Published online Nov 21, 2013. doi: 10.3748/wjg.v19.i43.7795
Revised: August 23, 2013
Accepted: September 15, 2013
Published online: November 21, 2013
AIM: To study the differential expression of Annexin A1 (ANXA1) protein in human gastric adenocarcinoma. This study was also designed to analyze the relationship between ANXA1 expression and the clinicopathological parameters of gastric carcinoma.
METHODS: Purified gastric adenocarcinoma cells (GAC) and normal gastric epithelial cells (NGEC) were obtained from 15 patients with gastric cancer by laser capture microdissection. All of the peptide specimens were labeled as 18O/16O after trypsin digestion. Differential protein expressions were quantitatively identified between GAC and NGEC by nanoliter-reverse-phase liquid chromatography-mass/mass spectrometry (nano-RPLC-MS/MS). The expressions of ANXA1 in GAC and NGEC were verified by western blot analysis. The tissue microarray containing the expressed ANXA1 in 75 pairs of gastric carcinoma and paracarcinoma specimens was detected by immunohistochemistry (IHC). The relationship between ANXA1 expression and clinicopathological parametes of gastric carcinoma was analyzed.
RESULTS: A total of 78 differential proteins were identified. Western blotting revealed that ANXA1 expression was significantly upregulated in GAC (2.17/1, P < 0.01). IHC results showed the correlations between ANXA1 protein expression and the clinicopathological parameters, including invasive depth (T stage), lymph node metastasis (N stage), distant metastasis (M stage) and tumour-lymph node metastasis stage (P < 0.01). However, the correlations between ANXA1 protein expression and the remaining clinicopathological parameters, including sex, age, histological differentiation and the size of tumour were not found (P > 0.05).
CONCLUSION: The upregulated ANXA1 expression may be associated with carcinogenesis, progression, invasion and metastasis of GAC. This protein could be considered as a biomarker of clinical prognostic prediction and targeted therapy of GAC.
Core tip: The anti-inflammatory protein Annexin A1 (ANXA1) mediates various important physiological and pathophysiological processes. Evidence has shown that ANXA1 is related to the development and progression of human multi-tumours. However, the ANXA1 expression in gastric adenocarcinoma of Chinese patients and the relationship between this protein and its clinicopathological parameters remain unclear. In the present study, the ANXA1 expression in gastric adenocarcinoma of Chinese patients was investigated by proteomics and western blot analysis. Authors examined 75 pairs of gastric adenocarcinoma and paracarcinoma tissues by tissue microarray to determine the presence of ANXA1 by immunohistochemistry. They found that ANXA1 expression was upregulated and involved in human gastric adenocarcinoma invasion and metastasis. Our findings suggested that ANXA1 might be used as a valuable biomarker in clinical diagnosis, prognostic prediction and targeted therapy of gastric cancer.
- Citation: Zhang ZQ, Li XJ, Liu GT, Xia Y, Zhang XY, Wen H. Identification of Annexin A1 protein expression in human gastric adenocarcinoma using proteomics and tissue microarray. World J Gastroenterol 2013; 19(43): 7795-7803
- URL: https://www.wjgnet.com/1007-9327/full/v19/i43/7795.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i43.7795
Gastric cancer (GC) is a common digestive tract cancer because of the lack of early diagnosis strategies; a previous study revealed that GC cases are usually diagnosed when the disease is at an advanced stage[1]. On the basis of metastasis, recurrence and other causes, the treatment and prognosis of GC remain poor. Therefore, effective biomarkers of GC should be determined; the mechanism of incidence and development should also be investigated to promote early diagnosis, effective treatment and prognosis improvement of GC.
Annexin A1 (ANXA1) is a key member of the A subfamily and belongs to the multi-gene family of Annexins. ANXA1 exhibits calcium-mediated phospholipid binding properties and participates in many physiological and pathological processes. Further studies have shown that ANXA1 is abnormally expressed in various tumours. This abnormal expression is closely related to tumourigenesis, development, invasion and metastasis of human tumours. The expression of ANXA1 in tumours is tissue specific; for instance, a low ANXA1 expression is observed in oesophageal squamous cell carcinoma[2], whereas a high expression is found in colorectal cancer[3]. The relationship between ANXA1 expression in GC and GC invasion as well as metastasis remains unclear. ANXA1 also exhibits a low expression in GC and negatively correlates with invasion and metastasis[4]. However, other studies have revealed opposite results[5,6].
We performed laser capture microdissection (LCM) to investigate the relation of ANXA1 expression in GC to the clinical parameters and to obtain purified gastric adenocarcinoma cells (GAC) and normal gastric epithelial cells (NGEC). 18O/16O was used to label the digested peptides in the mixture of GAC and NGEC. Nanoliter-reverse-phase liquid chromatography-mass/mass spectrometry (nano-RPLC-MS/MS) was performed to identify and quantify the differentially expressed proteins. Nano-RPLC-MS/MS was also conducted to validate the results of proteomics. To verify the differential protein expression of ANXA1, we performed western blot. Immunohistochemistry (IHC) was performed to detect the expression of ANXA1 in 75 pairs of tissue microarray of GC tissues and paracarcinoma tissues. This study aimed to analyze the correlations of ANXA1 with clinical pathological parameters, including age, gender, differentiation degree, metastasis, invasion depth, tumour-lymph node metastasis (TNM) staging and tumour size (maximum diameter). This study also investigated the relations and possible mechanisms of the expression differences of ANXA1 protein in carcinogenesis, progression and prognosis of GC.
