Published online Dec 28, 2013. doi: 10.3748/wjg.v19.i48.9328
Revised: August 21, 2013
Accepted: September 4, 2013
Published online: December 28, 2013
AIM: To study the potential association between hepatocellular carcinoma (HCC) in patients with chronic hepatitis C (CHC), cirrhosis and latent hepatitis B (LHB) infection, defined as the absence of detectable serum hepatitis B surface antigen (HBsAg) and the presence of hepatitis B core antibody (HBcAb).
METHODS: This retrospective analysis is comprised of 185 cirrhotic patients with HCC who were hepatitis C virus antibody (HCV Ab) (+) and HBsAg(-) at Wayne State University between 1999 and 2008. From these, 108 patients had HCV polymerase chain reaction confirmation of viremia while the remaining (77) were considered to have CHC on the basis of a positive HCV Ab and the absence of any other cause of liver disease. Controls were drawn from our institutional database from the same time period and consisted of 356 HBsAg(-) age, race and gender matched patients with HCV RNA-confirmed CHC and without evidence of HCC. A subgroup of controls included 118 matched patients with liver cirrhosis. χ2 test and t test were used for data analysis.
RESULTS: Seventy-seven percent of patients in all 3 groups were African Americans. Patients with HCC had a significantly higher body mass index (P = 0.03), a higher rate of co-infection with human immunodeficiency virus (HIV) (P = 0.05) and a higher prevalence of alcohol abuse (P = 0.03) than the controls. More patients with HCC had LHB than controls (78% vs 39%, P = 0.01). Sixty three percent of patients with HCC were both hepatitis B surface antigen (HBsAb)(-) and HBcAb(+) compared to 23% of controls (P < 0.01). When compared to cirrhotic controls, the frequency of HBcAb(+) remained higher in patients with HCC (78% vs 45%, P = 0.02). Patients with HCC were more likely to be both HBsAb(-) and HBcAb(+) than the cirrhotic controls (63% vs 28%, P = 0.01). Although not statistically significant, 100% of CHC and HIV co-infected patients with HCC (n = 11) were HBcAb(+) when compared to controls (44%; n = 9).
CONCLUSION: These data suggest that LHB occurs at a significantly increased frequency in patients with CHC and HCC than in patients with CHC without HCC.
Core tip: Latent hepatitis B (LHB) has recently received significant attention among researchers and clinicians managing chronic liver disease. It is defined as a combination of hepatitis B surface antigen negative and hepatitis B core antibody positive. The potential association of LHB with hepatocellular carcinoma among patients with chronic hepatitis C infection has been studied and reported in this manuscript.
- Citation: Reddy A, May E, Ehrinpreis M, Mutchnick M. Latent hepatitis B is a risk factor for hepatocellular carcinoma in patients with chronic hepatitis C. World J Gastroenterol 2013; 19(48): 9328-9333
- URL: https://www.wjgnet.com/1007-9327/full/v19/i48/9328.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i48.9328
Emerging data suggest that the mortality rate in cirrhotic patients with hepatocellular carcinoma (HCC) is rising whereas the mortality rate from other complications of cirrhosis is either stable or declining[1]. In the United States, chronic hepatitis C (CHC) accounts for the majority of cases of HCC. Among patients with CHC, factors such as older age, male gender, severity of liver disease, metabolic syndrome and poor response to interferon therapy are established risk factors for hepatocarcinogenesis[1]. “Latent hepatitis B (LHB)”, defined as the presence of detectable hepatitis B core antibody (HBcAb) with undetectable hepatitis B surface antigen (HBsAg)(-) in serum and usually with detectable HBV DNA in hepatocytes, has not been studied as a risk factor for HCC in the United States[2]. Patients with previous exposure to hepatitis B virus but with no evidence of chronic infection are HBsAg(-) and HBcAb(+). This finding alone is now considered as unrecognized LHB[3]. In a large study, the majority of patients with LHB had detectable hepatitis B DNA (HBV DNA) in serum as well as in liver tissue[4]. Various other studies have also confirmed the same findings[5,6]. This led to the identification of a unique group of patients who are HBcAb(+) and at risk for latent hepatitis B.
