Published online Mar 14, 2014. doi: 10.3748/wjg.v20.i10.2542
Revised: January 13, 2014
Accepted: January 20, 2014
Published online: March 14, 2014
Viable and non-viable pathological bacterial translocation promote a self-perpetuating circle of dysfunctional immune activation and systemic inflammation facilitating infections and organ failure in advanced cirrhosis. Bacterial infections and sepsis are now recognized as a distinct stage in the natural progression of chronic liver disease as they accelerate organ failure and contribute to the high mortality observed in decompensated cirrhosis. The increasing knowledge of structural, immunological and hemodynamic pathophysiology in advanced cirrhosis has not yet translated into significantly improved outcomes of bacterial infections over the last decades. Therefore, early identification of patients at the highest risk for developing infections and infection-related complications is required to tailor the currently available measures of surveillance, prophylaxis and therapy to the patients in need in order to improve the detrimental outcome of bacterial infections in cirrhosis.
Core tip: We will discuss susceptibility and impact of specific bacterial infections in cirrhosis, their natural course and the identification of risk factors for organ failure and death in order to help clinicians identifying patients at the highest risk that may benefit from intensified surveillance, prophylaxis and therapy.
- Citation: Bruns T, Zimmermann HW, Stallmach A. Risk factors and outcome of bacterial infections in cirrhosis. World J Gastroenterol 2014; 20(10): 2542-2554
- URL: https://www.wjgnet.com/1007-9327/full/v20/i10/2542.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i10.2542
Bacterial infections are diagnosed in 25% to 47% of hospitalized patients with cirrhosis[1-4] and represent the most important precipitating event for acute decompensation[5]. Infections are increasingly recognized as a major trigger of systemic inflammation and organ failure in advanced cirrhosis leading to a four-fold increased mortality[6]. Despite recent advances in understanding the underlying pathogenic mechanisms of bacterial infections in cirrhosis[7-9], the progression of infections to multiple organ failure and septic shock is still associated with a short-term mortality of patients exceeding 75%[5,10]. In this review we will discuss susceptibility and impact of specific bacterial infections in cirrhosis, their natural course and the identification of risk factors for organ failure and death in order to help clinicians identifying patients at the highest risk that may benefit from intensified surveillance, prophylaxis and therapy.
Decompensated liver cirrhosis predisposes to delayed intestinal transit time, increased intestinal permeability and disturbed expression of intestinal antimicrobial peptides thereby facilitating the translocation of bacteria and bacterial products from the gastro-intestinal lumen through the lamina propria into mesenteric lymph nodes (MLN), ascitic fluid and the systemic circulation[11-13]. Whereas bacterial translocation (BT) can be controlled by the immune system of healthy individuals, pathological BT in cirrhotic patients is accompanied by a cirrhosis-associated immune dysfunction (CAID) supporting systemic inflammation as a consequence of non-viable BT as well as bacterial infections due to viable BT[8,9,14].
Among the intestinal microbiota, Gram-negative enteric bacilli more easily translocate to MLN than Gram-positive bacteria and obligate anaerobes[15]. Although the capability to translocate across the gastrointestinal barrier varies between different Escherichia coli (E. coli) strains[15], no single virulence factor responsible for BT in cirrhosis could be identified yet[16]. Furthermore, catabolic and inflammatory stress to the epithelial barrier can increase BT of various coliform bacterial strains[17-19]. With classical bacterial culture techniques, enteric organisms can be detected in MLN from up to 30% of patients with Child-Pugh C cirrhosis compared to 9%-15% of non-cirrhotic patients undergoing laparotomy[20,21].
This increased translocation of intestinal bacteria and bacterial products in cirrhosis has been attributed to both clinically significant portal hypertension (PH) and advanced liver failure. The role of PH is supported by the observations that the development of ascites is a prerequisite for significant translocation of viable bacterial to the MLN in the CCl4 model[22], and that the use of non-selective beta blockers (NSBB) reduces the translocation of viable BT to MLN in cirrhotic rats[23] and prevents infectious complications in humans with decompensated cirrhosis[24]. Furthermore, it has been shown that non-viable BT and immune activation also occur in the pre-ascitic stage[25] and correlate with the increase in portal pressure[26]. On the other hand, pathological BT to MLN also occurs in models of experimental acute liver failure in the absence of PH[27] but not in chronic PH in the absence of cirrhosis[28]. Moreover, NSBB are also effective in preventing spontaneous bacterial peritonitis (SBP) in patients showing no hemodynamic response to treatment[24] underlining that PH may not be the principal driver of viable pathological BT in advanced cirrhosis.