Fifteen cases of GAC and paired gastric mucosa tissues were obtained from the First Affiliated Hospital of Xinjiang Medical University from June 2009 to October 2009 and used as the surgical resection specimens. Six female and nine male subjects aged 40-81 years (mean age of 56 years) and classified in TNM stages I to IV participated in this study. GC and paragastric mucosa tissues (located away from the primary tumour > 5 cm) with a size of approximately 1.0 cm2 were obtained within 30 min of surgical resection. The tissues were washed immediately and repeatedly with normal saline to remove blood and other tissues. Afterwards, these tissues were stored at -80 °C in a refrigerator for future proteomics and western blot analysis. Informed consent was obtained from the patients to allow the collection and use of the samples. The procedures were also reviewed and approved by Xinjiang Medical Ethics Committee.
Frozen sections of GAC and paragastric mucosa tissues (8-10 μm) were prepared, affixed on LCM-specific film slides, fixed with 75% ethanol and stained with methyl green dye (Sigma-Aldrich, United States). The LCM system (Leica AS, Germany) was manipulated to determine GAC and NGEC. Tissue cell lysate was added and the total proteins of the purified GAC and NGEC were extracted. 2D Quant Kit (Amersham Biosciences, Sweden) was used to determine the protein concentration. We performed 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) to pre-separate the total proteins of cleaved GAC and NGEC with a sample loading volume of 100 μg. The gel was then stained with Coomassie R-250. The parallel gel bands were cut to obtain 36 pairs of protein gel bands. The obtained bands were washed, bleached, dehydrated, reduced with dithiothreitol (Amersham Biosciences, Sweden), alkylated with IAA and vacuum dried by centrifugation. Trypsin (Promega Corporation, United States) was added to initiate digestion. The peptides were extracted, mixed and dried. Afterwards, 18O/16O notation was performed by adding 8 μL of H218O (or H216O; Huayi Isotope Corporation, Jiangsu) and 2 μL of acetonitrile (Sigma-Aldrich Inc., United States) to the mixed polypeptides, which also contained immobilized trypsin (Pierce, United States). The resulting mixture was then incubated at 37 °C for 24 h. At the end of the labelling reaction, 1 μL of formic acid was added to terminate the reaction.
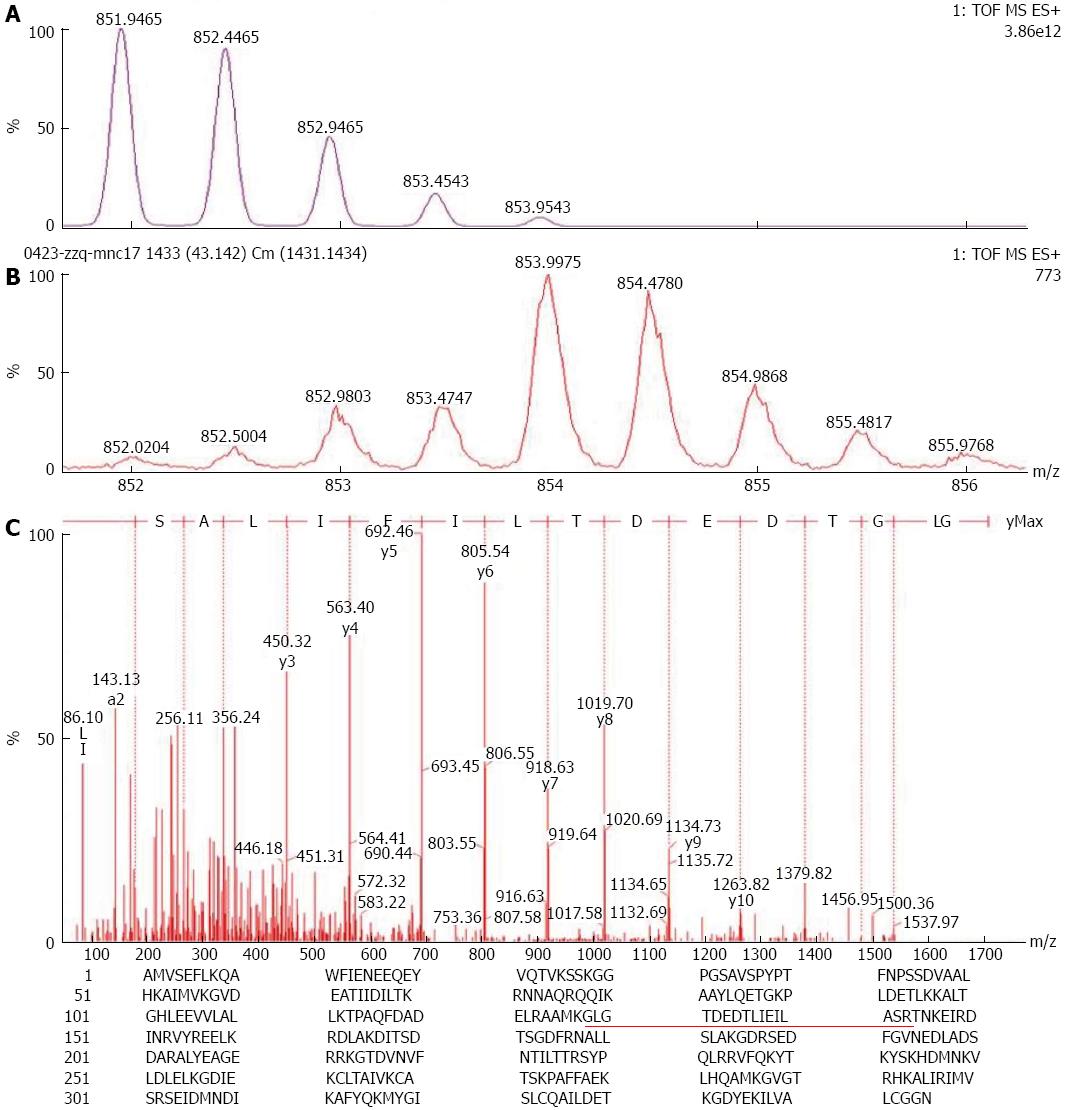
