Published online Dec 14, 2013. doi: 10.3748/wjg.v19.i46.8638
Revised: July 17, 2013
Accepted: September 4, 2013
Published online: December 14, 2013
AIM: To compare efficacy and complications of partially covered self-expandable metal stent (pcSEMS) to plastic stent (PS) in patients treated for malignant, infrahilar biliary obstruction.
METHODS: Multicenter prospective randomized clinical trial with treatment allocation to a pcWallstent® (SEMS) or a 10 French PS. Palliative patients aged ≥ 18, for infrahilar malignant biliary obstruction and a Karnofsky performance scale index > 60% from 6 participating North American university centers. Primary endpoint was time to stent failure, with secondary outcomes of death, adverse events, Karnofsky performance score and short-form-36 scale administered on a three-monthly basis for up to 2 years. Survival analyses were performed for stent failure and death, with Cox proportional hazards regression models to determine significant predictive characteristics.
RESULTS: Eighty-five patients were accrued over 37 mo, 42 were randomized to the SEMS group and 83 patients were available for analyses. Time to stent failure was 385.3 ± 52.5 d in the SEMS and 153.3 ± 19.8 d in the PS group, P = 0.006. Time to death did not differ between groups (192.3 ± 23.4 d for SEMS vs 211.5 ± 28.0 d for PS, P = 0.70). The only significant predictor was treatment allocation, relating to the time to stent failure (P = 0.01). Amongst other measured outcomes, only cholangitis differed, being more common in the PS group (4.9% vs 24.5%, P = 0.029). The small number of patients in follow-up limits longitudinal assessments of performance and quality of life. From an initially planned 120 patients, only 85 patients were recruited.
CONCLUSION: Partially covered SEMS result in a longer duration till stent failure without increased complication rates, yet without accompanying measurable benefits in survival, performance, or quality of life.
Core tip: This randomized trial is one of very few comparing partially covered self-expandable metal stent (SEMS) to 10 French plastic stent (PS) in the contemporary palliation of malignant biliary obstruction. In 85 patients, time to stent failure was significantly longer (385.3 ± 52.5 d) in SEMS vs PS (153.3 ± 19.8 d), P = 0.006. Time to death did not differ (192.3 ± 23.4 d for SEMS vs 211.5 ± 28.0 d for PS, P = 0.70). Amongst other measured outcomes, only cholangitis differed and was more common in PS (4.9% vs 24.5%, P = 0.029).
-
Citation: Moses PL, AlNaamani KM, Barkun AN, Gordon SR, Mitty RD, Branch MS, Kowalski TE, Martel M, Adam V. Randomized trial in malignant biliary obstruction: Plastic
vs partially covered metal stents. World J Gastroenterol 2013; 19(46): 8638-8646 - URL: https://www.wjgnet.com/1007-9327/full/v19/i46/8638.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i46.8638
Malignant obstructive jaundice is associated with many symptoms that negatively impact quality of life such as anorexia, pruritus and malabsorption[1-3]. Endoscopic retrograde cholangio-pancreaticography (ERCP) with placement of a biliary stent is the procedure of choice for palliation of infrahilar common bile duct (CBD) malignant biliary obstruction[4].
Both plastic and self-expandable metallic stents can palliate malignant biliary obstruction, and although randomized trial data have shown uncovered metallic stents to remain patent for longer periods compared to plastic stents[3], the latter remain widely utilized[3,5,6] at least in part due to their lower upfront costs. The more recently introduced covered self-expandable metallic stents remain poorly studied in a randomized clinical trial setting, and may be associated with added complications such as pancreatitis and cholecystitis, as well as stent migration. This holds true for both fully covered and partially covered stents[7-15].
The primary aim of our study was thus to compare the stent patency’s of a partially covered metal stent and a commonly used plastic stent in a randomized controlled trial for patients with low to mid-CBD malignant biliary obstruction. We additionally sought to better characterize the safety of the partially covered metal stent and attempted to identify clinical variables that would allow clinicians to choose a metallic or plastic biliary stent.
The study was a randomized clinical trial. Randomization was performed using sealed envelopes in which patients were allocated in a 1:1 proportion to either a partially covered Wallstent® Endoscopic Biliary Endoprosthesis with Permalume™ covering comprised of two components: the implantable metallic stent and the Unistep™ Plus Delivery System, (Boston Scientific, Natick, MA, United States) (self-expandable metal stent, SEMS), or a 10 French (Fr) Amsterdam-type polyethylene plastic stent (PS) biliary stent. The sealed envelopes were opened only at the time of intent of stent insertion in the ERCP suite after confirmation that all selection criteria had been fulfilled. The allocation sequence was performed centrally and patient enrolment and participant assignment was carried out by a third party not directly involved with the patient’s care or the measurement of outcomes. Neither patient, treating team, or the evaluators of outcomes were blinded to treatment allocation due to the nature of the intervention and follow-up care required. Each investigator received written approval for the study from his respective Institutional Review Boards prior to study initiation and patient enrollment. The trial did not require prior registration as it was started before 2004 and both stents are FDA approved.
