Published online Oct 28, 2013. doi: 10.3748/wjg.v19.i40.6876
Revised: July 29, 2013
Accepted: September 16, 2013
Published online: October 28, 2013
AIM: To investigate the association between nuclear β-catenin overexpression in rectal adenocarcinoma and radioresistance.
METHODS: A retrospective analysis was conducted. The analysis involved 136 patients with locally advanced rectal adenocarcinoma who underwent short-course preoperative radiotherapy and radical resection. The expression of β-catenin in both pretreatment biopsy specimens and resected primary tumor tissues was examined by immunohistochemistry. The correlation of β-catenin expression with radioresistance was evaluated using the tumor regression grading (TRG) system. The relationship between β-catenin expression and clinicopathological characteristics was also analyzed. Univariate and logistic multivariate regression analyses were adopted to determine the independent factors of radioresistance.
RESULTS: Nuclear β-catenin overexpression was more evident in radioresistant rectal adenocarcinoma than in radiosensitive rectal adenocarcinoma (57.6% vs 16.7%, P < 0.001). Nuclear β-catenin was overexpressed in favor of poor TRG (≤ 2), whereas membrane β-catenin was expressed in favor of good TRG (≥ 3). Nuclear β-catenin expression in tumor cell differentiation (P = 0.018), lymph node metastasis (P = 0.022), and TRG (P < 0.001) showed significant differences. Univariate analyses demonstrated that radioresistance is associated with nuclear β-catenin overexpression (P < 0.001). In addition, logistic multivariate regression analysis indicated that only three factors, namely, tumor size (P < 0.001), tumor cell differentiation (P < 0.001), and nuclear β-catenin overexpression (P < 0.001), are associated with radioresistance. By using radioresistance as a prediction target, nuclear β-catenin-based prediction alone achieved 83% accuracy, 65% sensitivity, and 88% specificity.
CONCLUSION: Nuclear β-catenin overexpression may be a valuable candidate to predict the response of rectal adenocarcinoma to preoperative radiotherapy.
Core tip: In this paper we investigated the relationship between overexpression of nuclear β-catenin in rectal adenocarcinoma and radioresistance. We first confirmed that nuclear β-catenin overexpression in rectal adenocarcinoma is associated with radioresistance. Most importantly, we found that nuclear β-catenin-based prediction achieved a 83% accuracy, 65% sensitivity and 88% specificity for radioresistance. We provided a novel possible molecular mechanism to explain the radioresistance in rectal adenocarcinoma and thus may provide a new therapeutic target for enhancing radiosensitivity.
- Citation: Wang L, Zhang XM, Li Z, Liu XJ, Chai J, Zhang GY, Cheng YF. Overexpression of nuclear β-catenin in rectal adenocarcinoma is associated with radioresistance. World J Gastroenterol 2013; 19(40): 6876-6882
- URL: https://www.wjgnet.com/1007-9327/full/v19/i40/6876.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i40.6876
Colorectal cancer (CRC) is the third most common malignancy and the fourth most frequent cause of cancer deaths worldwide. Surgery is the predominant therapy for rectal cancer. However, rectal cancer treatment with surgery alone is insufficient because of the high incidence of local recurrence. During the past decades, preoperative radiotherapy and surgery have been increasingly used together for locally advanced rectal cancer to reduce local treatment failure[1-4]. However, many rectal cancer patients are resistant to preoperative radiotherapy because of the heterogeneity of treatment response. Therefore, predicting neoadjuvant radiotherapy response may allow individualization and more rational selection of patients that will most likely benefit from this therapy. A growing number of studies have investigated molecular markers for the prediction of tumor response to radiotherapy. XIAP[5], human phosphatidylethanolamine-binding protein 4[6], and epidermal growth factor receptor[7] are the molecular markers associated with tumor response to radiotherapy in rectal cancer.