The mobile phase solution A (0.1% formic acid in water) was added into Eppendorf tubes containing different peptide mixtures, shaken and centrifuged. The supernatant was extracted and added into a tapered bottom vial in the Ultimate FAMOS LC system autosampler. Isolation was performed using nano-RPLC-MS/MS instrument, in which an auxiliary pump was used to load the samples with mobile phase solution A at a flow rate of 20 μL/min for 10 min. The samples were then loaded in a pre-column and desalted. The pre-column was switched and connected to a capillary analytical column for gradient elution using the solvent gradient as follows: solution B [water/acetonitrile (v/v) containing 0.1% formic acid], 5% B, 0-10 min; 5%-90% B, 55 min; 90% B, 5 min; and 90%-0% B, 10 min. After equilibrium was reached for 15 min, separation was performed. Nanoliter analytical column flow rate was approximately 250 nL/min; therefore, the eluent could be placed directly in the Electrospray ionization ion Q-TOF mass spectrometer (Micromass Corporation, United Kingdom) for analysis. The standard peptide Glu-Fibrino peptide B was used as an external calibration standard of the mass spectrometer. The mass data provided peaklist files in Masslynx 4.0 software of the local Mascot 2.0 IPI database to search the protein database and identify the proteins. Quantitative analysis was performed in Masslynx according to the following procedures: the MS spectra containing the peptides used for quantification were obtained from the total ion chromatograph to integrate and form the spectrum of quantitative analysis; 16O/18O ratio was then calculated according to Eq. (1)[7].
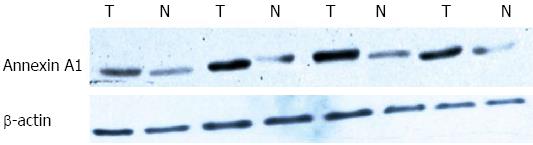
Fifteen pairs of microdissected and purified GAC and NGEC were added to the tissue lysate pre-cooled at 4 °C, vortexed and cleared in an ice bath for 30 min. Afterwards, the samples were centrifuged at 12000 r/min and 4 °C for 30 min. The supernatant (the total cellular protein) was then transferred to a new tube. The protein concentration was determined using Bradford method and the total protein was determined by separation via 10% PAGE at 100 V for approximately 2 h (loading volume of 40 μg). The protein was electronically transferred to a Polyvinylidene difluoride membrane. Rabbit anti-human ANXA1 antibody (1/500) was added and incubated at 4 °C overnight. HRP-labeled goat anti-mouse secondary antibody (1/2000) incubated at room temperature for 2 h was also added. Enhanced chemiluminescence reagent lightening, developing and fixing were conducted. The obtained images were scanned to calculate the relative expression levels of the differential proteins in Quantity One software.