Inclusion criteria were age 18 or older, and the provision of a signed written voluntary informed consent form approved by the Institutional Review Boards at participating centers. All patients demonstrated laboratory, imaging and/or histological evidence of malignant biliary obstruction. The cause of obstruction could be any intrinsic or extrinsic malignancy extending no more proximal than 1cm below the common hepatic ductal bifurcation. A Karnofsky performance scale was applied. A Karnofsky score > 60% is a validated measure of patient function, previously used in Pancreatico-biliary cancer patients[16]. Patients were required to haven anticipated life expectancy that would allow for completion of full follow-up. Exclusion criteria were jaundice related to intrahepatic cholestasis or obstruction, or a prior attempt at a curative surgical resection for the biliary obstructing lesion. There were 6 participating North American university centers (Fletcher Allen Health Care at the University of Vermont, McGill University Health Centre, St. Elizabeth’s Medical Center, Dartmouth Hitchcock Medical Center, Duke University Medical Center, and Thomas Jefferson University).
The primary endpoint of the trial was the time to occurrence of stent failure as defined by the appearance of one or more cholestatic symptoms accompanied by a 50% increase in bilirubin from the lowest post-stent insertion value recorded prior to this follow-up event, and/or cholangitis (defined as the new onset of pain, fever, and jaundice) whether associated with a stent replacement or not. Repeat ERCP for stent replacement or suspected obstruction using a stent of any type was considered to represent a stent failure. Secondary outcomes were death, cholestatic symptoms, laboratory data, technical success (defined as the successful delivery and deployment of the initial stent to the desired location in the biliary tree) measured at the time of the procedure, the presence of adverse events, and the Karnofsky performance score. We also administered the short form (SF)-36 general quality of life measurement scale, which is a questionnaire measuring patient’s perceptions about functional health and well-being previously administered to bilio-pancreatic cancer patients[1].
ERCP was performed by experienced endoscopists; stents were placed with or without prior dilatation or sphincterotomy after sealed-envelope randomization to stent type was done after confirmation of obstruction meeting inclusion criteria. The patient then received either the SEMS or the PS. The length of each type of stent was determined by the biliary anatomy and left to the discretion of the endoscopist as part of the medical effectiveness philosophy of the trial thereby enhancing generalizability of the results. A cholangiogram was performed to document stent patency and position. No prophylactic antibiotics were used. Each patient had one- and three-month follow-up, followed by quarterly scheduled follow-up sessions up to 2 years following stent insertion.
Failed plastic stents were replaced with covered metal stents, while failed covered metal stents were replaced with either one or more plastic or metal stent(s) inserted through the metal stent. The decision for the choice of stent type following stent failure was left to the discretion of the endoscopist and recorded.
Dedicated, standardized electronic case report forms were completed by trained research assistants and downloaded into a web-based remote data entry repository. Internal validity of recorded data and missing data quality checks were performed centrally by trained research personnel. At baseline, investigation variables included any significant medical history, the tumor type, stage, and location, the date of diagnosis, the length and maximum diameter of stricture, and administration of any prior anticancer treatment, as well as the Karnofsky Score. Variables assessed at baseline and at periodic follow-up visits (months 1, 3, 6, 9, 12, and if the patient survived, 3-monthly up to month 24) following the index procedure included a cholestatic symptom assessment, the use of any adjuvant treatment such as radiation and/or chemotherapy, laboratory test results (chemistry, hematology), and the Karnofsky index. All adverse events were recorded, including the occurrences of cholangitis, pancreatitis, and cholecystitis using standardized definitions[17].
The planned enrollment was 120 patients. Sample size predictions were calculated using a model of binomial proportions and independent samples. Assuming a 25% improvement in stent patency duration with expandable metallic stenting and using a 1-sided type I error rate of 5% and a type II error rate of 20%, approximately 60 patients were thought to be needed in each group.
Amongst descriptive variables, continuous variables are reported as means and standard deviations as well as medians where appropriate, and categorical variables as proportions. Inferential testing was carried out using t-tests for continuous and χ2 for categorical variables. Karnofsky scores and quality of life scores were assessed for both intra- and between-group differences comparing baseline values to the last visit on record at the 1-mo, the 3-mo visit, and the 6-mo visits; both within and across groups. We used a t-test with either the pooled or Satterthwaite method, depending on the results of the equality of variances test at each follow-up period.
Survival analyses were performed for both stent failure and patient survival using both intention-to-treat (ITT) and per protocol (PP) analyses. In the first group, only subjects who had at least a 50% drop in bilirubin at 1 mo were included. In the second, all subjects were included as originally randomized. Kaplan-Meier curves were created for the SEMS and PS groups, and compared with a log-rank test. Cox proportional hazards regression models were also used to determine if significant covariates were associated with either time-to-stent failure or time-to-death. The proportional hazards assumption was tested with the use of a Kolmogorov-Smirnov Supremum test. The following covariates were included for these analyses in addition to stent randomization group: obstruction (for prediction of mortality), tumor type, known metastatic cancer, chemotherapy or radiation therapy, and the baseline Karnofsky score. Covariates that were associated with the outcome with a P-value of 0.15 or less in a univariate model were entered into a multivariable model. There was no planned interim analysis.
