Published online Sep 14, 2013. doi: 10.3748/wjg.v19.i34.5622
Revised: July 10, 2013
Accepted: July 17, 2013
Published online: September 14, 2013
AIM: To assess the possible effect of two different types of preoperative transcatheter arterial chemoembolization (TACE) on recurrence-free survival after liver transplantation (LT) in patients with hepatocellular carcinoma (HCC) and to analyze the effects of TACE on tumor histology.
METHODS: We retrospectively analyzed the histological features of 130 HCC nodules in 63 native livers removed at transplantation. Patients who received any other type of treatment such as radiofrequency tumor ablation, percutaneous ethanol ablation or who were not treated at all were excluded. All patients in the present study were within the Milan Criteria at the last imaging findings before transplantation. Doxorubicin-eluting bead TACE (DEB-TACE) was performed in 22 patients (38 nodules), and conventional TACE (c-TACE) in 16 (25 nodules). Patients’ and tumors’ characteristics were retrospectively reviewed. We performed a per-nodule analysis of the explanted livers to establish the mean percentage of necrosis of any nodule treated by TACE (conventional or DEB) and a per-patient analysis to establish the percentage of necrosis in the cumulative tumor area, including 21 nodules not reached by TACE. Inflammatory and fibrotic changes in the tissue surrounding the tumor nodule were analyzed and categorized as poor/absent, moderate and enhanced reaction. Uni- and multivariate analysis of risk factors for HCC-recurrence were performed.
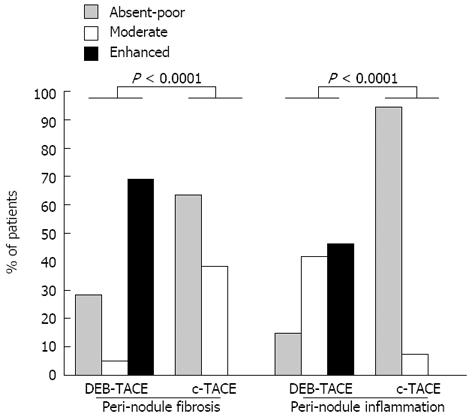
RESULTS: The number and diameter of the nodules, the time spent on the waiting list and the number of treatments were similar in the two groups. A trend towards higher appropriate response rates (necrosis ≥ 90%) was observed in the DEB-TACE group (44.7% vs 32.0%, P = 0.2834). The mean percentage of necrosis in the cumulative tumor area was 58.8% ± 36.6% in the DEB-TACE group and 50.2% ± 38.1% in the c-TACE group (P = 0.4856). Fibrotic and inflammatory reactions surrounding the tumor nodule were markedly more common in the DEB-TACE group (P < 0.0001, for both the parameters). The three-year recurrence-free survival was higher in DEB-TACE-treated patients than in conventionally treated patients (87.4% vs 61.5%, P = 0.0493). Other factors affecting recurrence-free survival included viable tumor beyond Milan Criteria on histopathological examination, the percentage of necrosis on CTA ≤ 50% and a pre-transplant serum α-fetoprotein level greater than 70 ng/mL. On multivariate analysis, the lack of treatment with DEB-TACE, high levels of α-fetoprotein and viable tumor beyond Milan Criteria at histology examination were identified as independent predictors of tumor recurrence.
CONCLUSION: DEB-TACE can effectively promote tumor necrosis and improves recurrence-free survival after LT in HCC.
Core tip: The manuscript reports the experience with a newer technique of transcatheter arterial chemoembolization (TACE) that uses doxorubicin-eluting beads (DEB) for the treatment of hepatocellular carcinoma in liver transplant candidates. The results of DEB-TACE were compared to those of conventional TACE, and remarkably, a significantly higher recurrence-free survival after liver transplantation was observed in patients who were treated with DEB-TACE. The histological pattern observed in the area surrounding the tumor nodules of DEB-TACE patients was characterized by an intense inflammatory and fibrotic reaction, which could play a role in limiting tumor spread during waiting list time.
-
Citation: Nicolini D, Svegliati-Baroni G, Candelari R, Mincarelli C, Mandolesi A, Bearzi I, Mocchegiani F, Vecchi A, Montalti R, Benedetti A, Risaliti A, Vivarelli M. Doxorubicin-eluting bead
vs conventional transcatheter arterial chemoembolization for hepatocellular carcinoma before liver transplantation. World J Gastroenterol 2013; 19(34): 5622-5632 - URL: https://www.wjgnet.com/1007-9327/full/v19/i34/5622.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i34.5622
Hepatocellular carcinoma (HCC) accounts for approximately 5% of all cancers, with more than 500000 new cases diagnosed each year[1]. The link between liver cirrhosis and HCC is well known; more than 90% of HCCs develop in cirrhotic livers, and 3%-8% of cirrhotic patients are diagnosed as HCC carriers each year[2]. Ideally, liver transplantation (LT) is the best treatment option for HCC, as it removes both the tumor and the underlying chronic condition[3].
Milan Criteria (MC) of LT candidates with HCC, based on the number and size of the tumor nodules, has led to 5-year survival rates well above 70% and recurrence rates below 15%[4]. Currently, more than 30% of LT recipients in the United States are HCC carriers[5].
In an intent-to-treat purpose, one of the major limitations of LT in HCC carriers is the time spent on the waiting list; the risk of tumor progression increases with time, resulting in a cumulative probability of dropout from the waiting list of 7.2% for a 6-mo waiting time, which rises to 37.8% and 55.1% for 12 and 18 mo of waiting time, respectively[6].
To attempt to cure to a larger number of HCC carriers, two strategies have been outlined. The first is to downstage those tumors that exceed the Milan Criteria at the time of the first observation, thereby allowing transplantation. The second strategy is to delay the tumor growth using locoregional treatments while the patient is on the waiting list to reduce the dropout rate. The response to locoregional treatment is related to patient prognosis and seems to denote favorable tumor biology[7-10].
Transcatheter arterial chemoembolization (TACE) is the most frequently used treatment of HCC in LT candidates[11]. TACE is usually performed by administering a mixture of epirubicin and Lipiodol to concentrate the drug within the tumor. This is followed by a gelatin sponge (conventional TACE, c-TACE) to obtain occlusion of the feeding arteries of the tumor, with the aim of producing infarction and necrosis of the tumor tissue. Recently, a novel doxorubicin-eluting bead (DEB) has been developed to bind, deliver and elute chemotherapeutic drugs in the tumor area during TACE. Pre-clinical and clinical studies have demonstrated that DEB-TACE produces a higher drug concentration within the tumor than c-TACE while maintaining a lower systemic concentration[12-14].