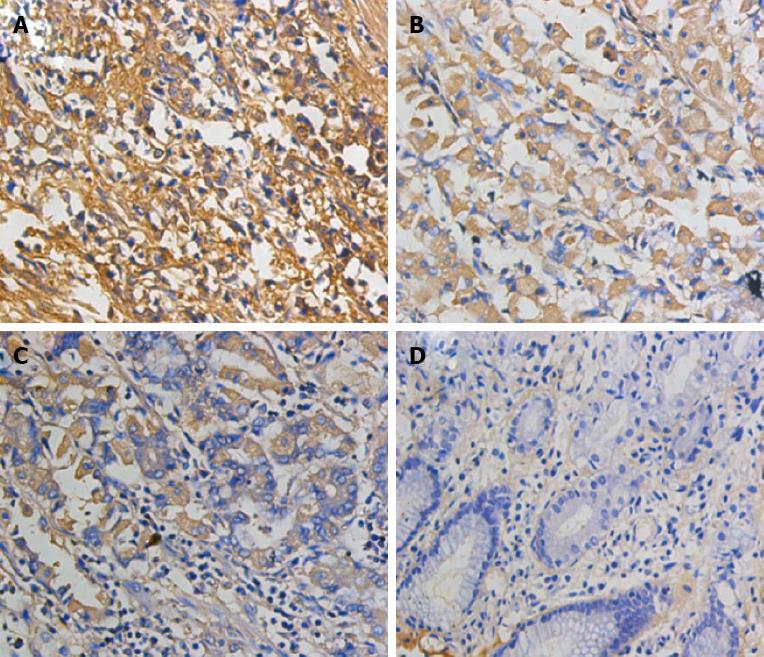
A total of 75 pairs of human GC tissue microarray (Shanghai Outdo Biotech Co., Ltd., China), including paired GC tissues and paraGC tissues were obtained from 50 males and 25 females aged 30-84 years (average age of 63.6 years). Among these subjects, 12 cases were in Phase I, 25 cases in Phase II, 32 cases in Phase III and 4 cases in Phase IV stage cases (according to the TNM classification Standard, 7th edition, developed by the International Union Against Cancer in 2009). The clinical pathological data were complete: the cases showing tumours that invaded the submucosa, muscularis, serosa and serosa were 6, 13, 46 and 10, respectively. A total of 34 cases showed high amounts of moderately differentiated adenocarcinoma and 41 cases showed low amounts of undifferentiated adenocarcinoma. No lymph node metastasis was observed in 30 cases, but lymph node metastasis was present in 45 cases. Distant metastasis was absent in 69 cases, but 6 patients exhibited distant metastasis. According to SP method and the manufacturer’s instructions, tissue microarrays were subjected to conventional dewaxing hydration and retrieved using citrate antigen. Afterwards, 3% H2O2-formaldehyde was used to block endogenous peroxidase. ANXA1 primary antibody (1/100) was added and incubated at 4 °C overnight. The biolabelled secondary antibody and streptavidin-peroxidase solution were added. Each sample was washed with PBS and incubated at room temperature. The sample was then stained with DAB staining, restained with haematoxylin and eosin, dehydrated with graded alcohol and mounted using neutral gum. The primary antibody was replaced with PBS as the negative control sample; the known positive reaction chip was used as the positive control sample. IHC staining score was based on Formowitz comprehensive scoring method[8] and determined according to the staining intensity and percentage of positive cells in each section. Staining intensity was scored as follows: no staining, 0; pale yellow, 1; brownish-yellow, 2; and tan, 3. At least 10 high-power fields (× 200) were randomly selected for each point and at least 1000 cells were counted. Among the total number of cells, the following percentages were obtained: 5% positively-stained cells scored as 0; 5%-25% scored as 1; 26%-50% scored as 2; 51%-75% scored as 3; > 75% scored as 4. The total score of staining intensity and the score of the percentage of positive cells were shown as follows: 2 as negative (-); 2-3 as weakly positive (+); 4-5 as moderately positive (++); and 6 to 7 as strongly positive (+++).
SPSS 15.0 statistical software was used to analyze the experimental results. The relationship between differential protein expression and the clinicopathological parameters of GC from different samples was determined by conducting Wilcoxon rank sum test. The relative protein expression levels were expressed as mean ± SD. t test was performed and P < 0.05 was considered statistically significant.
The total proteins of highly homogeneous GAC and NGEC (> 90%) purified by LCM were determined to separate by 1D SDS-PAGE, gel digestion, extraction and 18O labelling. These proteins were then identified by nano-RPLC-MS/MS. According to the standard differential proteins (where 2 < 18O/16O ratio < 0.5), a total of 78 differential proteins were identified, in which the expressions of 42 proteins, including ANXA1, ANXA2, ANXA4, Protein S100-A9, and HSP 90-alpha2 were upregulated in GC. By contrast, the expressions of 36 proteins were downregulated in GC, including RKIP, ADP/ATP translocase 2 and L-lactate dehydrogenase B. These differentially expressed proteins function as metabolic enzymes, enzyme proteins, cytoskeletal proteins, and signal transduction proteins; other proteins also exhibit unknown functions, in which ANXA1 expression was 2.17 times higher in GAC than in NGEC (Figure 1).