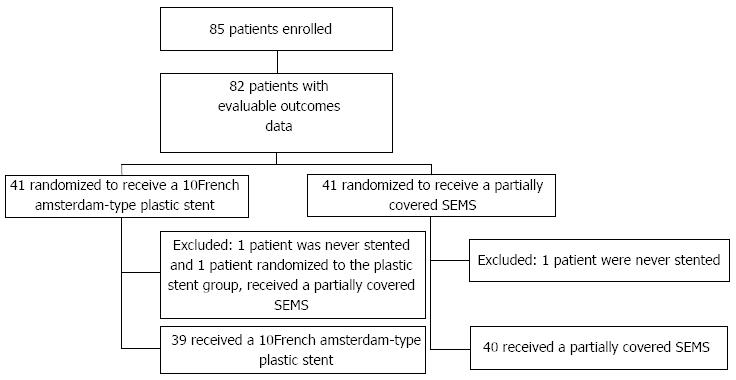
A total of 85 patients were accrued over 37 mo. The study was closed prior to completion of enrollment of the estimated 120 patients due to a marked slowing of patient accrual (trial fatigue). Of the 85 patients included, 42 were randomized to the SEMS group, and 43 to the PS group. Three patients had evaluable baseline patient data but were excluded from further analyses because of inclusion protocol violations (2 were never stented, and one received a metal covered stent when in fact randomized to plastic stent). Of the 82 patients with analyzable outcomes data, 41 received a SEMS and 41 a PS; the CONSORT diagram is shown in Figure 1. Population characteristics at baseline for both groups are shown in Table 1.
| Characteristic | Partially covered SEMS (n = 42) | 10-French polyethylene plastic stent (n = 43) | P value | |
| Gender (male) | 51.2% | 50.0% | 0.9119 | |
| Age (yr) | 70.8 ± 12.9 | 73.3 ± 10.7 | 0.3896 | |
| Co-morbidities: | Cardiovascular | 53.7% | 47.5% | 0.5676 |
| Respiratory | 22.0% | 20.0% | 0.8209 | |
| Neurologic | 19.5% | 22.5% | 0.7343 | |
| GI, liver, biliary | 75.6% | 77.5% | 0.8362 | |
| Renal, urinary | 15.0% | 30.0% | 0.0982 | |
| Musculoskeletal | 25.0% | 35.0% | 0.3148 | |
| Endocrine | 47.5% | 30.0% | 0.0976 | |
| Cholestatic symptoms | 97.5% | 100.0% | 0.2968 | |
| Jaundice | 85.4% | 97.5% | 0.0453 | |
| Clay-colored stools | 36.6% | 52.5% | 0.1404 | |
| Abdominal pain | Abdominal pain | 53.7% | 40.0% | 0.2056 |
| Pruritus | 51.2% | 50.0% | 0.9119 | |
| Dark urine | 75.6% | 75.0% | 0.9489 | |
| Fever | Fever | 9.8% | 5.0% | 0.3972 |
| Constitutional symptoms | ||||
| Weight Loss | 73.2% | 47.5% | 0.0155 | |
| Anorexia | 51.2% | 50.0% | 0.9119 | |
| Obstruction location | Papilla | 2.78% | 7.9% | 0.2951 |
| Distal common bile duct | 72.2% | 47.4% | 0.0198 | |
| Mid common bile duct | 22.2% | 39.5% | 0.0845 | |
| Proximal common bile duct | 2.8% | 5.3% | 0.5595 | |
| Type of primary tumor | Ampullary carcinoma | 2.6% | 7.5% | 0.3036 |
| Cholangiocarcinoma | 0.0% | 5.0% | 0.1422 | |
| Gallbladder adenocarcinoma | 2.6% | 2.5% | 0.9767 | |
| Metastatic Cancer | 10.3% | 7.5% | 0.6501 | |
| Pancreatic adenocarcinoma | 69.2% | 67.5% | 0.8662 | |
| Other | 0.0% | 2.5% | 0.3024 | |
| Unknown | 15.4% | 7.5% | 0.2519 | |
| Metastat. cancer prim. location | Colon | 28.6% | 0.0% | 0.0002 |
| Lung | 28.6% | 20.0% | 0.355 | |
| Other | 42.9% | 80.0% | 0.0004 | |
| Tumor stage | T1 | 4.0% | 19.2% | 0.0292 |
| T2 | 32.0% | 11.5% | 0.0217 | |
| T3 | 16.0% | 42.3% | 0.0077 | |
| T4 | 48.0% | 26.9% | 0.0443 | |
| Nodes | N0 | 36.4% | 61.9% | 0.0187 |
| N1 | 63.6% | 38.1% | 0.0187 | |
| Metastatic tumor | M0 | 29.2% | 36.0% | 0.5038 |
| M1 | 70.8% | 64.0% | 0.5038 | |
| Chemotherapy or radiation | 11.1% | 8.82% | 0.7255 | |
| Laboratory data | Alkaline phosphatase (IU/L) | 630.5 ± 347.7 | 532.7 ± 331.4 | 0.1486 |
| Bilirubin (mg/dL) | 9.56 ± 6.99 | 11.33 ± 7.82 | 0.3082 | |
| Hematocrit | 43.97% ± 50.36% | 37.06% ± 5.95% | 0.3280 | |
| Hemoglobin (g/dL) | 12.01 ± 1.63 | 12.39 ± 2.09 | 0.3305 | |
| INR | 1.17 ± 0.19 | 1.25 ± 0.36 | 0.5922 | |
| AST (IU/L) | 168.64 ± 98.34 | 191.26 ± 149.25 | 0.6687 | |
| ALT (IU/L) | 240.03 ± 178.35 | 265.03 ± 240.48 | 0.8926 | |
| Karnosky performance scores | 81.8 ± 10.8 | 82.0 ± 12.03 | 0.9151 |
Twenty-nine point eight percent were inpatients; amongst these, the mean hospital stays related to the procedure were 2.5 ± 1.6 d in the SEMS group, and 4.9 ± 4.7 d in the PS group.