Early studies from the 1990s suggested that patients with HCC in the absence of chronic hepatitis B and C had detectable covalent closed circular hepatitis B DNA (ccc DNA) in liver parenchyma although they were HBsAg(-) in serum. These patients were considered to have “occult hepatitis B”[7]. A single prospective study by Squadrito et al[8] revealed that among HBsAg(-) patients with CHC, patients with occult hepatitis B with ccc DNA in liver biopsy specimens were at a higher risk for the development of HCC. With the availability of highly sensitive real-time polymerase chain reaction (PCR) assays for the measurement of HBV DNA, tissue analysis for HBV DNA is largely unnecessary to make a diagnosis of latent hepatitis B[2]. Patients with cirrhosis from alcoholic and non-alcoholic fatty liver disease are also at a significantly higher risk for developing HCC when associated with LHB particularly in those who were HBcAb(+) but HBsAg(-)[9]. Injection drug users, patients on hemodialysis, patients with CHC and human immunodeficiency virus (HIV)-infected patients are at increased risk for LHB[10]. In patients with CHC, occult hepatitis B seems to be associated with rapid progression of liver disease[11]. Studies from areas with high prevalence of chronic hepatitis B have associated occult hepatitis B with HCC among patients with CHC[12,13]. This association is much stronger among CHC patients who are non-responders to currently available therapy[14]. Another study reported that although occult hepatitis B may not have a significant impact on response of CHC to interferon, it does increase the risk for HCC among non-responders but not among responders[15].
A large multicenter Japanese study concluded that CHC patients with LHB are at a significantly higher risk for developing HCC[16]. In the same study, interferon was less effective in preventing HCC in patients with LHB when compared to those without evidence of previous HBV exposure. This association was independent of the presence of HBV DNA in serum and therefore, LHB is clinically and prognostically more relevant than serum DNA status.
The above referenced studies establishing LHB as a risk factor for development of HCC are from countries with high endemicity for chronic hepatitis B infection. We studied this potential association among predominantly African American patients with CHC and cirrhosis in an area with low endemicity for chronic hepatitis B.
This retrospective study, included patients with CHC who were diagnosed with HCC between January 1999 and December 2008 at the Detroit Medical Center, Detroit, Michigan. The primary sites were the Wayne State University Gastroenterology clinic and the Department of Pathology at Harper University Hospital in Detroit. Patients with a diagnosis of HCC who were > 18 years old, hepatitis C Ab(+) and HBsAg(-) were included in our study. The diagnosis of HCC was made either by histopathology or by non-invasive criteria i.e., alpha feto-protein (AFP) > 200 ng/mL and a mass lesion in the liver with radiological features typical for HCC observed on two or more imaging modalities (European Association for the Study of the Liver, EASL criteria)[17]. The study group consisted of both inpatients and outpatients although the majority were outpatients.
A control comparison group consisted of patients with CHC who were HBsAg(-) without evidence of HCC. These patients were drawn from our institutional database of CHC patients. They were age, race and gender matched to the cases and were from the same time period. Patients with HIV and CHC coinfection were included in the study and were part of a subset analysis. The study was approved by the Institutional Review Board at Wayne State University and the Detroit Medical Center.
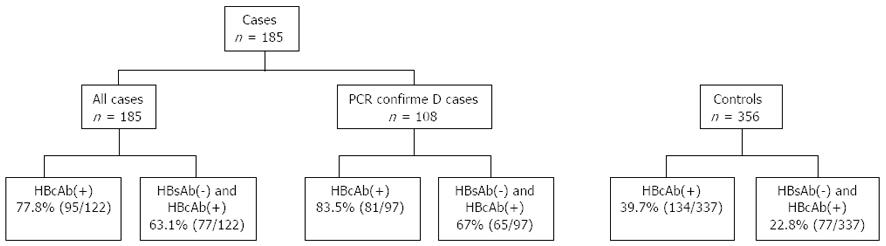
A total of 185 patients fulfilled the selection criteria and were evaluated for inclusion in the study. Of these, 108 had serum RNA confirmation of CHC by PCR, while CHC viremia was presumed in the remaining 77. A total of 356 matched (1:2) non-HCC controls with CHC were selected from our database. All controls had PCR confirmation of CHC, were HBsAg(-) and were selected from the same time period. Non HCC controls included 118 matched patients with cirrhosis diagnosed by histopathology or by clinical criteria. Since the majority of patients with CHC who develop HCC have underlying advanced liver disease[10], a selected sub-group of matched controls with cirrhosis and CHC was utilized in the analysis (Figure 1). In addition to demographic data, alcohol intake was assessed by chart review. Patients were classified into three categories based on alcohol consumption. Those who consumed 1 to 2 servings of liquor or wine a week or ≤ 6 beers (12 oz) a week were categorized as “mild drinkers”. Patients with alcohol consumption that exceeded this amount were considered “heavy drinkers”. The third group was designated as “non drinkers”. Lab values closest to the date of the diagnosis of HCC were collected. In controls, lab values at the time of their initial evaluation were recorded.
