomycosis, schistosomiasis, balantidiasis, tuberculosis, adenovirus, melanosis, neurofibroma, endometriosis, adenomatous or hyperplastic polyps, villous or tubulovillous adenoma, gastrointestinal stromal tumor, leukemia, and foreign body reactions. In this study, the authors conducted and investigation of the incidence and implications of unusual histopathological findings detected in resected appendectomy specimens obtained from patients who underwent surgery for suspected acute appendicitis.
The authors emphasize and strongly recommend that all appendectomy specimens be examined by histopathological analysis, even if specimens have a normal gross appearance.
This is a quite well-done manuscript of appropriate interest and with images of reasonable quality.
P- Reviewers Higgins PJ, Mura B S- Editor Huang XZ L- Editor A E- Editor Zhang DN
| 1. | Akbulut S, Tas M, Sogutcu N, Arikanoglu Z, Basbug M, Ulku A, Semur H, Yagmur Y. Unusual histopathological findings in appendectomy specimens: a retrospective analysis and literature review. World J Gastroenterol. 2011;17:1961-1970. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 79] [Cited by in F6Publishing: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 2. | Duzgun AP, Moran M, Uzun S, Ozmen MM, Ozer VM, Seckin S, Coskun F. Unusual findings in appendicectomy specimens: Evaluation of 2458 cases and review of the literature. Indian J Surg. 2004;66:221-226. [Cited in This Article: ] |
| 3. | Hafezi Ahmadi M, Seifmanesh H. Taeniasis caused appendicitis without local tenderness: A rare case. Hospital Chronicles. 2011;6:207-209. [Cited in This Article: ] |
| 4. | Sforza M, Andjelkov K, Zaccheddu R, Ivanov D, Krstić S, Paganelli A. An unusual case of ascariasis of the appendix. Srp Arh Celok Lek. 2011;139:809-811. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Gialamas E, Papavramidis T, Michalopoulos N, Karayannopoulou G, Cheva A, Vasilaki O, Kesisoglou I, Papavramidis S. Enterobius vermicularis: a rare cause of appendicitis. Turkiye Parazitol Derg. 2012;36:37-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Demetrashvili Z, Chkhaidze M, Khutsishvili K, Topchishvili G, Javakhishvili T, Pipia I, Qerqadze V. Mucocele of the appendix: case report and review of literature. Int Surg. 2012;97:266-269. [PubMed] [Cited in This Article: ] |
| 7. | Kelemouridou E, Mogrampi SA, Tsavis G, Verroiotou M, Rallis T, Fardellas I. Mucinous cystadenoma of the appendix. A diagnostic dilemma? Chirurgia (Bucur). 2011;106:251-254. [PubMed] [Cited in This Article: ] |
| 8. | Driman DK, Melega DE, Vilos GA, Plewes EA. Mucocele of the appendix secondary to endometriosis. Report of two cases, one with localized pseudomyxoma peritonei. Am J Clin Pathol. 2000;113:860-864. [PubMed] [Cited in This Article: ] |
| 9. | Al Imari A, Vajpeyi R. Neuroendocrine Tumor (Carcinoid) of the Appendix With Mucocele: Sonographic and Pathological. Chaosheng Zhenduan Zazhi. 2011;27:176. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Shapiro R, Eldar S, Sadot E, Papa MZ, Zippel DB. Appendiceal carcinoid at a large tertiary center: pathologic findings and long-term follow-up evaluation. Am J Surg. 2011;201:805-808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | In’t Hof KH, van der Wal HC, Kazemier G, Lange JF. Carcinoid tumour of the appendix: an analysis of 1,485 consecutive emergency appendectomies. J Gastrointest Surg. 2008;12:1436-1438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Ozer MT, Demirbas S, Celik E, Safali M, Harlak A, Coskun K, Ersoz N, Uzar AI. Natural behaviour and surgical treatment of appendiceal carcinoids: an analysis of 2,376 consecutive emergency appendectomies. Bratisl Lek Listy. 2011;112:619-622. [PubMed] [Cited in This Article: ] |
| 13. | AbdullGaffar B. Granulomatous diseases and granulomas of the appendix. Int J Surg Pathol. 2010;18:14-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Tucker ON, Healy V, Jeffers M, Keane FB. Granulomatous appendicitis. Surgeon. 2003;1:286-289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Shivakumar P, Shanmugam RP, Mani CS. Idiopathic granulomatous appendicitis: a rare appendicular pseudo tumor. Trop Gastroenterol. 2010;31:130-131. [PubMed] [Cited in This Article: ] |