The assessment of the tumor response after TACE remains a critical issue; the Response Evaluation Criteria in Solid Tumors (RECIST), based on the sum of the largest diameter of target lesions on computed tomography (CT) or magnetic resonance (MR) before and during treatment, can be misleading when assessing the treatment-related tumor necrosis, which is not necessarily associated with a reduction in tumor diameter[15,16]. In 2001, a panel of experts concluded that an estimation of the reduction of viable tumor (recognized as the non-enhancing areas on a CT scan) should be considered the optimal method to assess local response (modified-RECIST)[17].
However, CT findings often underestimate the residual tumor extent, which can be accurately determined only at histology[18]; in this regard, LT represents a unique setting to correctly assess tumor necrosis induced by TACE, as the whole native liver becomes available for histological examination.
Although excellent tumor necrosis rates induced by TACE are reported in LT recipients[19], the impact of TACE on recurrence-free survival remains to be established[20-22].
The aim of this study was to compare DEB-TACE with c-TACE by assessing the histological features of the tumor nodules in native livers removed at transplantation, focusing on the degree of necrosis, as well as to assess the recurrence-free survival of HCC recipients after LT.
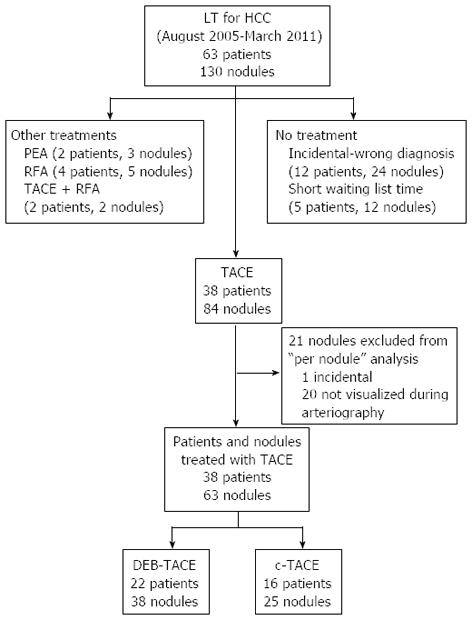
From August 2005 to March 2011, 63 liver transplants were performed in patients with HCC on cirrhosis in our center. We retrospectively analyzed the histological features of 130 HCC nodules in 63 native livers removed at transplantation (2.06 nodules per patient). Patients who received any other type of treatment, including radiofrequency tumor ablation (RFA) or percutaneous ethanol ablation (PEA), or those who were not treated at all were excluded from the present analysis, as described in Figure 1. Thirty-eight patients who received one or more TACEs as the only neoadjuvant therapy before LT represent the study population.
The policy of our center for TACE is to downstage those tumors that are initially beyond the MC. In the present study, only those patients who were successfully downstaged within the MC and therefore underwent LT are considered; TACE was also performed in those patients who fulfilled the MC and entered the waiting list with an expected waiting time longer than 2 mo. Based on the imaging findings, all the patients in the present study were within the Milan Criteria at the time of transplantation.
As the initial endpoint of our study was to confirm the safety of DEB-TACE and to assess its efficacy in achieving tumor necrosis in comparison with c-TACE, we performed a per nodule analysis of the explanted livers to establish the mean percentage of necrosis of any nodule treated by TACE (conventional or DEB). Twenty-one nodules were not reached by the treatment due to the failure to visualize the tumor’s feeding arteries during arteriography (20 cases) or failure to visualize the tumor itself in the pre-LT imaging (1 case); these 21 nodules were not included in the “per nodule” analysis to assess the mean percentage of necrosis produced by TACE. However, these nodules were taken into account in the “per patient analysis” and in the “survival analysis” to precisely quantify the neoplastic burden of each patient, which could influence the prognosis.
Demographics (age, sex), etiology of cirrhosis, the Child-Pugh and the Model for End Stage Liver Disease (MELD score), radiological and pathological tumor classification according to MC, laboratory tests, imaging studies and pathology reports were recorded for each patient. Factors related to tumor biology, such as serum alpha-fetoprotein, microvascular invasion (MVI) and grading, that play a key role in determining tumor recurrence[23-26] were compared between the 2 groups. The waiting time for LT and the interval between the last TACE and LT were also calculated. Computed tomography scans were performed one month after TACE and every 3 mo thereafter; candidates who were initially beyond MC at imaging were reassessed by two interventional radiologist according to the European Association for the Study of the Liver guidelines[17] to define the amount of tumor necrosis after TACE. When radiologic findings demonstrated viable tumor beyond MC, chemoembolization was repeated.
In addition to the type of TACE, the impact of the following risk factors on recurrence-free survival was also assessed: adherence to MC at pathology (considering only the viable portion of each nodule), tumor grading (G3-G4), the presence of MVI, the presence of multiple nodules at pathology, a percent necrosis in the cumulative tumor area (CTA) less than or equal to 50%, high levels of α-fetoprotein (> 70 ng/mL) and the need to repeat TACE before LT.
All patients underwent baseline celiac and superior mesenteric arteriography via a femoral artery approach. Prior to embolization, liver vascular anatomy was identified to check the patency of the portal vein and visualize the arterial feeders of the tumor(s). The procedure was defined as “superselective” when the tip of a highly flexible coaxial microcatheter (2.7 Fr; Progreat; Terumo) was successfully placed in the branches supplying the tumor. When nodules were fed by multiple tiny arteries or when multinodular disease was present, TACE was performed with segmental or lobar (only for c-TACE group) catheterization (non-superselective TACE). Conventional TACE was performed by administering a mixture of 50 mg of epirubicin (Pfizer, New York, NY, United States) in an emulsion with lipiodol (Guebert, Aulnay-sousBois, France), followed by embolization with gelatin sponge particles (SPONGOSTAN; Johnson and Johnson, Gargrave, United Kingdom). DC beads (Biocompatibles, Farnham, Surrey, United Kingdom) became available at our institution beginning in June 2007; thereafter, patients were randomly assigned to one of the two techniques. The caliber of beads was chosen based on the type of catheterization, the vascularity of the lesion, and the tumor diameter. DEB-TACE was performed using 100- to 300-μm beads for single lesions < 50 mm without arteriovenous shunts, whereas in larger tumors, multiloculated lesions or suspected satellites, one vial of 100- to 300-μm beads and one vial of 300- to 500-μm beads were injected. DC beads were impregnated with 75 mg of doxorubicin in each vial to a maximum of 150 mg of doxorubicin loaded in two vials of DC beads (4 mL total).