β-actin was used as the internal standard in western blot and the maximum gray value of the strip was set as 1. The other gray value was divided by the maximum gray value, and the obtained ratio corresponded to the relative protein expression level. The results showed that ANXA1 was upregulated at a higher extent in GAC than in NGEC (P < 0.01). The quantitative relationships calculated from the grayscale analysis were the same as the proteomic analysis results (Figure 2, Table 1).
| Group | n | Annexin A1 expression |
| NGEC | 15 | 0.49 ± 0.082 |
| GAC | 15 | 1.06 ± 0.068b |
IHC was performed to detect 150 points of the tissue microarray in human GC. The results showed that ANXA1 protein was positively expressed mainly in the cytoplasm and the nuclei of GC tissues and paratissues (normal mucosa). Positive expression was also observed in the stroma. ANXA1 protein was highly expressed in GC. The negative, weakly positive, moderately positive and strongly positive expression rates in GC and para-tissues were: 30.7%, 14.7%, 34.7% and 20.0% vs 49.3%, 33.3%, 17.3% and 0%, respectively (w = 4599.0, P < 0.01; Table 2) The ANXA1 expression was significantly related to the invasion depth, lymph node metastasis, distant metastasis and TNM staging (P < 0.01). By contrast, ANXA1 expression was not significantly related to age, gender, histological grade and tumour size (P > 0.05; Figure 3, Table 3).
| Tissue type | n | Annexin A1 cases | W | P value | |||
| - | + | + + | + + + | ||||
| NGEC | 75 | 37 (49.3) | 25 (33.3) | 13 (17.3) | 0 (0) | 4599 | 0 |
| GAC | 75 | 23 (30.7) | 11 (14.7) | 26 (34.7) | 15 (20.0) | ||
| Parametes | n | ANXA1 cases | Wilcoxon | P value | |||
| - | + | + + | + + + | ||||
| Sex | 1039.5 | 0.290 | |||||
| Male | 50 | 15 (30.0) | 7 (14.0) | 17 (34.0) | 11 (22.0) | ||
| Female | 25 | 8 (32.0) | 4 (16.0) | 9 (36.0) | 4 (16.0) | ||
| Age (yr) | 840.0 | 0.394 | |||||
| ≤ 60 | 24 | 7 (29.2) | 5 (20.8) | 10 (41.7) | 2 (8.3) | ||
| > 60 | 51 | 16 (31.4) | 6 (11.7) | 16 (31.4) | 13 (25.5) | ||
| Histological differentiation | 1350.0 | 0.520 | |||||
| High | 34 | 10 (29.4) | 4 (11.8) | 12 (35.3) | 8 (23.5) | ||
| Moderate/poor | 41 | 13 (31.7) | 7 (17.1) | 14 (34.1) | 7 (17.1) | ||
| Invasive depth | 514.5 | 0.008 | |||||
| T1-2 | 19 | 10 (52.6) | 4 (21.1) | 3 (15.8) | 2 (10.5) | ||
| T3-4 | 56 | 13 (23.2) | 7 (12.5) | 23 (41.1) | 13 (23.2) | ||
| N stage | 929.0 | 0.017 | |||||
| N0 | 30 | 12 (40.0) | 6 (20.0) | 10 (33.3) | 2 (6.7) | ||
| N1-3 | 45 | 11 (24.4) | 5 (11.1) | 16 (35.6) | 13 (28.9) | ||
| M stage | 348.5 | 0.017 | |||||
| M0 | 69 | 23 (33.3) | 10 (14.5) | 25 (36.2) | 11 (15.9) | ||
| M1 | 6 | 0 (0) | 1 (16.7) | 1 (16.7) | 4 (66.7) | ||
| TNM stage | 1014.5 | 0.000 | |||||
| I-II | 37 | 20 (54.1) | 4 (10.8) | 11 (29.7) | 2 (5.4) | ||
| III-IV | 38 | 3 (2.6) | 7 (10.5) | 15 (42.1) | 13 (44.7) | ||
| Tumor size | 1403.0 | 0.9735 | |||||
| ≤ 5 cm | 38 | 11 (28.9) | 6 (15.8) | 14 (36.8) | 7 (18.4) | ||
| < 5 cm | 37 | 12 (32.4) | 5 (13.5) | 12 (32.4) | 8 (21.6) | ||
The mortality rate of GC ranks second among the mortality rates of malignant tumours and approximately 75 million people die of stomach cancer worldwide yearly[9]. The five-year survival rate of GC is only 20%-30%, and the five-year survival rate of radical resection of early GC can reach 90%-95%[10]. Molecular markers are considered as one of the most sensitive and effective indicators of tumour diagnosis, recurrence, metastasis and prognosis prediction. Therefore, the molecular markers closely associated with the development of GC and the relative pathogenesis should be determined in the early diagnosis of GC to improve treatment outcomes. Proteomics technology has an important function in the identification of tumour-related protein species in cancer development as well as in the discovery of tumour molecular markers and therapeutic targets. The present study employed simple LCM to solve problems about tumour proteomics heterogeneity, in which the purified cells exhibiting > 90% homogeneity were rapidly obtained. Using the advanced 18O stable isotope labelling/MS quantitative proteomics technique, we set the normal gastric mucosa and GC tissue of humans as the targets in this study to screen and identify the differentially expressed proteins. A total of 78 differentially expressed proteins were found and 42 of such proteins were highly expressed in GC cell compared with NGEC. By contrast, 36 differentially expressed proteins were found at lower concentrations. These results provided information about differentially expressed proteins and the mechanism of GC carcinogenesis, development and screening of molecular markers.