β-catenin, a component of the Wingless/Wnt signaling pathway, can activate target genes linked to the APC gene in CRC. The localization of β-catenin is related to its function in cancer growth[8]. β-catenin in the cytoplasm and membrane binds with the intracellular domain of E-cadherin, a cell-to-cell adhesion molecule, and maintains normal tissue architecture. In the presence of a Wnt signal, β-catenin translocates to the nucleus and interacts with the lymphoid enhancing factor/T-cell factor to promote the transcription of several target genes involved in cell proliferation[9].
A previous study indicated that Wnt/β-catenin mediates the radiation resistance of mouse mammary progenitor cells and Sca1+ progenitors in an immortalized mammary gland cell line[10,11]. Thus, an active interaction probably exists between the radiotherapy response of cancer cells and Wnt/β-catenin signal pathway. However, no direct evidence about the correlation between nuclear β-catenin expression in rectal cancer and radiotherapy sensitivity has been reported to date. In this study, we enrolled 136 rectal adenocarcinoma patients treated with fractionated preoperative radiotherapy and radical resection. We investigated the expression of nuclear β-catenin in rectal adenocarcinoma using biopsy specimens and resected primary tumor tissues to determine whether nuclear β-catenin expression can be a predictive marker of tumor response to preoperative radiotherapy.
The study cohort comprised 136 rectal cancer patients who had undergone consecutive preoperative radiotherapy (25 Gy in five fractions for 1 wk) and radical surgery at Qilu Hospital and Shandong Tumor Hospital and Institute from November 2008 to June 2011. The subjects included 98 men and 38 women with a mean age of 64 years (range: 35 years to 78 years). First, rectal cancer was diagnosed by contrast computed tomography (CT) and colonoscopy. Afterward, archival paraffin-embedded biopsy tumor specimens and post-surgery resected tumor tissues were histologically identified as rectal adenocarcinoma.
The inclusion criteria were as follows: (1) patients histologically diagnosed with rectal adenocarcinoma; and (2) rectal cancer clinically diagnosed as stage III. The exclusion criteria were as follows: (1) hereditary nonpolyposis CRC patients and patients with familial adenomatous polyposis; and (2) patients with distant metastasis. All 136 patients met the inclusion criteria and were included in this study. However, 11 cases with distant metastasis and 1 case with familial adenomatous polyposis were excluded.
All cancers were clinically diagnosed as stage III with no distant metastasis. Tumor stage was based on the American Joint Committee on Cancer (AJCC) TNM staging system (7th edition, 2009). The TNM stages of the patients in this cohort were as follows: 28 patients with stage IIIA, 46 patients with stage IIIB, and 62 patients with stage IIIC.
All patients received preoperative radiotherapy and underwent Miles’ operation. The distance from distal margin of rectal cancer to anal edge was less than 7 cm in all patients. The interval between preoperative radiotherapy and surgery was 10 d to 14 d.
This study was approved by the Ethics Committees of Qilu Hospital, Shandong University, and Shandong Tumor Hospital and Institute. Informed consent was obtained from each patient. Clinical information related to diagnostic procedures and tumor characteristics was collected from medical records. Clinical and pathological data, including sex, age, tumor size, tumor invasion depth, tumor cell differentiation, and lymph node metastasis, were collected. Firstly, the association between expression of nuclear β-catenin in biopsy specimens and respective resected tumor tissues was retrospectively analyzed. Meanwhile, tumor regression grading (TRG) of rectal adenocarcinoma after radiotherapy was evaluated. Then, the relationship between nuclear β-catenin expression in rectal adenocarcinoma and clinicopathological characteristics was retrospectively analyzed. Finally, univariate analysis and logistic multivariate regression analysis were adopted to discriminate independent factors of radioresistance.