After LT, a dedicated liver pathologist performed the analysis of all the explanted livers, which were serially cut into sections of approximately 0.5 cm in thickness. The presence of cirrhosis was confirmed in all cases. Every lesion suspected to be HCC was completely paraffin-embedded, and multiple histological sections were made. Tumor grade according to the Edmonson and Steiner[27] classification and the presence of MVI were also assessed, except when complete necrosis of the tumor was achieved. The necrosis rate of each nodule was expressed as the percentage of necrotic tissue within the whole area of the nodule; necrosis was categorized as complete (100%), appropriate (90% or greater), partial (between 51% and 89%), or inadequate (50% or lower) as described previously[28,29]. The sum of the tumor diameters, CTA, and the cumulative necrotic and viable areas (including not-treated nodules) were measured in each patient to calculate the percentage of tumor necrosis within the cumulative tumor area (% necrosis on CTA). Adherence to MC was assessed during the pathological examination using only the viable portion of each nodule. Inflammatory and fibrotic changes in the tissue surrounding the tumor nodule were analyzed and categorized as poor/absent, moderate or enhanced reaction. The localization of microspheres with respect to the 38 HCC nodules treated with DEB-TACE was assessed and defined as intratumoral (exclusively within the tumor capsule), peritumoral (outside the nodule but within 5 mm of the tumor capsule), intra- and peritumoral or intratumoral (microspheres found in the cirrhotic parenchyma beyond 5 mm from the tumor capsule).
Continuous variables were reported as the mean and standard deviation or as median and range and were compared using Student’s t test or Mann-Whitney U test when appropriate. Categorical variables were reported as numbers and percentages and compared using Fisher’s exact test. Recurrence-free survival was calculated from the day of surgery to the first follow-up visit at which tumor recurrence was diagnosed or, in patients without recurrence, to the most recent follow-up visit. Follow-up of those patients who died without evidence of recurrence was censored at the time of death. The impact of each individual variable in determining HCC recurrence-free survival was assessed using the Kaplan-Meier method and compared using the log-rank test. Continuous variables, including pre-LT serum alpha-fetoprotein levels and the percentage of necrosis on CTA, were dichotomized; cutoff values were defined according to receiver operating characteristic curve analysis[30]. To identify factors independently related to HCC recurrence, the multivariate Cox proportional-hazard regression analysis was applied, taking in account only the variables that proved significant in the univariate analysis. A two-sided P value of less than 0.05 was considered statistically significant in all cases.
Twenty-two patients underwent DEB-TACE, and 16 underwent c-TACE. Age at LT, gender, etiology and severity of cirrhosis were comparable in the two treatment arms (Table 1).
| Variable | All treated patients (n= 38) | Type of TACE | P value | |
| DEB-TACE (n= 22) | c-TACE (n= 16) | |||
| Age at LT (yr) | 56.5 ± 6.5 | 57.2 ± 6.5 | 55.6 ± 6.5 | 0.4545 |
| Male gender | 34 (89.5) | 19 (86.4) | 15 (93.7) | 0.6245 |
| MELD score | 10 (6-27) | 9 (6-27) | 10 (7-16) | 0.4688 |
| Etiology of cirrhosis | 0.1834 | |||
| HCV-related | 22 (57.9) | 10 (45.5) | 12 (75.0) | |
| HBV-related | 11 (28.9) | 8 (36.3) | 3 (18.7) | |
| Non-viral | 5 (13.2) | 4 (18.2) | 1 (6.3) | |
| Waiting list time (mo) | 3.1 (0.1-26.7) | 2.9 (0.1-24.3) | 3.3 (0.6-26.7) | 0.5844 |
| Interval between last TACE and LT (mo) | 3.6 (0.1-15.9) | 2.3 (0.2-13.8) | 5.5 (0.9-15.9) | 0.0625 |
| Repeated TACE | 20 (52.6) | 12 (54.5) | 8 (50.0) | 0.7817 |
| Adherence to MC at imaging before TACE | 0.3243 | |||
| Within MC | 21 (55.3) | 14 (63.6) | 7 (43.7) | |
| Beyond MC | 17 (44.7) | 8 (36.4) | 9 (56.3) | |
| BCLC stage before TACE | 0.3243 | |||
| A | 21 (55.3) | 14 (63.6) | 7 (43.7) | |
| B | 17 (44.7) | 8 (36.4) | 9 (56.3) | |
| Number of nodules before TACE | 2 (1-5) | 2 (1-5) | 2 (1-5) | 0.8708 |
| Nodule number class before TACE | 0.2222 | |||
| 1 nodule | 16 (42.1) | 8 (36.4) | 8 (50.0) | |
| 1 < nodules < 4 | 13 (34.2) | 10 (45.4) | 3 (18.8) | |
| Nodules ≥ 4 | 9 (23.7) | 4 (18.2) | 5 (31.2) | |
| 1Serum α-fetoprotein > 70 ng/mL | 8/33 (24.2) | 3/18 (16.7) | 5/15 (33.3) | 0.4811 |
| Post-LT follow-up (mo) | 39.9 ± 22.5 | 34.9 ± 19.0 | 46.8 ± 25.6 | 0.1065 |
No major complications were observed after TACE. The post-embolization syndrome (transient fever, abdominal pain, nausea) was the most common complication following chemoembolization in both groups; all side effects were successfully treated with medical therapy. The median time spent on the waiting list was similar in the two groups (3.3 mo in the c-TACE, vs 2.9 mo in the DEB-TACE group, respectively, P = 0.5844).
DEB-TACE and c-TACE were repeated in 12 (54.5%) and 8 (50%) patients, respectively; the maximum number of treatments per patient was 3. The post-LT mean follow-up time was 34.9 ± 19.0 and 46.8 ± 25.6 mo for the DEB-TACE and c-TACE groups, respectively (P = 0.1065). No patients were lost at follow-up.