Therefore, alternative mechanisms must be involved in triggering pathological BT in liver failure and advanced fibrosis. There is evidence that quantitative and qualitative changes in the intestinal microflora might contribute to this phenomenon. Small intestinal bacterial overgrowth (SIBO) frequently occurs in decompensated cirrhosis and correlates with systemic endotoxemia[29] and the presence of bacterial DNA fragments (bactDNA) in the peripheral blood[30]. Small intestinal dysmotility[31] and altered bile composition in cirrhosis[32] contribute to SIBO and drive endotoxemia and infectious complications[33]. Once a systemic or local immune response has been established, proinflammatory cytokines like tumor necrosis factor (TNF) and interleukin (IL)-6 disrupt the intestinal integrity of the intestinal barrier by altering the structure of the apical junctional complex[34,35] thereby further increasing intestinal permeability (IP) generally observed in cirrhosis[12,26]. Although some cohort studies show an association of IP with septic complications[12,36,37] the only prospective study available did not find an association of IP with infectious complications[38] questioning whether increased IP is really the cause - or rather the consequence - of pathological BT and intestinal inflammation in cirrhosis.
Whether the composition of the intestinal microbiome, modulates BT and infectious complications in cirrhosis has not been thoroughly investigated yet. There is some evidence that the fecal microbiome in cirrhosis is less diverse and shows an abundance of Enterobacteriaceae, Streptococcus spp. and Enterococcus faecalis[39-41], which mirrors the microbial pattern observed in SBP[1,42,43]. The microbiome associated with the colonic mucosa might be of particular interest because the enrichment of Enterococcus, Proteus, Clostridium, and Burkholderia and the loss of non-pathogenic commensal bacteria in cirrhosis was associated with more severe liver disease, increased inflammation and endothelial activation[44,45]. The recent findings that the gut microbiome is modulated by the enterocyte inflammasome[46,47] which leads to increased nonviable BT and augmented hepatic inflammation[48], suggest a critical interaction of gut mucosa, intestinal dysbiosis and immune activation in cirrhosis.
To control viable BT in cirrhosis, immune control takes place at the epithelial barrier including the mucus layer, at gut-associated lymphatic tissue and the MLN and at the systemic level after bacteria or bacterial products have passed MLN via the portal vein or the thoracic duct[49]. In the context of pathological BT, organ-resident macrophages recognize pathogen-associated molecular patterns, such as lipopolysaccharide (LPS), muramyl dipeptide, bacterial lipoproteins and bactDNA, via extra- and intracellular Toll-like receptors (TLR) (e.g., TLR2, TLR4, TLR9) or intracellular NOD-like receptors (e.g., NOD2). Ligation of these conserved pattern recognition receptors (PRR) leads to classical macrophage activation, the secretion of pro-inflammatory cytokines (TNF, IL-1β, IL-12) and polarization to pro-inflammatory macrophages supported by interferon (IFN)-γ released from activated T cells[50]. In healthy individuals, binding of LPS to TLR4 increases expression of scavenger receptors, MHC class II and co-stimulatory molecules to accelerate phagocytosis and subsequent presentation of bacteria and bacterial products[51]. Several abnormalities of the innate immune system in cirrhosis contribute to non-control of BT, subsequent inflammation and the increased incidence of infections. These abnormalities have been described as CAID syndrome (CAIDS) stressing the role of insufficient processing of bacteria and bacterial products by phagocytes[8]. Importantly, CAIDS is often not associated with inflammatory anergy but with marked immune activation and a high degree of systemic inflammation, which correlates with the severity of liver disease and predicts survival in these patients[52].