Data was analyzed using SPSS version 12. χ2 test was used to analyze nominal data while t test was used to compare means among groups. Univariate analysis was then performed after controlling for covariates in the final analysis. Among cases, baseline characteristics were compared in patients with PCR confirmation of CHC and in those without PCR confirmation (Table 1). Since these groups were identical in baseline characteristics, they were combined for subsequent analysis. Additionally, subset analysis of African-American patients, patients with PCR confirmation of CHC, and cirrhotic patients was performed.
| HCV Ab(+), HCV RNA(+) (n = 108) | HCV Ab(+) (n = 77) | P value | |
| Age (yr), mean ± SD | 60.88 ± 8.6 | 59.42 ± 7.4 | 0.88 |
| Males | 73.10% | 67.53% | 0.32 |
| African American | 75.90% | 72.70% | 0.22 |
| n = 70 | n = 52 | 0.20 | |
| BMI (kg/m2) | 29.56 ± 6.11 | 27.88 ± 5.2 | |
| HIV coinfection | 10.20% | 7.60% | 0.16 |
| Heavy alcohol use | 37.03% | 48.05% | 0.04 |
The mean age of patients with HCC was 60 years, and 71% were male (Table 2). More than seventy five percent of patients in each group were African-American. HCC was diagnosed by biopsy in 129 patients and by non-invasive (EASL) criteria in the remainder. Patients with HCC had a significantly higher body mass index (BMI), AFP, aspartate aminotransferase, alanine aminotransferase and a more prolonged prothrombin time (PT), but they had a lower albumin and platelet count. HIV-HCV coinfection was seen more commonly in patients with HCC (8.1%) than in controls (2.5%, P = 0.05). While mild alcohol consumption was not different in both groups, patients with HCC were more likely to be heavy drinkers (42% vs 27%; Table 2). Furthermore, HCV patients without HCC were more likely to be non-drinkers (32%) compared to patients with HCC (11%, P < 0.01; Table 2).
| CHC with HCC (n = 185) | CHC without HCC (n = 356) | P value | |
| Age (yr), mean ± SD | 60.3 ± 9.71 | 59.72 ± 9.2 | |
| Males | 70.80% | 71.10% | |
| Race AA | 74.60% | 78.70% | |
| CAU | 21.60% | 18.50% | |
| Other | 3.80% | 2.80% | |
| BMI (kg/m2) | n = 122 | n = 320 | |
| 28.8 ± 6.01 | 27.26 ± 5.9 | ||
| Albumin | n = 178 | n = 289 | |
| 2.67 ± 0.7 | 3.88 ± 0.6 | < 0.01 | |
| PT (s) | n = 171 | n = 242 | |
| 16.69 ± 8.6 | 11.57 ± 2.5 | < 0.01 | |
| AFP (ng/mL) | n = 163 | n = 284 | |
| 99035.1 ± 263605.5 | 21.12 ± 90.4 | ||
| Platelets | n = 172 | n = 337 | |
| 176.5 ± 127 | 202.98 ± 83.4 | 0.04 | |
| ALT (IU/L) | n = 166 | n = 345 | |
| 263.8 ± 518.2 | 78.19 ± 58.3 | < 0.01 | |
| AST (IU/L) | n = 160 | n = 324 | |
| 283.8 ± 63.5 | 75.2 ± 55.9 | < 0.01 | |
| HIV co-infection | 8.10% | 2.50% | 0.05 |
| Alcohol | |||
| Mild | 29.7% | 27.50% | NS |
| Heavy | 41.60% | 27% | 0.03 |
| Non drinkers | 10.80% | 31.70% | < 0.01 |
HBcAb was positive in 78% of patients with HCC but in only 40% of controls (P = 0.01). When hepatitis B surface antibody (HBsAb) status was determined, 63% of HCC cases were both HBsAb(-) and HBcAb(+) as compared to only 23% of controls (P < 0.01). When analysis was restricted to patients with cirrhosis, the prevalence of HBcAb was higher in cirrhotic controls at 42%, and the combination of HBsAb(-) and HBcAb(+) was also more prevalent when compared to total controls (27.6% vs 63.1%, P < 0.01). Despite this difference in prevalence of HBsAb and HBcAb among control groups, overall prevalence remained significantly higher in patients with HCC (63.1% vs 22.8%). Although statistical significance was not achieved, 100% of HIV-HCV coinfected patients with HCC (44.4%) were HBcAb(+) when compared to < 50% among coinfected controls.