The size and focality of the tumors were comparable in the two groups at explant examination. The mean tumor necrosis was 55.7% ± 41.9% and 52.2% ± 40.9% in DEB- and c-TACE groups, respectively (P = 0.7420). A trend towards a higher probability of an appropriate response was observed in the DEB-TACE group (17/38, 44.7% of nodules) in comparison with the c-TACE group (8/25, 32.0% of nodules), although this difference was not statistically significant (P = 0.2834). No difference in necrosis was found comparing the superselective procedures performed with DEB-TACE or c-TACE (76.2% ± 33.8% vs 69.1% ± 36.5%, P = 0.5803). However, independent of the type of TACE (DEB or conventional), superselective procedures resulted in a higher percentage of necrosis than did non-superselective procedures (73.9% ± 34.3% vs 31.3% ± 37.0%, P = 0.0018) (Table 2).
| Variable | All treated nodules 63 in 38 patients | Type of TACE | P value | |
| DEB-TACE 38 in 22 patients | c-TACE 25 in 16 patients | |||
| Degree of necrosis | 54.3% ± 41.2% | 55.7% ± 41.9% | 52.2% ± 40.9% | 0.7420 |
| Complete necrosis (100%) | 21 (33.3) | 14 (36.8) | 7 (28.0) | 0.5877 |
| Histological response | 0.2834 | |||
| Appropriate (necrosis ≥ 90%) | 25 (39.7) | 17 (44.7) | 8 (32.0) | |
| Partial (50% < necrosis < 90%) | 9 (14.3) | 3 (7.9) | 6 (24.0) | |
| Inadequate (necrosis ≤ 50%) | 29 (46.0) | 18 (47.4) | 11 (44.0) | |
| Diameters of nodules (cm) | 2 (0.7-10) | 1.8 (0.7-4.5) | 2.2 (1-10) | 0.1752 |
| Number of nodules | 0.2492 | |||
| Single | 17 (27.0) | 8 (21.1) | 9 (36.0) | |
| Degree of necrosis | 63.1% ± 37.8% | 69.7% ± 34.8% | 57.2% ± 41.5% | 0.5144 |
| Multiple | 46 (73.0) | 30 (78.9) | 16 (64.0) | |
| Degree of necrosis | 51.1% ± 42.3% | 52.0% ± 43.4% | 49.4% ± 41.7% | 0.8454 |
| Modality of TACE | 0.3015 | |||
| Superselective | 34 (54.0) | 23 (60.5) | 11 (44.0) | |
| Degree of necrosis | 73.9% ± 34.3% | 76.2% ± 33.8% | 69.1% ± 36.5% | 0.5803 |
| Non-superselective | 29 (46.0) | 15 (39.5%) | 14 (56.0) | |
| Degree of necrosis | 31.3% ± 37.0% | 24.3% ± 33.3% | 38.9% ± 40.5% | 0.2970 |
Microscopically, tumor necrosis was mixed, colliquative and coagulative in all cases. In the DEB-TACE group only, a “foreign body reaction” with granulomatosis giant cells was observed in the tissue surrounding the tumor in 20 out of 22 patients (90.1%). As described in Figure 2, the nonspecific acute inflammatory infiltrate, containing foamy histiocytes and lymphocytes, was more enhanced at the tumor periphery of DEB-TACE-treated nodules in comparison with the c-TACE-treated ones. In most cases, DEB-TACE-treated nodules were surrounded by thick walls of tissue made of degenerated collagen fibers, inflamed granulation tissue, and hyalinization. The peri-tumor fibrous tissue of the nodules treated with c-TACE was thinner and apparently less affected by the secondary changes induced by DEB-TACE. These features were not dependent on the time between last treatment and LT; patients with nodules surrounded by an enhanced or mild fibrotic reaction were transplanted 4.2 ± 4.0 mo after TACE, whereas patients with poor-absent fibrosis were transplanted 6.0 ± 4.1 mo after TACE (P = 0.2024).
The distribution of microspheres with respect to the 38 nodules treated by DEB-TACE was intratumoral (11/38, 28.9%), intra- and peritumoral (14/38, 36.8%), peritumoral (10/38, 26.3%) and intratumoral (3/38, 7.9%). Nodules’ necrosis differed with respect to the distribution of beads (85.9%, 57.0%, 34.5% and 10.0% for intratumoral, intra- and peritumoral, peritumoral and intratumoral distribution, respectively, P = 0.0041).
Ten of 17 patients were successfully downstaged within the MC; the effectiveness of DEB-TACE and c-TACE was similar to this regard. Seven out of 17 patients who were beyond MC at the imaging performed before TACE (8 patients in DEB-TACE and 9 in c-TACE group) remained outside the MC at pathology; the failure to accurately stage the tumor during the imaging performed before the LT was related to the misdiagnosis of complete necrosis (Table 3).