Biopsy specimens obtained through colonoscopy and resected tumor tissues collected after surgery were embedded in paraffin and cut into sections for immunohistochemical staining using the streptavidin peroxidase complex method. Tissue sections (4 μm thick) were deparaffinized and microwaved for 15 min, incubated twice in 10 mmol/L citrate buffer at 100 °C to retrieve the antigens, and then incubated in 3% H2O2 for 10 min to quench endogenous peroxidase. Nonspecific binding of antibodies was inhibited by incubation in 5% normal goat serum for 20 min in a humid chamber. The tissue sections were then incubated with mouse monoclonal anti-β-catenin antibodies (diluted 1:50, mouse IgG1; Cell Signaling Technology, Boston, United States) overnight at 4 °C. The tissue sections were washed three times with PBS and then incubated with biotinylated goat antimouse IgG for 30 min at room temperature. After washing, the slides were incubated in a streptavidin-peroxidase complex for 20 min at 37 °C, washed three times, visualized using 3,3’-diaminobenzidine, and then counterstained with hematoxylin. Sections that were stained without the primary antibodies served as the negative control. β-catenin expression is defined as brown colored staining on the membrane or in the cytoplasm and nucleus. For immunohistochemical assessment, six slides were randomly selected and observed at a magnification of × 200 by two pathologists. The immunostained slides were scored as previously described[12]. In brief, the immunostained slides were scored using the sum of the signal intensity (0 = no expression; 1 = weak expression; 2 = moderate expression; 3 = strong expression) and the percentage of positive cells (% tumor cells: 0 = 0%; 1 = 1% to 25%; 2 = 26% to 50%; 3 = 51% to 75%; and 4 = 76% to 100%). Nuclear β-catenin immunoreactivity at the invasive front was considered positive if moderate or strong expression was observed in the nuclei. Nuclear β-catenin overexpression was considered positive when the expression was observed in > 50% of the tumor cells.
The response of rectal cancer to preoperative radiotherapy was evaluated in the hematoxylin and eosin-stained slides using the TRG system as previously described[13]. The characteristics of each grade were as follows: TRG 0, no regression; TRG 1, dominant tumor mass with obvious fibrosis in 25% or less of the tumor mass; TRG 2, dominant tumor mass with obvious fibrosis in 26 to 50% of the tumor mass; TRG 3, dominant fibrosis outgrowing the tumor mass; and TRG 4, no viable tumor cells (only a fibrotic mass).
Statistical analysis was performed using SPSS16.0. χ2 test and Fisher exact test were used to analyze the correlation between nuclear β-catenin expression and clinicopathological characteristics. Univariate and logistic multivariate regression analyses were performed to determine the independent factors of TRG. P < 0.05 was considered statistically significant.
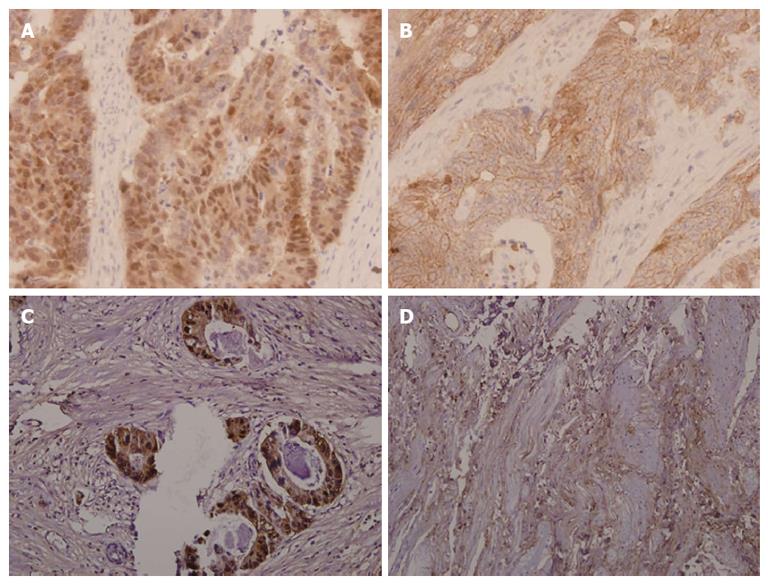