During the screening of the identified differential proteins, ANXA1 was significantly upregulated in GC. ANXA1 is involved in various physiological and pathological processes, such as cell signal transduction, cell proliferation, differentiation and apoptosis as well as inflammation and immune response. Previous studies found that ANXA1 is upregulated in breast cancer[11], lung cancer[12], pancreatic cancer[13], colorectal cancer[3,14] and bladder cancer[15]. By contrast, ANXA1 is downregulated in oral squamous cell carcinoma as well as nasopharyngeal, laryngeal and other head and neck cancer[16-18], oesophageal squamous cell carcinoma[2] and prostate cancer[19]. The dysfunction of ANXA1 is closely related to breast cancer, lung cancer, and pancreatic cancer as well as in other tumour invasion and metastasis. Therefore, ANXA1 is considered as a risk factor affecting the survival of patients. Wang et al[20] used a immunohistochemical method and found that ANXA1 expression is 39% higher in the stomach/gastroesophageal junction adenocarcinoma and closely correlated with the pathological staging and distant metastasis of tumour, i.e., higher clinical stages, particularly in distant lymph node metastasis, correspond to higher ANXA1 expression levels; higher tumour recurrence rates correspond to lower survival rate. This result suggested that the upregulation of ANXA1 could be used as a prognosis indicator in the stomach-oesophageal junction adenocarcinoma. Currently, the expression and function of ANXA1 in GC remain controversial.
In this study, quantitative proteomics was performed to screen ANXA1, revealing that ANXA1 was expressed 2.17 times higher in GC than in normal gastric mucosa. Western blotting and IHC of the tissue microarray also revealed the same results as proteomics analysis. In particular, the results showed that ANXA1 was significantly expressed at a higher extent in GC tissues than in paratissues; further analysis about the relations of ANXA1 and clinical parameters of human GAC revealed that ANXA1 expression was upregulated in tumour-penetrating serosa and thus invaded the paratissues. This result is different from human GAC normally confined to the mucosa, submucosa and muscularis; human GAC also possibly invaded the tumour in the lower serosa. ANXA1 was also highly expressed in human GAC with lymph node metastasis and distant metastasis compared with human GAC without lymph node metastasis and distant metastasis. As TNM staging increased, the positive expression rate of ANXA1 increased. However, no relationship was observed among ANXA1, patient’s age, gender, histological grade and tumour size. This result suggested that ANXA1 was closely related to the biological behaviour of human GAC and involved in the development, invasion and metastasis of human GAC. Currently, the mechanism by which ANXA1 functions in the biological behaviour of GC remains unclear. Cheng et al[21] reported that ANXA1 can regulate GC invasion by mediating formyl peptide receptor (FPR)/extracellular signal-regulated kinase/integrin protein β-1 bind protein pathway; all of the three FPRs are involved in the regulation process. Kang et al[11] further found that ANXA1 gene can decrease the activity and protein expression of matrix metalloproteinase-9 (MMP-9) transcriptional promoter by inhibiting the activity of nuclear factor κ-B. MMP-9 also has an important function in GC invasion and metastasis. Vascular endothelial growth factor (VEGF) is another important factor regulating angiogenesis, and angiogenesis is important in tumour growth and metastasis. Pin et al[22] found that VEGF-induced cell migration and angiogenesis of miR-196a can change the expression level of ANXA1, which is controlled by p38-ANXA1 signal conduction pathway. The function of Helicobacter pylori (H. pylori) infection in GC has also been demonstrated, showing that H. pylori can change the cellular ANXA1 localization[23]. This result suggests that ANXA1 may be involved in H. pylori infection-induced GC. Furthermore, these results can explain the possible mechanism by which ANXA1 participates in GAC biological behaviour to some extent.
A few reports about ANXA1 in GC have been published. For instance, Yapar et al[4] conducted an immunohistochemical study and found that ANXA1 expressed in GC is 56.3% higher than that in paratissues; this expression is also positively correlated with tumour invasion and lymph node metastasis; a high ANXA1 expression suggests poor prognosis of GC. Jorge et al[6] performed RT-PCR and immunohistochemistry, revealing that ANXA1 mRNA and protein expressions are increased in GC compared with normal gastric mucosa. The results of the present study are consistent with those in the aforementioned previous studies, although other studies have revealed contrasting results. In some studies, ANXA1 is downregulated in GC[4,24] and negatively correlated with tumour staging and lymph node metastasis[4]. The reasons may be described as follows: (1) different test conditions, antibodies, and test methods; and (2) different samples or different types and pathological staging parameters of GC samples. The intracellular ANXA1, which is mainly in the cytoplasm, is possibly redistributed at different stimulations. For example, ANXA1 likely enters the nucleus as induced by an epidermal growth factor (EGF) under oxidation conditions or heat shock; by contrast, ANXA1 is transferred to the membrane and then secreted out of the cells as stimulated by GC or phorbol-12-myristate-13-acetate[25,26]. Therefore, the upregulation and downregulation of ANXA1 in GC may be associated with different stages of GC pathology. Different levels of EGF and GC in vivo may possibly induce cellular ANXA1 relocalization. ANXA1 may also be involved in GC occurrence and development via different pathways and mechanisms.