| Variable | All treated patients (n= 38) | Type of TACE | P value | |
| DEB-TACE (n= 22) | c-TACE (n= 16) | |||
| Number of nodules per patients | 2.2 ± 1.3 | 2.2 ± 1.2 | 2.1 ± 1.4 | 0.7019 |
| Untreated nodules | 21/84 (25.0) | 13/51 (25.4) | 8/33 (24.2) | 0.8934 |
| Nodule number class at pathology | 0.1473 | |||
| 1 nodule | 17 (44.7) | 8 (36.4) | 9 (56.3) | |
| 1 < nodules < 4 | 14 (36.8) | 11 (50.0) | 3 (18.7) | |
| ≥ 4 nodules | 7 (18.4) | 3 (13.6) | 4 (25.0) | |
| Sum of tumor diameters (cm) | 4.2 ± 2.8 | 4.1 ± 2.4 | 4.5 ± 3.3 | 0.5813 |
| CTA (cm2) | 5.5 (0.8-78.5) | 4.6 (1.5-19.1) | 7.2 (0.8-78.5) | 0.8592 |
| Necrosis on CTA | 55.2% ± 37.0% | 58.8% ± 36.6% | 50.2% ± 38.1% | 0.4856 |
| Adherence to MC at pathology | 0.4250 | |||
| Within MC | 31 (81.6) | 19 (86.4) | 12 (75.0) | |
| Beyond MC | 7 (18.4) | 3 (13.6) | 4 (25.0) | |
| Histological response | 0.2896 | |||
| Appropriate (necrosis on CTA ≥ 90%) | 11 (28.9) | 8 (36.4) | 3 (18.7) | |
| Partial (50% < necrosis on CTA < 90%) | 8 (21.1) | 3 (13.6) | 5 (31.3) | |
| Inadequate (necrosis on CTA ≤ 50%) | 19 (50.0) | 11 (50.0) | 8 (50.0) | |
| Risk factors for recurrence | ||||
| Microvascular invasion | 7 (18.4) | 5 (22.7) | 2 (12.5) | 0.4271 |
| 1Grading > 2 | 7 (18.4) | 2/18 (11.1) | 5/14 (35.7) | 0.1948 |
The number of nodules that were not reached by TACE (21/84, 25%) was similar in the 2 groups (13/51, 25.4% in DEB-TACE and 8/33, 24.2% in c-TACE group, P = 0.8934). The mean diameter of the missed nodules was 1.1 ± 0.5 cm, and 10 out of these 21 nodules (47.6%) were equal or inferior to 1 centimeter in size. The number of HCC nodules, the sum of the tumor diameters and the CTA (also including untreated nodules) were similar in the two groups. The mean percentage of necrosis on CTA was 58.8% and 50.2% in the DEB- and c-TACE group, respectively (P = 0.4856); no difference in terms of response to treatment in various subcategories was observed. Risk factors for recurrence, such as a pre-transplant serum α-fetoprotein greater than 70 ng/mL (Table 1), tumor grading and microvascular invasion were similarly distributed in the two treatment arms.
Overall 3-year survival after LT was 73.9% and 58.7% in the DEB-TACE and c-TACE groups, respectively (P = 0.7511). Seven (18.4%) patients experienced tumor recurrence after LT; the main site of recurrence was the liver (5 patients), the spinal cord and the liver concurrently (1 patient) and the adrenal gland (1 patient). The mean time to recurrence was 17.0 ± 5.5 mo; three out of seven patients were alive with recurrence at the time of publication.
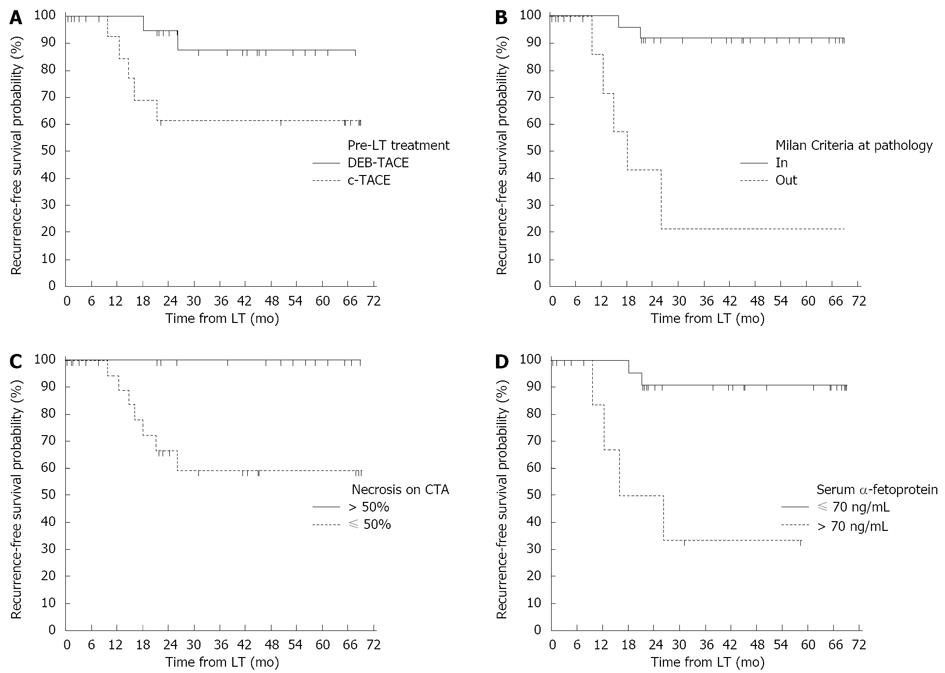
The three-year recurrence-free survival was significantly higher in patients who were treated preoperatively with DEB-TACE than with c-TACE (87.4% vs 61.5%, P = 0.0493, Figure 3A).
Other factors affecting recurrence-free survival included Milan Criteria unfulfilled at pathology, percentage of necrosis on CTA lower than 50% and pre-transplant serum α-fetoprotein levels greater than 70 ng/mL (Figure 3B-D). On multivariate analysis, a lack of treatment with DEB-TACE, serum α-fetoprotein levels exceeding 70 ng/mL and Milan Criteria unfulfilled at pathology were independent predictors of tumor recurrence (Table 4).
| Risk factors | Univariate analysis | Multivariate analysis | |||
| 3-yr recurrence-free survival rate | HR (95%CI) | Log rank P value | Exp(b) (95%CI) | P value | |
| Tumor grading G3-G4 (vs G1-G2) | 66.7% vs 74.2% | 1.76 (0.289-13.149) | 0.4934 | ||
| Presence of microvascular invasion (vs absence) | 60.0% vs 80.7% | 2.74 (0.451-39.277) | 0.2071 | ||
| Multiple nodules at pathology (vs single) | 69.1% vs 85.7% | 2.15 (0.460-9.070) | 0.3478 | ||
| Repeated TACE (vs single) | 74.1% vs 80.0% | 1.08 (0.244-4.821) | 0.9148 | ||
| Necrosis on CTA ≤ 50% (vs > 50%) | 59.3% vs 100.0% | NA1 | 0.0098 | NA1 | NA1 |
| MC unfulfilled at pathology (vs fulfilled) | 21.4% vs 92.0% | 13.84 (10.121-636.416) | < 0.0001 | 11.6 (1.932-69.646) | 0.0077 |
| Absence of DEB-TACE (vs presence) | 61.5% vs 87.4% | 4.47 (1.005-22.188) | 0.0493 | 15.45 (1.457-163.766) | 0.0237 |
| α-fetoprotein > 70 ng/mL (vs ≤ 70 ng/mL) | 33.3% vs 90.9% | 10.31 (4.749-370.242) | 0.0008 | 15.31 (1.766-132.614) | 0.0137 |
Among patients with HCC awaiting LT, TACE is the most commonly used neo-adjuvant therapy[11]. Although TACE can successfully downstage 24% to 63% of HCCs, pre-LT treatment is not clearly associated with any survival benefit[22,31-33]; in a large multicenter study, the 5-year recurrence-free survival was 67% in patients treated with TACE prior to LT and 64% in those not treated[20].