The expression of β-catenin in the biopsy and respective resected tumor tissues is presented in Figure 1. β-catenin expression was observed in all rectal cancer specimens. β-catenin was expressed mostly in a nuclear-associated staining pattern in patients who exhibited resistance to preoperative radiotherapy (Figure 1A). Conversely, β-catenin was expressed mostly in a membrane-associated staining pattern in patients who exhibited hyper-radiosensitivity (Figure 1B). Nuclear β-catenin overexpression was observed in 68 cases who exhibited radioresistance (57.6%). However, this overexpression was only observed in three patients who exhibited radiosensitivity (16.7%). Therefore, nuclear β-catenin overexpression is more evident in radioresistant patients than in radiosensitive patients (P < 0.001, Table 1). After preoperative radiotherapy, nuclear β-catenin was overexpressed in favor of poor TRG (≤ 2) (Figure 1C), whereas membrane β-catenin was overexpressed in favor of good TRG (≥ 3) (Figure 1D). This result suggests that nuclear β-catenin overexpression is a predictive marker of radioresistance.
| Characteristics | n | Nuclearβ-catenin expression | P value1 | |
| ≤50% | > 50% | |||
| Gender | ||||
| Male | 108 | 54 | 54 | 0.312 |
| Female | 28 | 11 | 17 | |
| Age (yr) | ||||
| ≤ 55 | 19 | 11 | 8 | 0.342 |
| > 55 | 117 | 54 | 63 | |
| Tumor size (cm) | ||||
| ≤ 5 | 78 | 38 | 40 | 0.802 |
| > 5 | 58 | 27 | 31 | |
| Tumor cell differentiation | ||||
| Well/moderately differentiated | 98 | 53 | 45 | 0.018 |
| Poorly differentiated | 38 | 12 | 26 | |
| Tumor invasion depth | ||||
| T1/T2 | 23 | 15 | 8 | 0.066 |
| T3/T4 | 113 | 50 | 63 | |
| Lymph node metastasis | ||||
| N1 | 74 | 42 | 32 | 0.022 |
| N2 | 62 | 23 | 39 | |
| TRG | ||||
| TRG0-2 | 118 | 50 | 68 | < 0.001 |
| TRG3-4 | 18 | 15 | 3 | |
χ2 test was performed to investigate the relationship between nuclear β-catenin expression and clinicopathological features. The correlations between nuclear β-catenin expression level and clinicopathological variables are shown in Table 1. Nuclear β-catenin expression in tumor cell differentiation (P = 0.018), lymph node metastasis (P = 0.022), and TGR (P < 0.001) showed significant differences.
Clinicopathological characteristics were divided into two groups according to tumor response to radiotherapy to confirm the predictive value of nuclear β-catenin overexpression as a biomarker of radioresistance. Univariate and multivariate analyses were performed to determine the factors related to radioresistance. Univariate analysis results (Table 2) demonstrated that radioresistance was associated with tumor size (P < 0.001), tumor cell differentiation (P < 0.001), tumor invasion depth (P = 0.027), and nuclear β-catenin expression (P < 0.001). However, logistic multivariate regression analysis retained only three factors in the model, namely, tumor size (P < 0.001), tumor cell differentiation (P < 0.001), and nuclear β-catenin overexpression (P < 0.001). By using radioresistance as a prediction target, nuclear β-catenin-based prediction alone achieved 83% accuracy, 65% sensitivity, and 88% specificity (Table 3).