The liver itself plays a major role in phagocytizing pathogens and scavenging macromolecules because organ-resident Kupffer cells and liver sinusoidal endothelial cells account for 80% of the human reticuloendothelial system (RES)[53]. The overwhelming sinusoidal influx of bacterial components in liver cirrhosis owing to mechanisms described above, switches the physiological immune-modulatory state of the local hepatic immune system elicited by Kupffer cells to a state in which the production of pro-inflammatory cytokines predominates accumulating in further demise of hepatic parenchyma cells and deterioration of liver architecture[54]. Due to portosystemic shunting, decreased opsonization and dysfunctional phagocytic activity, the hepatic clearance function is markedly reduced in cirrhosis and correlates with the severity of liver disease, bacteremia and survival[55-58]. In parallel, non-classical pro-inflammatory subsets of monocytes expand and contribute to an inflammatory state in cirrhosis[52,59-61]. This state of dysfunctional activation has been observed in neutrophils as well. Neutrophils from patients with cirrhosis are potent producers of pro-inflammatory cytokines and reactive oxygen species but also display decreased chemotaxis and inefficient phagocytosis[62-67]. Dysfunctional phagocytosis and increased activation can be transmitted to neutrophils from healthy individuals with plasma from cirrhotic patients suggesting repetitive priming mediated by soluble factors[66,67], which may result in the cellular depletion of antioxidants and increased oxidative damage[65,68].
Whereas phenotypic and functional abnormalities have been well described for the aspects of the innate immunity in cirrhosis, the state of the adaptive immunity is less well defined. Phenotypically, patients with cirrhosis display decreased numbers of total and naïve T cells due to defective thymic generation and splenic sequestration in parallel with increased activation, proliferation and turnover of memory T cells presumably due to repetitive antigen stimulation[69,70]. Data on the functional consequences of these findings are sparse. Although clinical studies show attenuated immune responses after vaccination in cirrhotic patients[71-73], in vitro analyses could not identify specific T cell defects[74,75] but suggest soluble factors such as IL-10 as modulators of inconsistent T cell responses in cirrhosis[75-77].
Whether an observed depletion and functional alteration of innate natural killer (NK) cells[78,79] and memory B cells[79,80] in cirrhosis contribute to pathological BT, inflammation and bacterial infections in cirrhosis has not been demonstrated.
In response to bacterial infection, patients with cirrhosis have a pronounced inflammatory response with elevated systemic concentrations of the pyrogenic cytokines IL-6 and TNF superimposing on high basal levels resulting in fever, leukocytosis and acute phase reaction[81]. As a consequence, systemic inflammatory response syndrome (SIRS) can be found in up to 67% of cirrhotic patients with bacterial infections compared to 37% of patients without, making sepsis a common complication of advanced cirrhosis[82-84].
Even in the absence of overt bacterial infection, the occurrence of systemic inflammation as indicated by SIRS is of prognostic relevance in patients with cirrhosis[83,85]. There is some evidence that beyond its role as an indicator of occult bacterial infection and advanced organ failure, systemic inflammation might even aggravate portal hypertension, renal failure and hepatic encephalopathy, thereby contributing to organ failure and death in cirrhotic patients by immunological, metabolic and hemodynamic mechanisms[83]. Due to hyperdynamic circulation with tachycardia or tense ascites leading to hyperventilation in patients with decompensated cirrhosis, SIRS criteria are most certainly less specific for inflammatory and infectious complications than in the general population and need to be interpreted with caution.