Univariate analysis predicting HCC showed that HBcAb(+) status had an odds ratio of 1.9 (95%CI: 1.28-3.04, P = 0.02) where as a combination of HBsAb(-) and HBcAb(+) had a higher odds ratio of 3.24 (95%CI: 2.28-4.62, P < 0.01). In this analysis, BMI, albumin, PT, alcohol consumption and HIV coinfection were identified covariates. When regression analysis was performed after controlling for these covariates, these odds ratios were 1.84 (95%CI: 1.22-3.08, P = 0.01) and 2.98 (95%CI: 2.12-5.08, P < 0.01) respectively. Analysis of cirrhotic patients when controlled for covariates showed that HBcAb(+) had an odds ratio of 1.66 and a combination of HBsAb(-) and HBcAb(+) was at 2.10 (95%CI: 2.12-4.04, P < 0.01). Subset analysis of African-American patients with cirrhosis when controlled for covariates resulted in an odds ratio of 2.08 (95%CI: 1.42-3.60, P < 0.01) when HBcAb was positive and 2.58 (95%CI: 1.82-4.44, P < 0.01) when HBsAb was negative in addition to a positive HBcAb.
In this study, HCC and advanced liver disease, shown by higher transaminases, lower albumin and platelet count and prolonged prothrombin time were associated with increased frequency of HBcAb(+) among patients with CHC. Previous studies have also noted an increased association of HBcAb(+) with advanced liver disease and HCC[1,11,12,14,16,18]. However, these data, as well as a prospective study[16], originated from regions of relatively high prevalence for both chronic hepatitis B and HCC. Our study presents findings from an area of relatively low endemicity in a population comprised predominantly of urban African-Americans, yet the prevalence of HBcAb(+) was even higher in our study (74%) than previously reported[11,14]. Multivariate analysis restricted to this group of patients revealed a much stronger association of LHB with HCC (Table 3). Our study included a total of 418 African-American patients and is by far the largest analysis studying this association in a select group.
| OR (95%CI) | P value | |
| HCC in patients with CHC | ||
| HBcAb(+) | 1.90 (1.28-3.04) | 0.02 |
| HBsAb(-) and HBcAb(+) | 3.24 (2.28-4.62) | < 0.01 |
| HCC in cirrhotic patients with CHC | ||
| HBcAb(+) | 1.54 (1.18-2.54) | 0.02 |
| HBsAb(-) and HBcAb(+) | 2.14 (1.68-3.82) | 0.01 |
| HCC in patients with CHC when controlled for covariates1 | ||
| HBcAb(+) | 1.84 (1.22-3.08) | 0.01 |
| HBsAb(-) and HBcAb(+) | 2.98 (2.12-5.08) | < 0.01 |
| HCC in cirrhotic patients with CHC when controlled for covariates1 | ||
| HBcAb(+) | 1.66 (1.22-3.24) | 0.01 |
| HBsAb(-) and HBcAb(+) | 2.10 (1.72-4.04) | < 0.01 |
| HCC in cirrhotic African American patients with CHC when controlled for covariates1 | ||
| HBcAb(+) | 2.08 (1.42-3.6) | < 0.01 |
| HBsAb(-) and HBcAb(+) | 2.58 (1.82-4.44) | < 0.01 |
Certain potential limitations to our study need further discussion. A majority of patients with HCC (66%) were diagnosed by histopathology. This is largely due to inclusion of patients before the EASL non-invasive criteria for diagnosis of HCC were proposed[17]. One could argue that lack of RNA confirmation of CHC viremia in 40% of our cases detracts from our conclusions. However, patients with or without RNA confirmation of HCV viremia had similar baseline characteristics and no other etiology for their liver disease, and were therefore appropriately grouped together. Alcohol consumption is a well established risk factor for HCC among patients with chronic liver disease. In the present study, details pertaining to alcohol consumption were not available in some patients despite extensive review of both inpatient and outpatient medical records. Every effort was made to classify patients based on alcohol consumption when information was available. Controls, who were drawn from a prospectively maintained institutional database were more likely to have reliable information regarding alcohol intake. Nevertheless, in concurrence with previous literature, our results suggest that BMI and alcohol consumption play an important role in the progression to HCC in cirrhotics with CHC.