| Clinicopathological factors | Univariate analysis | Multivariate analysis | ||
| Good response1 | P value | OR | P value | |
| Gender | ||||
| Male | 15 (13.8) | 0.697 | NS | |
| Female | 3 (10.7) | |||
| Age (yr) | ||||
| ≤ 55 | 5 (26.3) | 0.129 | NS | |
| > 55 | 13 (11.1) | |||
| Tumor size (cm) | ||||
| ≤ 5 | 16 (20.5) | < 0.001 | 5.058 | < 0.001 |
| > 5 | 2 (3.5) | |||
| Tumor cell differentiation | ||||
| Well/moderately differentiated | 4 (4.1) | < 0.001 | 4.692 | < 0.001 |
| Poorly differentiated | 14 (36.8) | |||
| Tumor invasion depth | ||||
| T1/T2 | 7 (30.4) | 0.027 | 1.143 | 0.053 |
| T3/T4 | 11 (9.7) | |||
| Nuclear β-catenin expression | ||||
| ≤ 50% | 15 (23.1) | < 0.001 | 6.375 | < 0.001 |
| > 50% | 3 (4.2) | |||
| Nuclearβ-catenin expression | Good response | Poor response |
| ≤ 50% | 15% | 50% |
| > 50% | 3% | 68% |
| Accuracy | 83% | |
| Sensitivity | 65% | |
| Specificity | 88% | |
| Positive predictive value | 79% | |
| Negative predictive value | 48% |
In the present study, the expression of β-catenin in 136 rectal adenocarcinoma patients was detected and its relationship with radioresistance was investigated. Our data demonstrated that overexpression of nuclear β-catenin is associated with radioresistance in rectal adenocarcinoma. To the best of our knowledge, few studies have been done to investigate the prognostic value of nuclear β-catenin overexpression for radioresistance in rectal cancer. The clinical significance of the current study is that we might provide promising biomarkers to predict sensitivity of rectal cancer to radiotherapy.
Preoperative radiotherapy has become a standard treatment for locally advanced rectal cancer. Obtaining an accurate prediction of cancer cell response to radiotherapy to determine if a patient would benefit from adjuvant therapy is challenging[14]. Previous studies indicated that molecules involved in signaling pathways are crucial to the sensitivity of cancer cells to radiotherapy[15,16]. Numerous studies have investigated biomarkers to predict rectal cancer sensitivity to radiotherapy[17-19]. However, only a few scholars have focused on the Wnt/β-catenin signaling pathway, which is vital in the progression of rectal cancer.
β-catenin is a component of the Wingless/Wnt signaling pathway. Previous studies have demonstrated that β-catenin is associated with the sensitivity of some cancers to radiotherapy. Kim et al[20] found that glioblastoma (GBM) cell lines enriched with cells positive for active β-catenin are increased by in vitro radiation treatment. This finding suggests that the radiation resistance of GBM is partly mediated by the activation of stem cell-associated pathways, including Wnt. Watson et al[21] found that the expression of a kinase dead mutant GSK3β endows Panc1 and BxPC3 pancreatic cancer cells with radioresistance. β-catenin silencing results in radiosensitization, whereas a nondegradable β-catenin construct induces radioresistance. These data support the hypothesis that GSK3β modulates the cellular response to radiation in a β-catenin-dependent mechanism. In the current study, only stage III rectal adenocarcinoma patients who accepted preoperative radiotherapy were included to eliminate the effects of different therapeutic methods, such as concurrent chemoradiotherapy. The results indicated that nuclear β-catenin overexpression is more evident in tumor biopsy specimens from patients who exhibited radioresistance. We also found that tumor tissues were relatively intact when nuclear β-catenin was overexpressed in tumor cells after preoperative radiotherapy. These immunohistochemical data indicated that the accumulation of nuclear β-catenin in tumor cells is critical in the radioresistance of rectal adenocarcinoma.