The mean length of Wallstents used was 61.4 ± 11.2 mm (median: 60 mm, range: 40-80 mm); 95.1% patients received a 10Fr diameter and 4.9% an 8F diameter. In the PC group, the stent length was 76.0 ± 18.2 mm (median: 70, range: 50-120 mm); all patients had a 10Fr diameter.
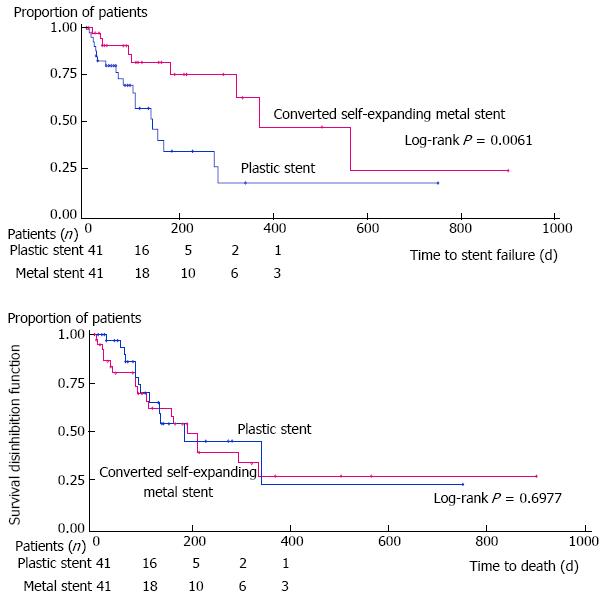
In ITT analysis, the time to stent failure was 385.3 ± 52.5 d in the SEMS and 153.3 ± 19.8 d in the PS group (P = 0.006) (Figure 2A). Corresponding results were 396.5 ± 56.8 d and 164.3 ± 24.1 d, respectively (P = 0.025) using a PP approach. After adjustment for possible confounding variables, in ITT analysis, the only independent significant predictor of a failed stent was the stent group allocation (HR = 0.29, 95%CI: 0.12-0.75, P = 0.011); similar findings were noted with the PP analysis (HR = 0.22, 95%CI: 0.06-0.80, P = 0.013)
Procedural outcomes: No differences in intra-procedural events were noted. Overall, 69.4% of all stent insertions were carried out in an out-patient setting. Optimal stent insertion and positioning was noted in 95.3% of patient with in the SEMS and 97.4% of patients in the PS groups, respectively. The length of the SEMS used was 61.4 ± 11.2 mm (median: 60 mm, range: 40-80 mm) and a diameter of 59.64 ± 11.2 mm (median: 60 mm, range: 40-80 mm); 95.1% patients received a 10Fr diameter and 4.9% a 8F diameter. In the PC group, the stent length was 76.0 ± 18.2 mm (median: 70 mm, range: 50-120 mm); All patients had a 10Fr diameter. Sphincterotomy was carried out prior to stent insertion in 18.8% of cases, and balloon dilatation in 3.5%. The distal end of the stent was positioned outside the CBD into the duodenum in 93.0%. Some form of tissue sampling was carried out at the time of ERCP in 56.0% of patients.
Time to death: The time to death did not differ between both groups: 192.3 ± 23.4 d for SEMS vs 211.5 ± 28.0 d for PS (P = 0.70) using an ITT approach (Figure 2B). Similar conclusions were reached using the PP approach 248.5 ± 26.8 d vs 251.3 ± 32.5 d, respectively (P = 0.66). After adjustment for possible confounding variables, no significant predictor of time to death was found in ITT or PP analysis.
Additional secondary outcomes: Complications including the development of pancreatitis, cholangitis, and cholecystitis are (2.4% vs 2.4%, P = 1.0000; 4.9% vs 24.5%, P = 0.029; 4.8% vs 0.0%, P = 0.4741). Only cholangitis differed, with a greater frequency in the PS group.
The percentage reduction in bilirubin value from baseline to the 1-mo visit was no different in SEMS than in the PS group [74.0%, (95%CI: 60.0-87.9) vs 63.7% (95%CI: 45.5-81.9), respectively, P = 0.37]. No statistical differences in Karnoksy performance scores were noted between the two treatment groups when comparing the differences in scores for the last, 1, 3, and 6 mo visits compared to baseline. In the pre-planned paired analysis to assess intra-group differences, patients receiving the SEMS, showed significant improvements noted at 6 mo and at the last available visit (P = 0.015, and P = 0.022, respectively). There were also significant improvements noted for patients in the PS group compared to baseline both at 1 mo and at the last available date of follow-up (P = 0.045, and P = 0.0014, respectively; full data available upon request).