Although the majority of patients with HCC were drawn from an outpatient setting, some were hospitalized and likely sicker. Controls were selected from a predominantly outpatient database. This may account for the significantly higher AFP and transaminases in the CHC patients with HCC. Although PCR measurement of serum HBV DNA assessment was not accomplished in our patients, we do not consider this as a significant limitation. In previous studies, HBcAb had a stronger association with HCC than serum HBV DNA among patients with CHC[5,6]. Latent hepatitis B, defined as a previous exposure to hepatitis B [HBcAb(+) and HBsAg(-)] is a clinically more relevant tool for predicting risk for HCC than is the assessment of HBV DNA in serum or liver tissue.
Another limitation to the study is that the frequency of smoking, injection drug use and diabetes was not analyzed. Patients with HIV-HCV co-infection have rapid progression of liver disease and development of HCC. Liver disease is the leading cause of mortality in these patients[19-21]. Of 20 patients with HIV-HCV coinfection, 11 patients had HCC and all 11 were HBcAb(+) compared to 40% among coinfected controls. Although this difference did not achieve statistical significance, due to low numbers, this raises the intriguing observation that HIV-HCV coinfected patients with HCC may have an increased frequency of LHB.
Our study is consistent with previous studies from areas with high prevalence of hepatitis B that suggests that LHB increases the risk for HCC. Furthermore, patients sero-negative for HBsAb are at even higher risk, which may suggest longer time since acquisition of HBV. Our data also suggest that African-Americans have a greater risk of HCC when associated with LHB. There is additional data from our institution (preliminary) to support the observation that patients with CHC and LHB are more likely to have advanced liver disease and respond poorly to Interferon-based therapies. The current study takes this concept further by associating LHB with HCC.
In conclusion, LHB occurs at a significantly increased frequency in patients with CHC and HCC than in patients with CHC without HCC. This association is even stronger in African Americans. It is important to recognize this risk during surveillance for HCC in these patients.
Patients with chronic hepatitis C (CHC) and cirrhosis have an increased risk of developing hepatocellular carcinoma (HCC). Several risk factors for this progression have so far been identified. The authors studied the potential association between HCC in patients with CHC, cirrhosis and latent hepatitis B (LHB) infection, defined as the absence of detectable serum hepatitis B surface antigen (HBsAg) and the presence of hepatitis B core antibody (HBcAb).
LHB has recently received significant attention among researchers and clinicians managing chronic liver disease. It is defined as a combination of HBsAg(-) and HBcAb(+). The potential association of LHB with HCC among patients with chronic CHC has been studied and reported in this manuscript.
Interestingly, subset analysis among human immunodeficiency virus-CHC co-infected patients showed a 100% association of HCC with LHB suggesting much higher association in this group. This identifies a unique group of CHC patients at much higher risk for development of HCC.
Risk factors for HCC in patients with chronic hepatitis C are only partially understood. Here the authors showed that there is a clear association between latent hepatitis B infection and HCC development in an area with low endemicity for chronic hepatitis B.
P- Reviewers: Gonzalez-Aseguinolaza G S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN
| 1. | Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35-S50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1691] [Cited by in F6Publishing: 1665] [Article Influence: 83.3] [Reference Citation Analysis (2)] |
| 2. | van Hemert FJ, Zaaijer HL, Berkhout B, Lukashov VV. Occult hepatitis B infection: an evolutionary scenario. Virol J. 2008;5:146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Vitale F, Tramuto F, Orlando A, Vizzini G, Meli V, Cerame G, Mazzucco W, Virdone R, Palazzo U, Villafrate MR. Can the serological status of anti-HBc alone be considered a sentinel marker for detection of occult HBV infection? J Med Virol. 2008;80:577-582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Toyoda H, Kumada T, Kiriyama S, Sone Y, Tanikawa M, Hisanaga Y, Kanamori A. Prevalence of low-level hepatitis B viremia in patients with HBV surface antigen-negative hepatocellular carcinoma with and without hepatitis C virus infection in Japan: analysis by COBAS TaqMan real-time PCR. Intervirology. 2007;50:241-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Adachi S, Shibuya A, Miura Y, Takeuchi A, Nakazawa T, Saigenji K. Impact of occult hepatitis B virus infection and prior hepatitis B virus infection on development of hepatocellular carcinoma in patients with liver cirrhosis due to hepatitis C virus. Scand J Gastroenterol. 2008;43:849-856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Chemin I, Trépo C. Clinical impact of occult HBV infections. J Clin Virol. 2005;34 Suppl 1:S15-S21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 7. | Tsega E, Nordenfelt E, Hansson BG, Mengesha B, Lindberg J. Chronic liver disease in Ethiopia: a clinical study with emphasis on identifying common causes. Ethiop Med J. 1992;30:1-33. [PubMed] [Cited in This Article: ] |