Regarding the correlation between nuclear β-catenin expression and clinicopathological characteristics, previous investigators reported contradictory results. Zhang et al[22] believed that nuclear β-catenin accumulation is related to tumor stage and/or metastasis. However, correlations between nuclear β-catenin and pertinent clinicopathological variables were not observed in Baldus’s study[23]. Our data demonstrated that nuclear β-catenin overexpression is related to low tumor cell differentiation and lymph node metastasis. Hyper-radiosensitivity is associated with low tumor cell differentiation. In the current study, a relationship was found between nuclear β-catenin overexpression and low tumor cell differentiation. However, tumor tissues in which nuclear β-catenin was overexpressed exhibited resistance to preoperative radiotherapy. This result suggests that nuclear β-catenin overexpression is a potential mechanism by which rectal adenocarcinoma cells avoid the destructive effect of radiotherapy.
Univariate and multivariate analyses were used to determine the factors related to radioresistance and thus confirm the predictive value of nuclear β-catenin overexpression for radioresistance. Univariate analysis demonstrated that nuclear β-catenin overexpression is associated with tumor response to radiotherapy. This finding coincides with the immunohistochemical result. A multivariate logistic regression analysis model was constructed to distinguish the independent factors for radioresistance and obtain a more precise estimate of the effect of nuclear β-catenin overexpression on tumor response to radiotherapy. As shown in Table 2, tumor response to radiotherapy is associated with nuclear β-catenin overexpression. This result suggests that nuclear β-catenin overexpression may affect radioresistance independently. When radioresistance was used as a prediction target in this study, nuclear β-catenin overexpression-based prediction alone achieved 83% accuracy, 65% sensitivity, and 88% specificity. These data confirmed that our conclusion is consistent with previous findings and suggested that nuclear β-catenin overexpression in rectal adenocarcinoma is a useful marker of radioresistance. Prediction of rectal cancer patient response is critical in tailoring preoperative radiotherapy to individuals. In addition, the search for predictive markers of radiotherapy is similar to the development of targeted therapy to some extent. We believe that β-catenin is a potential target for reducing radioresistance in rectal adenocarcinoma. In the future, β-catenin-targeting molecular drugs and radiation therapy may be used in combination to improve the efficacy of radiotherapy for rectal adenocarcinoma.
There are some potential limitations of this study. First, this is a retrospective study, and the confounding effects associated with a design of this kind are surely present. Second, only those patients who accepted preoperative radiotherapy were included in order to study the prognostic value of nuclear β-catenin overexpression for radioresistance in this study. Having only a small number of patients in the study also prevented us making further analysis about the correlation of nuclear β-catenin overexpression with clinical outcome. We believe that a larger prospective trial and long-term follow up study will allow us to confirm our conclusions.
In conclusion, nuclear β-catenin overexpression in rectal adenocarcinoma is associated with radioresistance. Both clinical and pathological data support our conclusion. Therefore, nuclear β-catenin overexpression in rectal adenocarcinoma can be used as a valuable predictor of radioresistance. However, these preliminary findings must be verified in a larger, prospective, controlled clinical study and in a subsequent experimental study.
Radiotherapy is a major treatment for rectal cancer. During previous decades, preoperative radiotherapy has increasingly been used together with surgery for locally advanced rectal cancer in order to reduce local treatment failures. However, many rectal cancer patients are actually resistant to preoperative radiotherapy due to the heterogeneity of treatment response. Therefore, the ability to predict response for neoadjuvant radiotherapy may allow individualization and more rational selection of patients that will most likely benefit from this therapy.
A previous study indicated that Wnt/β-catenin mediates radiation resistance of mouse mammary progenitor cells and Sca1+ progenitors in an immortalized mammary gland cell line. Thus, there seems to be active interaction between the response to radiotherapy of cancer cells and the Wnt/β-catenin signal pathway. However, there is no direct evidence about the correlation between nuclear β-catenin expression in rectal cancer and radiotherapy sensitivity up to now.