Unlike conventional TACE, which is the most commonly used technique, DEB-TACE is based on calibrated microspheres made of non-degradable polymers that produce permanent vascular embolization and increase intra-tumor drug delivery. There are 3 substantial pharmacokinetic advantages associated with DEB-TACE: a continuous elution of the drug for prolonged time, a higher concentration locally into the tumor and a lower systemic exposure to the drug in comparison with c-TACE[12]. In a preclinical study, Hong et al demonstrated that the peak of doxorubicin within the tumor is registered after three days, and drug levels remain high up to fourteen days after treatment[14].
Several clinical studies have compared DEB and conventional TACE in non-transplant settings. A recent randomized study including 212 patients failed to demonstrate a significant difference in the overall radiological response, although a better safety profile and a trend toward a better response rate was observed for DEB-TACE[13]. Malagari et al[34] demonstrated that DEB-TACE was able to stabilize disease in a higher percentage of patients when compared with bland embolization (embolic agents without drug), but the survival rate at 12 mo did not differ in the two groups. In another prospective study, complete and partial response rates, tumor recurrence and overall survival were similar with DEB-TACE and conventional TACE[35]. Although a retrospective study recently suggested a higher 2-year survival rate in DEB-TACE patients[36], the superiority of this technique remains to be further investigated.
Liver transplant candidates exhibit completely different characteristics than those patients considered in the above-mentioned studies. First, HCC in LT candidates is not advanced. Furthermore, the response to TACE in terms of tumor necrosis has clinical relevance only in those patients who require downstaging, whereas in the others, the goal is to halt tumor progression. Last, recurrence-free survival, measured following LT, is a realistic endpoint, as in the non-transplant setting, TACE is not intended to be curative[37].
Few reports are available about the results of DEB-TACE in LT candidates. A small study from Milan reported a higher complete histological necrosis rate (77%) in patients treated with DEB-TACE. However, only 8 patients had been treated with DEB-TACE in that study, while the 8 patients of the control group received bland embolization (non-loaded microspheres), with very low complete necrosis rates (27.2%)[38]. Unlike from the present study, Farris et al[39] recently reported a significantly higher necrosis rate after c-TACE in comparison with DEB-TACE (66.4% vs 46.1%). However, no mention of the HCC recurrence-free survival of the patients was made in these studies.
In our series, histopathological examination of the native livers did not indicate a significant difference between DEB-TACE and conventional TACE with regard to the effectiveness of the two different procedures in inducing histological necrosis and achieving tumor downstaging. However, a peculiar histological pattern was associated with DEB-TACE; DEB-TACE was characterized by an intense inflammatory and fibrotic reaction in the area surrounding the tumor tissue that was not observed in those patients treated with conventional TACE. Remarkably, a lower tumor recurrence rate after LT was associated with DEB-TACE. Furthermore, DEB-TACE was identified as an independent predictor of recurrence-free survival in the multivariate analysis. The others independent prognostic determinants found in the present study, serum alpha-fetoprotein levels and adherence to MC at histopathological examination, have been previously identified by others to be strictly linked with HCC recurrence after LT[3].
Pretransplant ablative treatments have the potential to decrease the release and growth of HCC metastases. As the release of tumor cells can be intermittent, the continuous elution of doxorubicin and the distribution of loaded beads in the vessels around the nodule might maintain a prolonged antineoplastic effect and explain the lower recurrence rate observed in the DEB-TACE group. The biological significance of the intense tissue reaction that surrounds the tumor treated with DEB-TACE must to be further investigated. However, one might speculate that the tissue reaction could play a role in limiting tumor spread.
The difference in the chemotherapeutic agent employed in the two different TACE techniques (epirubicin in c-TACE and doxorubicin in DEB-TACE) is unlikely to have influenced the results; in a large randomized controlled that compared c-TACE made using a lipiodol emulsion containing epirubicin or doxorubicin, no difference in the incidence of adverse reactions, changes in alpha-fetoprotein, extent of tumor reduction or the survival rates between the two drugs was reported[40].
Although further confirmation of our findings with randomized controlled trials is warranted, our report seems to indicate that the use of DEB-TACE in LT recipients with HCC can increase recurrence-free survival after liver transplantation.
Transcatheter arterial chemoembolization (TACE) is the most common locoregional treatment in cirrhotic patients with hepatocellular carcinoma (HCC) awaiting liver transplantation (LT), and the main objective of TACE is to prevent tumor progression in HCC patients who have already met the Criteria for transplantation or to downstage tumors initially outside Milan Criteria to allow LT. In addition to conventional TACE (c-TACE), based on mixtures of anticancer drugs, lipiodol and a gelatin sponge, a procedure with calibrated doxorubicin-loaded microspheres has been recently developed (DEB-TACE). In pre-LT setting, only a few reports have compared the impact of different TACE regimens on tumor histology and recurrence-free survival after transplantation.
Pre-clinical and clinical studies have demonstrated that DEB-TACE produces a higher drug concentration within the tumor than does c-TACE in presence of a lower systemic concentration, but its superiority in inducing tumor necrosis and increasing recurrence-free survival remains to be further investigated. As the whole native liver becomes available for histological examination, LT represents a unique setting to correctly assess necrosis and histological changes in tumor nodules of patients treated by TACE. This approach can be useful in developing new strategies to decrease the release of HCC metastases in patients awaiting LT.
A lower tumor recurrence rate after LT was observed in patients who were treated preoperatively with DEB-TACE. Although no significant differences were observed in terms of tumor necrosis between DEB and c-TACE, a peculiar histological pattern was associated with DEB-TACE, characterized by an intense inflammatory and fibrotic reaction in the area surrounding the tumor tissue. This finding, in addition to the prolonged antineoplastic effect of loaded beads in the vessels around the nodule, could limit tumor spread during time on the waiting list and could explain the lower postoperative recurrence rate observed in the DEB-TACE group.
According to the results of this study, DEB-TACE is an effective locoregional tool for the management of HCC patients awaiting liver transplantation and can increase recurrence-free survival after LT.