Overall, 29.4% of patients had one or more cholestatic symptoms at follow-up, 24.3% for the SEMS group and 33.3% for the PS group (P = 0.213). Additional symptom reporting showed no difference between both groups with regards to individual cholestatic symptoms (data not shown).
Quality of life-SF-36 measures: Seventy-four patients answered the quality of life questionnaires over a total of 174 visits during a 12-mo follow-up (31 patients answered only once to the SF-36 questionnaire, 17 answered to two questionnaire and 13 responded to 3 questionnaires). Among these, 38 had received a SEMS and 36 a PS. At baseline, patients in the SEMS group exhibited lower means than those in the PS group for all 8 summary scores, indicating worse quality of life parameters; the differences however were not statistically significant except for physical functioning (46.4 vs 63.9, P = 0.008). The SEMS group scores improved gradually such that, by 9 mo, most were arithmetically greater than scores from the PS group although without significant differences. There remained, however, only a very small number of patients able to complete the questionnaires in follow-up (9 patients at 9 mo, and 5 at 12 mo). In paired analysis, statistical significant improvements were noted amongst SEMS patients in physical functioning (6 mo vs baseline), and vitality (1 mo vs baseline). Significant bettering of quality of life was noted amongst PS patients at 1 month vs baseline for bodily pain, social functioning, and mental health, as well as in vitality for the 9 mo vs baseline comparison (full quality of life scores are available upon request).
Stenting for malignant biliary obstruction remains principally a palliative procedure[6,7]; temporary stenting until the time of exploratory or potentially curative surgery is performed (with the advent of useful adjuvant treatment methods), although the efficacy of this approach remains unproven and may in fact be harmful[18-20]. RCT data have suggested the superiority of uncovered metal over plastic biliary stenting3 owing to the larger internal luminal diameter, thus preventing premature blockage from bacterial biofilm encrustation and sludge formation[21]. Indeed, a Cochrane meta-analysis of 5 trials by Moss et al[7] concluded that uncovered metal stents had a lower risk of recurrent biliary obstruction than plastic stents (RR = 0.52, 95%CI: 0.39-0.69), with no difference in technical or therapeutic success, complications or 30-d mortality. An additional trial performed since, also confirmed the superiority of uncovered SEMS over Tannenbaum plastic stents[22], a plastic stent variant without side holes that may contribute to prolonged plastic stent patency.
Although these trials assessed uncovered metal biliary stents, these conclusions were largely presumed to be generalizable to (partially and completely) covered metal stents, and probably is the reason for a paucity of studies examining this latter comparison. This assumption, however, can be questioned and is of contemporary significance for two reasons: (1) plastic biliary stents remain very commonly inserted as initial method of stenting in the face of increasing use of covered and uncovered metal stents for non hilar biliary obstruction[6]; and (2) covered metal stents are reported to exhibit greater rates of migration, and perhaps other complications (such as cholecystitis and pancreatitis) compared to uncovered metal stents[5,11,23]. Both these realizations justify the aims of the current trial.
Only two randomized controlled trials have compared plastic to covered metal stents. In the multicenter trial by Isayama et al[24], investigators compared a covered metal biliary stent to a rarely used type of plastic stent with a double lumen (found to be superior to polyethylene stents with regards to stent patency[25]) in patients with lower biliary malignant obstruction attributable to pancreatic head cancer. In the Isayama multicentric trial[24], the cumulative stent patency was significantly greater in the covered metal stent group: the respective mean and median stent patency durations were 285 and 419 d, vs 202 and 133 d observed for the plastic stent group patients respectively (P = 0.0072). Interestingly, the covered metal stent group experienced more frequent cholecystitis (4 vs 0), pancreatitis (1 vs 0), and migration (5 vs 1), although these differences did not, at least taken separately, achieve statistical significance. These results validate the findings of the current trial that noted, using life-table analysis, that the time to stent failure was 385.3 ± 52.5 d in the SEMS, and 153.3 ± 19.8 d in the PS group (P = 0.006). Times to stent occlusion were all shorter, although the between-group differences remained, in the only other randomized trial assessing plastic vs covered metal stents by Soderlund et al[26]. In that study, 22 of 51 plastic stent and 9 of 49 covered metal stent group patients (P = 0.009) developed stent failure after medians of 1.1 and 3.5 mo (P = 0.007), with median patency times of 1.8 mo vs 3.6 mo (P = 0.002), respectively.
Even though insertion of a plastic stent is favored in patients with an estimated short survival, such as those with large tumors (over 30 mm), liver metastases, younger age, or adenocarcinoma histology[27-29], summary RCT data have shown that infrahilar biliary stenting, either pre-operatively or as sole palliation, does not improve mortality[3,18,19]. Interestingly, however, an observational trial and an as yet unpublished additional meta-analysis have suggested improvement in survival using expandable metal stent technologies, yet remain unconfirmed[30,31]. Furthermore, while other such comparisons have failed to demonstrate such a benefit5, two trials have recently suggested a patient survival benefit with the percutaneous insertion of covered rather than uncovered metal stents for infrahilar biliary obstruction, due to pancreatic cancers[15] and cholangiocarcinomas[32].