In this paper the authors investigated the relationship between overexpression of nuclear β-catenin in rectal adenocarcinoma and radioresistance. To the best of the knowledge, this is the first study that analyzes the relation between nuclear β-catenin overexpression in rectal adenocarcinoma and radioresistance. The authors provided a novel possible molecular mechanism to explain the radioresistance in rectal adenocarcinoma and thus may provide a new therapeutic target for enhancing radiosensitivity.
By studying the relationship between overexpression of nuclear β-catenin in rectal adenocarcinoma and radioresistance, this study may provide a new therapeutic target for enhancing radiosensitivity for rectal adenocarcinoma.
β-catenin is a component of the Wingless/Wnt signaling pathway and can activate target genes linking with the APC gene in colorectal cancer. Its localization relates to its function in cancer growth. β-catenin in the cytoplasm and membrane binds with the intracellular domain of E-cadherin, which is a cell-to-cell adhesion molecule, and to play a significant role in maintaining the normal tissue architecture.
The authors studied the clinical significance of β-catenin overexpression and radioresistance in patients with rectal adenocarcinoma. This paper is very interesting and the information is up to date. The study provided a novel possible molecular mechanism to explain radioresistance in rectal adenocarcinoma and thus may provide a new therapeutic target for enhancing radiosensitivity.
P- Reviewers Braet F, Cichoz-Lach H, Cui G, Triantafyllou K, Yu B S- Editor Gou SX L- Editor O’Neill M E- Editor Zhang DN
| 1. | Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-1740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4342] [Cited by in F6Publishing: 4228] [Article Influence: 211.4] [Reference Citation Analysis (1)] |
| 2. | Lim SB, Yu CS, Hong YS, Kim TW, Kim JH, Kim JC. Long-term outcomes in patients with locally advanced rectal cancer treated with preoperative chemoradiation followed by curative surgical resection. J Surg Oncol. 2012;106:659-666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Pacelli F, Sanchez AM, Covino M, Tortorelli AP, Bossola M, Valentini V, Gambacorta MA, Doglietto GB. Improved outcomes for rectal cancer in the era of preoperative chemoradiation and tailored mesorectal excision: a series of 338 consecutive cases. Am Surg. 2013;79:151-161. [PubMed] [Cited in This Article: ] |
| 4. | Bujko K, Richter P, Smith FM, Polkowski W, Szczepkowski M, Rutkowski A, Dziki A, Pietrzak L, Kołodziejczyk M, Kuśnierz J. Preoperative radiotherapy and local excision of rectal cancer with immediate radical re-operation for poor responders: a prospective multicentre study. Radiother Oncol. 2013;106:198-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 5. | Moussata D, Amara S, Siddeek B, Decaussin M, Hehlgans S, Paul-Bellon R, Mornex F, Gerard JP, Romestaing P, Rödel F. XIAP as a radioresistance factor and prognostic marker for radiotherapy in human rectal adenocarcinoma. Am J Pathol. 2012;181:1271-1278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Qiu J, Yang G, Shen Z, Xie Y, Wang L. hPEBP4 as a predictive marker for the pathological response of rectal cancer to preoperative radiotherapy. Int J Colorectal Dis. 2013;28:241-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Kuremsky JG, Tepper JE, McLeod HL. Biomarkers for response to neoadjuvant chemoradiation for rectal cancer. Int J Radiat Oncol Biol Phys. 2009;74:673-688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 186] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 8. | Harris TJ, Peifer M. Decisions, decisions: beta-catenin chooses between adhesion and transcription. Trends Cell Biol. 2005;15:234-237. [PubMed] [Cited in This Article: ] |