In summary, the present study provided valuable information to clarify GC pathogenesis. This study also presented the basic foundation to screen cancer biomarkers. After the differential proteins of GC were initially screened, the results suggested that identification and function in vivo and in vitro of some important differential proteins require further studies. Given that ANXA1 was also expressed in gastric stromal cells, ANXA1 expression in GC may be difficult to assess by IHC. Nevertheless, the study on immunohistochemical tissue microarray revealed the relationship between ANXA1 and GC clinicopathological parameters. We found that the upregulation of ANXA1 in human GAC was closely related to the depth of tumour invasion, lymph node metastasis, distant metastasis and TNM stage. The results also suggested that ANXA1 was possibly involved in tumour invasion and metastasis. The high expression of ANXA1 suggested poor prognosis of GC. The mechanism by which ANXA1 participated in the GC biological behaviour should be further studied. With the continuous development of this research, ANXA1 may be used in early cancer detection, diagnosis and treatment. ANXA1 may also become an indicator of cancer prognosis and a new target of cancer therapy, thereby providing new ideas of GC diagnosis and treatment.
Gastric cancer (GC) is a common digestive tract cancer because of the lack of early diagnosis strategies; a previous study revealed that GC cases are usually diagnosed when the disease is at an advanced stage.
During the screening of the identified differential proteins, Annexin A1 (ANXA1) was significantly upregulated in GC. ANXA1 is involved in various physiological and pathological processes, such as cell signal transduction, cell proliferation, differentiation and apoptosis as well as inflammation and immune response. Previous studies found that ANXA1 is upregulated in breast cancer, lung cancer, pancreatic cancer, colorectal cancer and bladder cancer. By contrast, ANXA1 is downregulated in oral squamous cell carcinoma as well as nasopharyngeal, laryngeal and other head and neck cancer, oesophageal squamous cell carcinoma and prostate cancer.
The study performed laser capture microdissection to investigate the relation of ANXA1 expression in GC to the clinical parameters and to obtain purified gastric adenocarcinoma cells (GAC) and normal gastric epithelial cells (NGEC). 18O/16O was used to label the digested peptides in the mixture of GAC and NGEC. Nanoliter-reverse-phase liquid chromatography-mass/mass spectrometry was performed to identify and quantify the differentially expressed proteins.
ANXA1 may be used in early cancer detection, diagnosis and treatment. ANXA1 may also become an indicator of cancer prognosis and a new target of cancer therapy, thereby providing new ideas of GC diagnosis and treatment.
This study is realistic significance to the GC, This manuscript is well written. The data is interesting and worthy for publication.
P- Reviewers: Okada H, Thuss-Patience PC S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
| 1. | Wadhwa R, Taketa T, Sudo K, Blum MA, Ajani JA. Modern oncological approaches to gastric adenocarcinoma. Gastroenterol Clin North Am. 2013;42:359-369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Moghanibashi M, Jazii FR, Soheili ZS, Zare M, Karkhane A, Parivar K, Mohamadynejad P. Proteomics of a new esophageal cancer cell line established from Persian patient. Gene. 2012;500:124-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Roth U, Razawi H, Hommer J, Engelmann K, Schwientek T, Müller S, Baldus SE, Patsos G, Corfield AP, Paraskeva C. Differential expression proteomics of human colorectal cancer based on a syngeneic cellular model for the progression of adenoma to carcinoma. Proteomics. 2010;10:194-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Yapar EG, Ekici E, Karasahin E, Gökmen O. Sonographic features of tuberculous peritonitis with female genital tract tuberculosis. Ultrasound Obstet Gynecol. 1995;6:121-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Sato Y, Kumamoto K, Saito K, Okayama H, Hayase S, Kofunato Y, Miyamoto K, Nakamura I, Ohki S, Koyama Y. Up-regulated Annexin A1 expression in gastrointestinal cancer is associated with cancer invasion and lymph node metastasis. Exp Ther Med. 2011;2:239-243. [PubMed] [Cited in This Article: ] |
| 6. | Jorge YC, Mataruco MM, Araújo LP, Rossi AF, de Oliveira JG, Valsechi MC, Caetano A, Miyazaki K, Fazzio CS, Thomé JA. Expression of annexin-A1 and galectin-1 anti-inflammatory proteins and mRNA in chronic gastritis and gastric cancer. Mediators Inflamm. 2013;2013:152860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Qian WJ, Monroe ME, Liu T, Jacobs JM, Anderson GA, Shen Y, Moore RJ, Anderson DJ, Zhang R, Calvano SE. Quantitative proteome analysis of human plasma following in vivo lipopolysaccharide administration using 16O/18O labeling and the accurate mass and time tag approach. Mol Cell Proteomics. 2005;4:700-709. [PubMed] [Cited in This Article: ] |