Despite difficulty in accrual leading to early termination of the study including 85 patients and not the projected 130 patients, the strengths of the current trial include the multi-institutional participation, the medical effectiveness design, and the adopted ITT analytical approach that all increase the generalizability of results. The a priori standardized definitions and independent measurements of outcomes all strengthen the validity, minimizing the chance of bias. Life table analysis and multivariable adjustment further ensure the clinical relevance of the findings. Other than improved stent patency, only the outcome of cholangitis differed amongst both groups, occurring more frequently in the plastic stent group. Cholecystitis was a rare outcome, as was pancreatitis, and the trial was not powered to demonstrate any differences in these less frequent endpoints. No differences in procedural outcomes were noted. The between-group quality of life comparisons were limited by the small number of survivors, yet the pre-post stenting analyses within each group using paired analyses confirm what few trials have shown: that a number of quality of life domains improve following successful biliary drainage[1,33,34], and that such benefits were observed both with metal and plastic stents. Post-stent insertion improvements in functional status using Karnovsky scores were also noted at 1 mo and extended to the last available patient visit recorded.
These results, taken as a whole, suggest that the observed benefits of the SEMS studied over the common type of PS used as comparator are attributable to the prolonged stent patency. The resulting decreased rates of cholangitis outweigh any possible risks attributable to increased stent migration, pancreatitis or cholecystitis. Further characterization of optimal patient groups may relate to issues such as cystic duct involvement that predicts cholecystitis[23,35].
Perhaps just as relevant as efficacy findings are cost-effectiveness issues that are being analyzed separately as part of the current trial. Indeed, past cost-effectiveness modeling have suggested that the presence of distant metastases, especially if numerous, is associated with shorter survival time in patients with pancreatic cancer, and that a metallic stent should not be used in this type of patients[27,36,37]. Rather, SEMS should be reserved for patients expected to live for at least 6 mo[38,39]. Nonetheless, as mentioned earlier, plastic stents remain commonly inserted, at least in part due to their lower upfront costs compared to their metal expandable equivalents.
In conclusion, the present study confirms that insertion of a partially covered SEMS for patients with infrahilar biliary obstructing tumors results in a longer duration until stent failure as compared to a commonly used plastic stent (in this case, an Amsterdam-type polyethylene stent) without increased complication rates. There were no measurable benefits in survival, performance, or quality of life. Additional trials and meta-analytical evaluation are required to more confidently assess these important additional patient outcomes.
Despite existing evidence in the literature favoring metal over platis stenting in distal biliary obstruction palliation, few data exist assessing partially covered metal stents, especially in a contemporary setting.
Plastuc stent palliation remains widespread, while partially covered metal biliary stents appear to migrate more frequently than their uncovered counterparts, but have gained popularity.
There have been 2 previously published studies in the literature that have compared partially covered metal to plastic biliary stenting with limited generalizability, suggesting the superiority of the metal stent alternative.
The current article validates the conclusion that partially covered metal stents result in a significantly longer time to stent patency, without a prolonged survival time in patients undergoing palliation for distal biliary malignant obstruction. Additional and summary data are required to confirm the robustness of the latter finding, while the advent of completely covered metal stents require further assessments, owing to the theoretical benefit of decreased stent in growth versus the possible increased risk of stent migration.
The findings of this randomized trial appear to confirm that of two previous studies in the literature and is limited by the small sample size, even though a signififcant difference in the primary outcome measure was noted. The achieved statistical power limits the interpretation of other endpoints.
P- Reviewers: Dormann AJ, Garcia-Cano J, Larentzakis A, Parsi MA S- Editor: Gou SX L- Editor: A E- Editor: Liu XM
| 1. | Abraham NS, Barkun JS, Barkun AN. Palliation of malignant biliary obstruction: a prospective trial examining impact on quality of life. Gastrointest Endosc. 2002;56:835-841. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 2. | Ballinger AB, McHugh M, Catnach SM, Alstead EM, Clark ML. Symptom relief and quality of life after stenting for malignant bile duct obstruction. Gut. 1994;35:467-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 199] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 3. | Moss AC, Morris E, Mac Mathuna P. Palliative biliary stents for obstructing pancreatic carcinoma. Cochrane Database Syst Rev. 2006;CD004200. [PubMed] [Cited in This Article: ] |