| 16. | Ariyarathenam AV, Nachimuthu S, Tang TY, Courtney ED, Harris SA, Harris AM. Enterobius vermicularis infestation of the appendix and management at the time of laparoscopic appendectomy: case series and literature review. Int J Surg. 2010;8:466-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Sodergren MH, Jethwa P, Wilkinson S, Kerwat R. Presenting features of Enterobius vermicularis in the vermiform appendix. Scand J Gastroenterol. 2009;44:457-461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Sartorelli AC, da Silva MG, Rodrigues MA, da Silva RJ. Appendiceal taeniasis presenting like acute appendicitis. Parasitol Res. 2005;97:171-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Lejbkowicz F, Abel AB, Tsilman B, Cohen HI. Taenia infestation in the appendix: a report of two cases. J Med Microbiol. 2002;51:90-91. [PubMed] [Cited in This Article: ] |
| 20. | Ajmera RK, Simon GL. Appendicitis associated with Taenia species: cause or coincidental? Vector Borne Zoonotic Dis. 2010;10:321-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Wani I, Maqbool M, Amin A, Shah F, Keema A, Singh J, Kitagawa M, Nazir M. Appendiceal ascariasis in children. Ann Saudi Med. 2010;30:63-66. [PubMed] [Cited in This Article: ] |
| 22. | Manzanares-Campillo Mdel C, Pardo-García R, Martín-Fernández J. Appendicular pseudodiverticula and acute appendicitis. Our 12-year experience. Rev Esp Enferm Dig. 2011;103:582-585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Coulier B, Pierard F, Malbecq S. Appendicular diverticulitis in an Amyand’s hernia. JBR-BTR. 2010;93:114. [PubMed] [Cited in This Article: ] |
| 24. | Lin CH, Chen TC. Diverticulosis of the appendix with diverticulitis: case report. Chang Gung Med J. 2000;23:711-715. [PubMed] [Cited in This Article: ] |
| 25. | Abdullgaffar B. Diverticulosis and diverticulitis of the appendix. Int J Surg Pathol. 2009;17:231-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 26. | O’Donnell ME, Badger SA, Beattie GC, Carson J, Garstin WI. Malignant neoplasms of the appendix. Int J Colorectal Dis. 2007;22:1239-1248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Komm M, Kronawitter-Fesl M, Kremer M, Lutz L, Holinski-Feder E, Kopp R. Primary mucinous adenocarcinoma of the vermiform appendix with high grade microsatellite instability. J Cancer. 2011;2:302-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Racek AR, Rabe KG, Wick MJ, Psychogios A, Lindor NM. Primary appendiceal mucinous adenocarcinoma in two first-degree relatives: case report and review. Hered Cancer Clin Pract. 2011;9:1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Guraya SY, Almaramhy HH. Clinicopathological features and the outcome of surgical management for adenocarcinoma of the appendix. World J Gastrointest Surg. 2011;3:7-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Graham RP, Williams NP, West KA. Primary epithelial tumours of the appendix in a black population: a review of cases. World J Gastroenterol. 2009;15:1472-1474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 10] [Cited by in F6Publishing: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Fu TY, Wang JS, Tseng HH. Primary appendiceal lymphoma presenting as perforated acute appendicitis. J Chin Med Assoc. 2004;67:629-632. [PubMed] [Cited in This Article: ] |
| 32. | Kitamura Y, Ohta T, Terada T. Primary T-cell non-Hodgkin’s malignant lymphoma of the appendix. Pathol Int. 2000;50:313-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Gupta K, Solanki A, Vasishta RK. Appendiceal neuroma: report of an elusive neuroma. Trop Gastroenterol. 2011;32:332-333. [PubMed] [Cited in This Article: ] |
| 34. | Patel AV, Friedman M, MacDermott RP. Crohn’s disease patient with right lower quadrant abdominal pain for 20 years due to an appendiceal neuroma (Fibrous obliteration of the appendix). Inflamm Bowel Dis. 2010;16:1093-1094. [PubMed] [Cited in This Article: ] |