TACE indicates transcatheter arterial chemoembolization. DEB-TACE indicates TACE with calibrated, doxorubicin-loaded microspheres used to bind, deliver and elute chemotherapeutic drugs in the tumor area. c-TACE indicates the conventional TACE procedure, performed by administering a mixture of epirubicin in an emulsion with lipiodol followed by a gelatin sponge to obtain occlusion of the feeding arteries of the tumor. HCC indicates hepatocellular carcinoma. LT indicates liver transplantation.
The authors present an interesting retrospective single-center study that clearly addresses pre-LT treatment of HCC. The authors highlight an important locoregional therapeutic tool, DEB-TACE, which has become increasingly utilized and can improve the outcomes of LT for HCC. The explanted livers underwent very close pathological scrutiny to judge the effects of the 2 different therapies; data analysis is well done. The paper is well written, and the manuscript improves significantly on the knowledge of the role of DEB-TACE in the management of HCC.
P- Reviewers Ramsay M, Sonzogni A, Wong RJ S- Editor Wen LL L- Editor A E- Editor Zhang DN
| 1. | Nordenstedt H, White DL, El-Serag HB. The changing pattern of epidemiology in hepatocellular carcinoma. Dig Liver Dis. 2010;42 Suppl 3:S206-S214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 367] [Cited by in F6Publishing: 394] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 2. | Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35-S50. [PubMed] [Cited in This Article: ] |
| 3. | Vivarelli M, Risaliti A. Liver transplantation for hepatocellular carcinoma on cirrhosis: strategies to avoid tumor recurrence. World J Gastroenterol. 2011;17:4741-4746. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 10] [Cited by in F6Publishing: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5110] [Cited by in F6Publishing: 5002] [Article Influence: 178.6] [Reference Citation Analysis (0)] |
| 5. | Thuluvath PJ, Guidinger MK, Fung JJ, Johnson LB, Rayhill SC, Pelletier SJ. Liver transplantation in the United States, 1999-2008. Am J Transplant. 2010;10:1003-1019. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 324] [Cited by in F6Publishing: 289] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 6. | Yao FY, Bass NM, Nikolai B, Merriman R, Davern TJ, Kerlan R, Ascher NL, Roberts JP. A follow-up analysis of the pattern and predictors of dropout from the waiting list for liver transplantation in patients with hepatocellular carcinoma: implications for the current organ allocation policy. Liver Transpl. 2003;9:684-692. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 213] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 7. | Yao FY, Kerlan RK, Hirose R, Davern TJ, Bass NM, Feng S, Peters M, Terrault N, Freise CE, Ascher NL. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology. 2008;48:819-827. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 385] [Cited by in F6Publishing: 386] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 8. | Majno PE, Adam R, Bismuth H, Castaing D, Ariche A, Krissat J, Perrin H, Azoulay D. Influence of preoperative transarterial lipiodol chemoembolization on resection and transplantation for hepatocellular carcinoma in patients with cirrhosis. Ann Surg. 1997;226:688-701; discussion 701-703. [PubMed] [Cited in This Article: ] |
| 9. | Otto G, Herber S, Heise M, Lohse AW, Mönch C, Bittinger F, Hoppe-Lotichius M, Schuchmann M, Victor A, Pitton M. Response to transarterial chemoembolization as a biological selection criterion for liver transplantation in hepatocellular carcinoma. Liver Transpl. 2006;12:1260-1267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 319] [Cited by in F6Publishing: 304] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 10. | Millonig G, Graziadei IW, Freund MC, Jaschke W, Stadlmann S, Ladurner R, Margreiter R, Vogel W. Response to preoperative chemoembolization correlates with outcome after liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2007;13:272-279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 198] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 11. | Fujiki M, Aucejo F, Kim R. General overview of neo-adjuvant therapy for hepatocellular carcinoma before liver transplantation: necessity or option? Liver Int. 2011;31:1081-1089. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, Ayuso C, Castells L, Montañá X, Llovet JM. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474-481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 694] [Cited by in F6Publishing: 749] [Article Influence: 44.1] [Reference Citation Analysis (1)] |
| 13. | Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, Pitton M, Sergent G, Pfammatter T, Terraz S. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1063] [Cited by in F6Publishing: 1115] [Article Influence: 74.3] [Reference Citation Analysis (0)] |
| 14. | Lewis AL, Taylor RR, Hall B, Gonzalez MV, Willis SL, Stratford PW. Pharmacokinetic and safety study of doxorubicin-eluting beads in a porcine model of hepatic arterial embolization. J Vasc Interv Radiol. 2006;17:1335-1343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 151] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 15. | Forner A, Ayuso C, Varela M, Rimola J, Hessheimer AJ, de Lope CR, Reig M, Bianchi L, Llovet JM, Bruix J. Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: are response evaluation Criteria in solid tumors reliable? Cancer. 2009;115:616-623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 323] [Cited by in F6Publishing: 352] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 16. | van Persijn van Meerten EL, Gelderblom H, Bloem JL. RECIST revised: implications for the radiologist. A review article on the modified RECIST guideline. Eur Radiol. 2010;20:1456-1467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 17. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [PubMed] [Cited in This Article: ] |
| 18. | Bhattacharjya S, Bhattacharjya T, Quaglia A, Dhillon AP, Burroughs AK, Patch DW, Tibballs JM, Watkinson AF, Rolles K, Davidson BR. Liver transplantation in cirrhotic patients with small hepatocellular carcinoma: an analysis of pre-operative imaging, explant histology and prognostic histologic indicators. Dig Surg. 2004;21:152-159; discussion 152-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Wong LL, Tanaka K, Lau L, Komura S. Pre-transplant treatment of hepatocellular carcinoma: assessment of tumor necrosis in explanted livers. Clin Transplant. 2004;18:227-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Decaens T, Roudot-Thoraval F, Bresson-Hadni S, Meyer C, Gugenheim J, Durand F, Bernard PH, Boillot O, Boudjema K, Calmus Y. Impact of pretransplantation transarterial chemoembolization on survival and recurrence after liver transplantation for hepatocellular carcinoma. Liver Transpl. 2005;11:767-775. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 159] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 21. | Oldhafer KJ, Chavan A, Frühauf NR, Flemming P, Schlitt HJ, Kubicka S, Nashan B, Weimann A, Raab R, Manns MP. Arterial chemoembolization before liver transplantation in patients with hepatocellular carcinoma: marked tumor necrosis, but no survival benefit? J Hepatol. 1998;29:953-959. [PubMed] [Cited in This Article: ] |