| 4. | Small AJ, Baron TH. Novel endoscopic approaches for assessing biliary tract diseases. Curr Opin Gastroenterol. 2008;24:357-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Saleem A, Leggett CL, Murad MH, Baron TH. Meta-analysis of randomized trials comparing the patency of covered and uncovered self-expandable metal stents for palliation of distal malignant bile duct obstruction. Gastrointest Endosc. 2011;74:321-327.e1-3. [PubMed] [Cited in This Article: ] |
| 6. | Ferreira LE, Baron TH. Endoscopic stenting for palliation of malignant biliary obstruction. Expert Rev Med Devices. 2010;7:681-691. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Moss AC, Morris E, Leyden J, MacMathuna P. Do the benefits of metal stents justify the costs? A systematic review and meta-analysis of trials comparing endoscopic stents for malignant biliary obstruction. Eur J Gastroenterol Hepatol. 2007;19:1119-1124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 132] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Lee SH, Cha SW, Cheon YK, Kim Y, Moon JH, Cho YD, Kim YS, Lee JS, Lee MS, Shim CS. A Randomized Controlled Comparative Study of Covered Versus Uncovered Self-Expandable Metal Stent for Malignant Biliary Obstruction. Gastrointest Endosc. 2004;59:AB188. [Cited in This Article: ] |
| 9. | Telford JJ, Carr-Locke DL, Baron TH, Poneros JM, Bounds BC, Kelsey PB, Schapiro RH, Huang CS, Lichtenstein DR, Jacobson BC. A randomized trial comparing uncovered and partially covered self-expandable metal stents in the palliation of distal malignant biliary obstruction. Gastrointest Endosc. 2010;72:907-914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 170] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 10. | Kullman E, Frozanpor F, Söderlund C, Linder S, Sandström P, Lindhoff-Larsson A, Toth E, Lindell G, Jonas E, Freedman J. Covered versus uncovered self-expandable nitinol stents in the palliative treatment of malignant distal biliary obstruction: results from a randomized, multicenter study. Gastrointest Endosc. 2010;72:915-923. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 206] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 11. | Isayama H, Komatsu Y, Tsujino T, Sasahira N, Hirano K, Toda N, Nakai Y, Yamamoto N, Tada M, Yoshida H. A prospective randomised study of “covered” versus “uncovered” diamond stents for the management of distal malignant biliary obstruction. Gut. 2004;53:729-734. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 460] [Cited by in F6Publishing: 477] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 12. | Smits ME, Rauws EAJ, Groen AK, Tytgat GNJ, Huibregtse K. Preliminary-Results of a Prospective Randomized Study of Partially Covered Wallstents Vs Noncovered Wallstents. Gastrointest Endosc. 1995;41:416. [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Gonzalez-Huix F, Huertas C, Figa M, Igea F, Juzgado-Lucas D, Espinos JC, Abadia CD, Madrigal RE, Perez-Miranda M. A Randomized Controlled Trial Comparing the Covered (CSEMS) Versus Uncovered Self-Expandable Metal Stents (USEMS) for the Palliation of Malignant Distal Biliary Obstruction (MDBO): Interim Analysis. Gastrointest Endosc. 2008;67:AB166. [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Cho YD, Cheon YK, Yoo KS, Bang SJ, Kim CD, Kim JS, Roh MH, Kim HG. Uncovered Versus Covered Self-Expanding Metallic Stents for Inoperable Malignant Distal Biliary Obstruction: A Prospective Randomized Multicenter Study. Gastrointest Endosc. 2009;69:AB137. [DOI] [Cited in This Article: ] |
| 15. | Krokidis M, Fanelli F, Orgera G, Tsetis D, Mouzas I, Bezzi M, Kouroumalis E, Pasariello R, Hatzidakis A. Percutaneous palliation of pancreatic head cancer: randomized comparison of ePTFE/FEP-covered versus uncovered nitinol biliary stents. Cardiovasc Intervent Radiol. 2011;34:352-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Koeberle D, Saletti P, Borner M, Gerber D, Dietrich D, Caspar CB, Mingrone W, Beretta K, Strasser F, Ruhstaller T. Patient-reported outcomes of patients with advanced biliary tract cancers receiving gemcitabine plus capecitabine: a multicenter, phase II trial of the Swiss Group for Clinical Cancer Research. J Clin Oncol. 2008;26:3702-3708. [PubMed] [Cited in This Article: ] |
| 17. | Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, Nickl N. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383-393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1890] [Cited by in F6Publishing: 1934] [Article Influence: 58.6] [Reference Citation Analysis (1)] |
| 18. | Mumtaz K, Hamid S, Jafri W. Endoscopic retrograde cholangiopancreaticography with or without stenting in patients with pancreaticobiliary malignancy, prior to surgery. Cochrane Database Syst Rev. 2007;CD006001. [PubMed] [Cited in This Article: ] |
| 19. | Wang Q, Gurusamy KS, Lin H, Xie X, Wang C. Preoperative biliary drainage for obstructive jaundice. Cochrane Database Syst Rev. 2008;CD005444. [PubMed] [Cited in This Article: ] |
| 20. | van der Gaag NA, Kloek JJ, de Castro SM, Busch OR, van Gulik TM, Gouma DJ. Preoperative biliary drainage in patients with obstructive jaundice: history and current status. J Gastrointest Surg. 2009;13:814-820. [PubMed] [Cited in This Article: ] |