| 8. | Fromowitz FB, Viola MV, Chao S, Oravez S, Mishriki Y, Finkel G, Grimson R, Lundy J. ras p21 expression in the progression of breast cancer. Hum Pathol. 1987;18:1268-1275. [PubMed] [Cited in This Article: ] |
| 9. | Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 1997;349:1269-1276. [PubMed] [Cited in This Article: ] |
| 10. | Peddanna N, Holt S, Verma RS. Genetics of gastric cancer. Anticancer Res. 1995;15:2055-2064. [PubMed] [Cited in This Article: ] |
| 11. | Kang H, Ko J, Jang SW. The role of annexin A1 in expression of matrix metalloproteinase-9 and invasion of breast cancer cells. Biochem Biophys Res Commun. 2012;423:188-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Biaoxue R, Xiling J, Shuanying Y, Wei Z, Xiguang C, Jinsui W, Min Z. Upregulation of Hsp90-beta and annexin A1 correlates with poor survival and lymphatic metastasis in lung cancer patients. J Exp Clin Cancer Res. 2012;31:70. [PubMed] [Cited in This Article: ] |
| 13. | Chen CY, Shen JQ, Wang F, Wan R, Wang XP. Prognostic significance of annexin A1 expression in pancreatic ductal adenocarcinoma. Asian Pac J Cancer Prev. 2012;13:4707-4712. [PubMed] [Cited in This Article: ] |
| 14. | Duncan R, Carpenter B, Main LC, Telfer C, Murray GI. Characterisation and protein expression profiling of annexins in colorectal cancer. Br J Cancer. 2008;98:426-433. [PubMed] [Cited in This Article: ] |
| 15. | Li CF, Shen KH, Huang LC, Huang HY, Wang YH, Wu TF. Annexin-I overexpression is associated with tumour progression and independently predicts inferior disease-specific and metastasis-free survival in urinary bladder urothelial carcinoma. Pathology. 2010;42:43-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Faria PC, Sena AA, Nascimento R, Carvalho WJ, Loyola AM, Silva SJ, Durighetto AF, Oliveira AD, Oliani SM, Goulart LR. Expression of annexin A1 mRNA in peripheral blood from oral squamous cell carcinoma patients. Oral Oncol. 2010;46:25-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Alves VA, Nonogaki S, Cury PM, Wünsch-Filho V, de Carvalho MB, Michaluart-Júnior P, Moyses RA, Curioni OA, Figueiredo DL, Scapulatempo-Neto C. Annexin A1 subcellular expression in laryngeal squamous cell carcinoma. Histopathology. 2008;53:715-727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Cheng AL, Huang WG, Chen ZC, Peng F, Zhang PF, Li MY, Li F, Li JL, Li C, Yi H. Identification of novel nasopharyngeal carcinoma biomarkers by laser capture microdissection and proteomic analysis. Clin Cancer Res. 2008;14:435-445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 124] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 19. | Schaeffer EM, Marchionni L, Huang Z, Simons B, Blackman A, Yu W, Parmigiani G, Berman DM. Androgen-induced programs for prostate epithelial growth and invasion arise in embryogenesis and are reactivated in cancer. Oncogene. 2008;27:7180-7191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 137] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 20. | Wang KL, Wu TT, Resetkova E, Wang H, Correa AM, Hofstetter WL, Swisher SG, Ajani JA, Rashid A, Hamilton SR. Expression of annexin A1 in esophageal and esophagogastric junction adenocarcinomas: association with poor outcome. Clin Cancer Res. 2006;12:4598-4604. [PubMed] [Cited in This Article: ] |
| 21. | Cheng TY, Wu MS, Lin JT, Lin MT, Shun CT, Huang HY, Hua KT, Kuo ML. Annexin A1 is associated with gastric cancer survival and promotes gastric cancer cell invasiveness through the formyl peptide receptor/extracellular signal-regulated kinase/integrin beta-1-binding protein 1 pathway. Cancer. 2012;118:5757-5767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 22. | Pin AL, Houle F, Fournier P, Guillonneau M, Paquet ÉR, Simard MJ, Royal I, Huot J. Annexin-1-mediated endothelial cell migration and angiogenesis are regulated by vascular endothelial growth factor (VEGF)-induced inhibition of miR-196a expression. J Biol Chem. 2012;287:30541-30551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 23. | Lin LL, Huang HC, Ogihara S, Wang JT, Wu MC, McNeil PL, Chen CN, Juan HF. Helicobacter pylori Disrupts Host Cell Membranes, Initiating a Repair Response and Cell Proliferation. Int J Mol Sci. 2012;13:10176-10192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 24. | Zhu F, Xu C, Jiang Z, Jin M, Wang L, Zeng S, Teng L, Cao J. Nuclear localization of annexin A1 correlates with advanced disease and peritoneal dissemination in patients with gastric carcinoma. Anat Rec (Hoboken). 2010;293:1310-1314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Solito E, de Coupade C, Parente L, Flower RJ, Russo-Marie F. IL-6 stimulates annexin 1 expression and translocation and suggests a new biological role as class II acute phase protein. Cytokine. 1998;10:514-521. [PubMed] [Cited in This Article: ] |
| 26. | Rhee HJ, Kim GY, Huh JW, Kim SW, Na DS. Annexin I is a stress protein induced by heat, oxidative stress and a sulfhydryl-reactive agent. Eur J Biochem. 2000;267:3220-3225. [PubMed] [Cited in This Article: ] |