| 22. | Graziadei IW, Sandmueller H, Waldenberger P, Koenigsrainer A, Nachbaur K, Jaschke W, Margreiter R, Vogel W. Chemoembolization followed by liver transplantation for hepatocellular carcinoma impedes tumor progression while on the waiting list and leads to excellent outcome. Liver Transpl. 2003;9:557-563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 347] [Cited by in F6Publishing: 326] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 23. | Esnaola NF, Lauwers GY, Mirza NQ, Nagorney DM, Doherty D, Ikai I, Yamaoka Y, Regimbeau JM, Belghiti J, Curley SA. Predictors of microvascular invasion in patients with hepatocellular carcinoma who are candidates for orthotopic liver transplantation. J Gastrointest Surg. 2002;6:224-232; discussion 232. [PubMed] [Cited in This Article: ] |
| 24. | Fiorentino M, Altimari A, Ravaioli M, Gruppioni E, Gabusi E, Corti B, Vivarelli M, Bringuier PP, Scoazec JY, Grigioni WF. Predictive value of biological markers for hepatocellular carcinoma patients treated with orthotopic liver transplantation. Clin Cancer Res. 2004;10:1789-1795. [PubMed] [Cited in This Article: ] |
| 25. | Cillo U, Vitale A, Bassanello M, Boccagni P, Brolese A, Zanus G, Burra P, Fagiuoli S, Farinati F, Rugge M. Liver transplantation for the treatment of moderately or well-differentiated hepatocellular carcinoma. Ann Surg. 2004;239:150-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in F6Publishing: 272] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 26. | Sotiropoulos GC, Malagó M, Molmenti E, Paul A, Nadalin S, Brokalaki E, Kühl H, Dirsch O, Lang H, Broelsch CE. Liver transplantation for hepatocellular carcinoma in cirrhosis: is clinical tumor classification before transplantation realistic? Transplantation. 2005;79:483-487. [PubMed] [Cited in This Article: ] |
| 27. | Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462-503. [PubMed] [Cited in This Article: ] |
| 28. | Bargellini I, Vignali C, Cioni R, Petruzzi P, Cicorelli A, Campani D, De Simone P, Filipponi F, Bartolozzi C. Hepatocellular carcinoma: CT for tumor response after transarterial chemoembolization in patients exceeding Milan Criteria--selection parameter for liver transplantation. Radiology. 2010;255:289-300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Golfieri R, Cappelli A, Cucchetti A, Piscaglia F, Carpenzano M, Peri E, Ravaioli M, D’Errico-Grigioni A, Pinna AD, Bolondi L. Efficacy of selective transarterial chemoembolization in inducing tumor necrosis in small (< 5 cm) hepatocellular carcinomas. Hepatology. 2011;53:1580-1589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 201] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 30. | Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29-36. [PubMed] [Cited in This Article: ] |
| 31. | Chapman WC, Majella Doyle MB, Stuart JE, Vachharajani N, Crippin JS, Anderson CD, Lowell JA, Shenoy S, Darcy MD, Brown DB. Outcomes of neoadjuvant transarterial chemoembolization to downstage hepatocellular carcinoma before liver transplantation. Ann Surg. 2008;248:617-625. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 225] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 32. | Roayaie S, Frischer JS, Emre SH, Fishbein TM, Sheiner PA, Sung M, Miller CM, Schwartz ME. Long-term results with multimodal adjuvant therapy and liver transplantation for the treatment of hepatocellular carcinomas larger than 5 centimeters. Ann Surg. 2002;235:533-539. [PubMed] [Cited in This Article: ] |
| 33. | De Luna W, Sze DY, Ahmed A, Ha BY, Ayoub W, Keeffe EB, Cooper A, Esquivel C, Nguyen MH. Transarterial chemoinfusion for hepatocellular carcinoma as downstaging therapy and a bridge toward liver transplantation. Am J Transplant. 2009;9:1158-1168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 34. | Malagari K, Pomoni M, Kelekis A, Pomoni A, Dourakis S, Spyridopoulos T, Moschouris H, Emmanouil E, Rizos S, Kelekis D. Prospective randomized comparison of chemoembolization with doxorubicin-eluting beads and bland embolization with BeadBlock for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2010;33:541-551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 237] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 35. | Sacco R, Bargellini I, Bertini M, Bozzi E, Romano A, Petruzzi P, Tumino E, Ginanni B, Federici G, Cioni R. Conventional versus doxorubicin-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2011;22:1545-1552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 181] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 36. | Dhanasekaran R, Kooby DA, Staley CA, Kauh JS, Khanna V, Kim HS. Comparison of conventional transarterial chemoembolization (TACE) and chemoembolization with doxorubicin drug eluting beads (DEB) for unresectable hepatocelluar carcinoma (HCC). J Surg Oncol. 2010;101:476-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 37. | Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M, Talwalkar J. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698-711. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1232] [Cited by in F6Publishing: 1298] [Article Influence: 81.1] [Reference Citation Analysis (0)] |
| 38. | Nicolini A, Martinetti L, Crespi S, Maggioni M, Sangiovanni A. Transarterial chemoembolization with epirubicin-eluting beads versus transarterial embolization before liver transplantation for hepatocellular carcinoma. J Vasc Interv Radiol. 2010;21:327-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 39. | Farris AB, Dursun N, Dhanasekaran R, Coban I, McIntosh EB, Adsay NV, Kim HS. Tumoral and angiogenesis factors in hepatocellular carcinoma after locoregional therapy. Pathol Res Pract. 2012;208:15-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 40. | Kawai S, Tani M, Okamura J, Ogawa M, Ohashi Y, Monden M, Hayashi S, Inoue J, Kawarada Y, Kusano M. Prospective and randomized trial of lipiodol-transcatheter arterial chemoembolization for treatment of hepatocellular carcinoma: a comparison of epirubicin and doxorubicin (second cooperative study). The Cooperative Study Group for Liver Cancer Treatment of Japan. Semin Oncol. 1997;24:S6-38-S6-S6-38-45. [PubMed] [Cited in This Article: ] |