| 21. | Swidsinski A, Schlien P, Pernthaler A, Gottschalk U, Bärlehner E, Decker G, Swidsinski S, Strassburg J, Loening-Baucke V, Hoffmann U. Bacterial biofilm within diseased pancreatic and biliary tracts. Gut. 2005;54:388-395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Katsinelos P, Paikos D, Kountouras J, Chatzimavroudis G, Paroutoglou G, Moschos I, Gatopoulou A, Beltsis A, Zavos C, Papaziogas B. Tannenbaum and metal stents in the palliative treatment of malignant distal bile duct obstruction: a comparative study of patency and cost effectiveness. Surg Endosc. 2006;20:1587-1593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Isayama H, Kawabe T, Nakai Y, Tsujino T, Sasahira N, Yamamoto N, Arizumi T, Togawa O, Matsubara S, Ito Y. Cholecystitis after metallic stent placement in patients with malignant distal biliary obstruction. Clin Gastroenterol Hepatol. 2006;4:1148-1153. [PubMed] [Cited in This Article: ] |
| 24. | Isayama H, Yasuda I, Ryozawa S, Maguchi H, Igarashi Y, Matsuyama Y, Katanuma A, Hasebe O, Irisawa A, Itoi T. Results of a Japanese multicenter, randomized trial of endoscopic stenting for non-resectable pancreatic head cancer (JM-test): Covered Wallstent versus DoubleLayer stent. Dig Endosc. 2011;23:310-315. [PubMed] [Cited in This Article: ] |
| 25. | Tringali A, Mutignani M, Perri V, Zuccalà G, Cipolletta L, Bianco MA, Rotondano G, Philipper M, Schumacher B, Neuhaus H. A prospective, randomized multicenter trial comparing DoubleLayer and polyethylene stents for malignant distal common bile duct strictures. Endoscopy. 2003;35:992-997. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Soderlund C, Linder S. Covered metal versus plastic stents for malignant common bile duct stenosis: a prospective, randomized, controlled trial. Gastrointest Endosc. 2006;63:986-995. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 218] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 27. | Prat F, Chapat O, Ducot B, Ponchon T, Fritsch J, Choury AD, Pelletier G, Buffet C. Predictive factors for survival of patients with inoperable malignant distal biliary strictures: a practical management guideline. Gut. 1998;42:76-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 144] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 28. | Weaver SA, Stacey BS, Hayward SJ, Taylor GJ, Rooney NI, Robertson DA. Endoscopic palliation and survival in malignant biliary obstruction. Dig Dis Sci. 2001;46:2147-2153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Mahajan A, Ho H, Jain A, Rehan ME, Northup PG, Phillips MS, Ellen K, Shami VM, Kahaleh M. Mortality in patients undergoing covered self-expandable metal stent revisions in malignant biliary stricture: does pathology matter? Dig Liver Dis. 2010;42:803-806. [PubMed] [Cited in This Article: ] |
| 30. | Schmassmann A, von Gunten E, Knuchel J, Scheurer U, Fehr HF, Halter F. Wallstents versus plastic stents in malignant biliary obstruction: effects of stent patency of the first and second stent on patient compliance and survival. Am J Gastroenterol. 1996;91:654-659. [PubMed] [Cited in This Article: ] |
| 31. | Waschke K, da Silveira E, Toubouti Y, Rahme E, Barkun A. Self-expanding metal stents confer a survival advantage in the palliation of distal malignant biliary obstruction. Gastrointest Endosc. 2005;61:AB222. [DOI] [Cited in This Article: ] |
| 32. | Krokidis M, Fanelli F, Orgera G, Bezzi M, Passariello R, Hatzidakis A. Percutaneous treatment of malignant jaundice due to extrahepatic cholangiocarcinoma: covered Viabil stent versus uncovered Wallstents. Cardiovasc Intervent Radiol. 2010;33:97-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 33. | Saluja SS, Gulati M, Garg PK, Pal H, Pal S, Sahni P, Chattopadhyay TK. Endoscopic or percutaneous biliary drainage for gallbladder cancer: a randomized trial and quality of life assessment. Clin Gastroenterol Hepatol. 2008;6:944-950.e3. [PubMed] [Cited in This Article: ] |
| 34. | Larssen L, Medhus AW, Hjermstad MJ, Körner H, Glomsaker T, Søberg T, Gleditsch D, Hovde O, Nesbakken A, Tholfsen JK. Patient-reported outcomes in palliative gastrointestinal stenting: a Norwegian multicenter study. Surg Endosc. 2011;25:3162-3169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Suk KT, Kim HS, Kim JW, Baik SK, Kwon SO, Kim HG, Lee DH, Yoo BM, Kim JH, Moon YS. Risk factors for cholecystitis after metal stent placement in malignant biliary obstruction. Gastrointest Endosc. 2006;64:522-529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 36. | Kaassis M, Boyer J, Dumas R, Ponchon T, Coumaros D, Delcenserie R, Canard JM, Fritsch J, Rey JF, Burtin P. Plastic or metal stents for malignant stricture of the common bile duct? Results of a randomized prospective study. Gastrointest Endosc. 2003;57:178-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 356] [Cited by in F6Publishing: 380] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 37. | Pereira-Lima JC, Jakobs R, Maier M, Benz C, Kohler B, Riemann JF. Endoscopic biliary stenting for the palliation of pancreatic cancer: results, survival predictive factors, and comparison of 10-French with 11.5-French gauge stents. Am J Gastroenterol. 1996;91:2179-2184. [PubMed] [Cited in This Article: ] |
| 38. | Yeoh KG, Zimmerman MJ, Cunningham JT, Cotton PB. Comparative costs of metal versus plastic biliary stent strategies for malignant obstructive jaundice by decision analysis. Gastrointest Endosc. 1999;49:466-471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 133] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 39. | Arguedas MR, Heudebert GH, Stinnett AA, Wilcox CM. Biliary stents in malignant obstructive jaundice due to pancreatic carcinoma: a cost-effectiveness analysis. Am J Gastroenterol. 2002;97:898-904. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 122] [Article Influence: 5.5] [Reference Citation Analysis (0)] |