| 9. | van Es JH, Barker N, Clevers H. You Wnt some, you lose some: oncogenes in the Wnt signaling pathway. Curr Opin Genet Dev. 2003;13:28-33. [PubMed] [Cited in This Article: ] |
| 10. | Woodward WA, Chen MS, Behbod F, Alfaro MP, Buchholz TA, Rosen JM. WNT/beta-catenin mediates radiation resistance of mouse mammary progenitor cells. Proc Natl Acad Sci USA. 2007;104:618-623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 471] [Cited by in F6Publishing: 505] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 11. | Chen MS, Woodward WA, Behbod F, Peddibhotla S, Alfaro MP, Buchholz TA, Rosen JM. Wnt/beta-catenin mediates radiation resistance of Sca1+ progenitors in an immortalized mammary gland cell line. J Cell Sci. 2007;120:468-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 12. | Horst D, Reu S, Kriegl L, Engel J, Kirchner T, Jung A. The intratumoral distribution of nuclear beta-catenin is a prognostic marker in colon cancer. Cancer. 2009;115:2063-2070. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997;12:19-23. [PubMed] [Cited in This Article: ] |
| 14. | Perez RO. Predicting response to neoadjuvant treatment for rectal cancer: a step toward individualized medicine. Dis Colon Rectum. 2011;54:1057-1058. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Wang Y, Liu L, Zhou D. Inhibition of p38 MAPK attenuates ionizing radiation-induced hematopoietic cell senescence and residual bone marrow injury. Radiat Res. 2011;176:743-752. [PubMed] [Cited in This Article: ] |
| 16. | Ho SY, Wu WJ, Chiu HW, Chen YA, Ho YS, Guo HR, Wang YJ. Arsenic trioxide and radiation enhance apoptotic effects in HL-60 cells through increased ROS generation and regulation of JNK and p38 MAPK signaling pathways. Chem Biol Interact. 2011;193:162-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Shioya M, Takahashi T, Ishikawa H, Sakurai H, Ebara T, Suzuki Y, Saitoh J, Ohno T, Asao T, Kuwano H. Expression of hypoxia-inducible factor 1α predicts clinical outcome after preoperative hyperthermo-chemoradiotherapy for locally advanced rectal cancer. J Radiat Res. 2011;52:821-827. [PubMed] [Cited in This Article: ] |
| 18. | Kim K, Chie EK, Wu HG, Kim SG, Lee SH, Kang GH, Hyun CL, Ha SW. High survivin expression as a predictor of poor response to preoperative chemoradiotherapy in locally advanced rectal cancer. Int J Colorectal Dis. 2011;26:1019-1023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Shinto E, Hashiguchi Y, Ueno H, Kobayashi H, Ishiguro M, Mochizuki H, Yamamoto J, Hase K. Pretreatment CD133 and cyclooxygenase-2 expression as the predictive markers of the pathological effect of chemoradiotherapy in rectal cancer patients. Dis Colon Rectum. 2011;54:1098-1106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Kim Y, Kim KH, Lee J, Lee YA, Kim M, Lee SJ, Park K, Yang H, Jin J, Joo KM. Wnt activation is implicated in glioblastoma radioresistance. Lab Invest. 2012;92:466-473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 21. | Watson RL, Spalding AC, Zielske SP, Morgan M, Kim AC, Bommer GT, Eldar-Finkelman H, Giordano T, Fearon ER, Hammer GD. GSK3beta and beta-catenin modulate radiation cytotoxicity in pancreatic cancer. Neoplasia. 2010;12:357-365. [PubMed] [Cited in This Article: ] |
| 22. | Zhang B, Ougolkov A, Yamashita K, Takahashi Y, Mai M, Minamoto T. beta-Catenin and ras oncogenes detect most human colorectal cancer. Clin Cancer Res. 2003;9:3073-3079. [PubMed] [Cited in This Article: ] |
| 23. | Baldus SE, Mönig SP, Huxel S, Landsberg S, Hanisch FG, Engelmann K, Schneider PM, Thiele J, Hölscher AH, Dienes HP. MUC1 and nuclear beta-catenin are coexpressed at the invasion front of colorectal carcinomas and are both correlated with tumor prognosis. Clin Cancer Res. 2004;10:2790-2796. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 137] [Article Influence: 6.9] [Reference Citation Analysis (0)] |