| 8. | Squadrito G, Pollicino T, Cacciola I, Caccamo G, Villari D, La Masa T, Restuccia T, Cucinotta E, Scisca C, Magazzu D. Occult hepatitis B virus infection is associated with the development of hepatocellular carcinoma in chronic hepatitis C patients. Cancer. 2006;106:1326-1330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Kim MJ, Kwon OS, Chung NS, Lee SY, Jung HS, Park DK, Ku YS, Kim YK, Kim YS, Kim JH. [The significance of anti-HBc and occult hepatitis B virus infection in the occurrence of hepatocellular carcinoma in patients with HBsAg and anti-HCV negative alcoholic cirrhosis]. Korean J Hepatol. 2008;14:67-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Raimondo G, Pollicino T, Romanò L, Zanetti AR. A 2010 update on occult hepatitis B infection. Pathol Biol (Paris). 2010;58:254-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Obika M, Shinji T, Fujioka S, Terada R, Ryuko H, Lwin AA, Shiraha H, Koide N. Hepatitis B virus DNA in liver tissue and risk for hepatocarcinogenesis in patients with hepatitis C virus-related chronic liver disease. A prospective study. Intervirology. 2008;51:59-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Matsuoka S, Nirei K, Tamura A, Nakamura H, Matsumura H, Oshiro S, Arakawa Y, Yamagami H, Tanaka N, Moriyama M. Influence of occult hepatitis B virus coinfection on the incidence of fibrosis and hepatocellular carcinoma in chronic hepatitis C. Intervirology. 2008;51:352-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Kannangai R, Molmenti E, Arrazola L, Klein A, Choti M, Thomas DL, Torbenson M. Occult hepatitis B viral DNA in liver carcinomas from a region with a low prevalence of chronic hepatitis B infection. J Viral Hepat. 2004;11:297-301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Miura Y, Shibuya A, Adachi S, Takeuchi A, Tsuchihashi T, Nakazawa T, Saigenji K. Occult hepatitis B virus infection as a risk factor for hepatocellular carcinoma in patients with chronic hepatitis C in whom viral eradication fails. Hepatol Res. 2008;38:546-556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Hasegawa I, Orito E, Tanaka Y, Hirashima N, Sakakibara K, Sakurai M, Suzuki S, Sugauchi F, Ohno T, Ueda R. Impact of occult hepatitis B virus infection on efficacy and prognosis of interferon-alpha therapy for patients with chronic hepatitis C. Liver Int. 2005;25:247-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Ikeda K, Marusawa H, Osaki Y, Nakamura T, Kitajima N, Yamashita Y, Kudo M, Sato T, Chiba T. Antibody to hepatitis B core antigen and risk for hepatitis C-related hepatocellular carcinoma: a prospective study. Ann Intern Med. 2007;146:649-656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 106] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 17. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3252] [Cited by in F6Publishing: 3170] [Article Influence: 137.8] [Reference Citation Analysis (0)] |
| 18. | Tamori A, Hayashi T, Shinzaki M, Kobayashi S, Iwai S, Enomoto M, Morikawa H, Sakaguchi H, Shiomi S, Takemura S. Frequent detection of hepatitis B virus DNA in hepatocellular carcinoma of patients with sustained virologic response for hepatitis C virus. J Med Virol. 2009;81:1009-1014. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Zhang X, Frank AC, Gille CM, Daucher M, Kabat J, Becker S, Lempicki RA, Cortez KJ, Polis MA, Subramanian GM. Altered regulation of extrinsic apoptosis pathway in HCV-infected HCC cells enhances susceptibility to mapatumumab-induced apoptosis. Hepatol Res. 2009;39:1178-1189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | MacDonald DC, Nelson M, Bower M, Powles T. Hepatocellular carcinoma, human immunodeficiency virus and viral hepatitis in the HAART era. World J Gastroenterol. 2008;14:1657-1663. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 42] [Cited by in F6Publishing: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Gomaa AI, Khan SA, Toledano MB, Waked I, Taylor-Robinson SD. Hepatocellular carcinoma: epidemiology, risk factors and pathogenesis. World J Gastroenterol. 2008;14:4300-4308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 456] [Cited by in F6Publishing: 470] [Article Influence: 29.4] [Reference Citation Analysis (1)] |