The magnitude of the acute phase response as indicated by C-reactive protein (CRP) or IL-6 levels might be more reliable tools than clinical SIRS criteria to estimate the risk for adverse outcome in cirrhotic patients. In patients with cirrhosis and SIRS concentrations of serum IL-6 correlate with organ failure, monocyte and neutrophil activation[86] but are also closely associated with the degree of portal hypertension[26] and hyperdynamic circulation[87]. The same observations have been made for CRP, where persistent elevation in the absence of infection indicates increased short-term mortality[88]. Discriminating infections from sterile systemic inflammatory response is only possible using CRP cut-offs are as high as 56 mg/L in advanced cirrhosis[89-91]. The large multicenter CANONIC study[5] identified elevated CRP levels and increasing WBC count as hallmark features to distinguish acute decompensation of cirrhosis from acute-on-chronic liver failure (ACLF) even when patients with bacterial infections were excluded. Patients who presented with ACLF had mean CRP levels of 33 mg/L (compared to 21 mg/L in patients without ACLF) and mean WBC counts of 9.4 Gpt/L (compared to 6.6 Gpt/L) at inclusion. Importantly, the probability of death in ACLF increased with the rise in WBC count[5] making WBC count an attractive, easily available linear variable to assess mortality risk associated with systemic inflammation in cirrhosis.
Since none of these established indicators of inflammation allow a precise distinction between sterile SIRS and bacterial infection, treating physicians must be aware of risk factors of bacterial infections to assess the likelihood of bacterial infections in patients with advanced cirrhosis presenting with signs of systemic inflammation. We have recently evaluated novel biomarkers that more precisely indicate specific infections and/or immune activation in advanced cirrhosis, such as mid-regional pro-adrenomedullin and soluble urokinase plasminogen activator receptor[52,90], which will expand the diagnostic armamentarium for predicting outcome independent of the presence of confounding systemic inflammation.
SBP and spontaneous bacteremia are the most thoroughly investigated complications in patients with cirrhosis because they occur frequently with a prevalence of 15%-20% in hospitalized patients[65,92] and cause high mortality after one month (33%-42%) and after one year (49%-66%)[6]. Supporting the concept of pathological BT of Enterobacteriaceae in cirrhosis, Gram-negative bacteria have been isolated from more than 70% of culture-positive bacterial infections in the past[1,42]. More recently however, bacterial infections caused by Gram-positive cocci dramatically increased in tertiary centers and now represent 60% of nosocomial culture-positive infections including the archetypal infectious complications spontaneous bacteremia and SBP[3,37,43]. This shift has been attributed to the use of antibiotics leading to intestinal dysbiosis favoring Gram-positive BT, but also to increased invasive procedures and associated episodes of Gram-positive bacteremia with secondary organ spread[3,43,93].
As a preventive measure, primary antibiotic prophylaxis is currently recommended in cirrhotic patients with gastrointestinal (GI) bleeding because bleeding facilitates pathological BT and infections on the one hand, and infections are associated with a higher rate of recurrent bleeding on the other hand[94]. Antibiotic prophylaxis over seven days in cirrhotic patients in with gastrointestinal bleeding is the current standard of care[94,95]. In nine controlled trials including 987 patients the pooled incidence of bacteremia following GI bleeding was 15% without antibiotic prophylaxis and 3% with antibiotic prophylaxis, indicating a risk reduction of 75% with antibiotic prophylaxis[96]. Results favoring antibiotic prophylaxis were also obtained when the outcomes SBP, pneumonia, urinary tract infection, overall bacterial infections and mortality were analyzed separately[96,97]. Although translocation of gut microbial flora into the bloodstream is likely to occur during endoscopy because of mucosal trauma related to the endoscopy procedure itself[98], the risk of bacteremia associated with endoscopic procedures is poorly investigated in non-bleeding cirrhotic patients. One study reported an incidence of 10% (6/58) bacteremia by possible contaminants (Gram-positive skin flora) after colonoscopy, but all cirrhotic patients with bacteremia remained asymptomatic[99]. Thus, international guidelines do not recommend routine antibiotic prophylaxis for cirrhotic patients undergoing colonoscopy[100] because of the lack of data in this setting, and it is recommended that clinicians should decide “on an individual case basis[101].
Patients with low ascitic fluid (AF) protein, elevated serum bilirubin levels and/or low platelets are at the highest risk of developing community-acquired SBP[102]. SBP has a recurrence rate of 70% within the first year after the first episode[103] making secondary prophylaxis with 400 mg norfloxacin daily a level A recommendation in current guidelines[95,104]. Because antibiotic prophylaxis in high-risk cirrhotic patients with low AF protein and with severe liver failure or with renal failure improves incidence of infections and short-term survival[105,106], primary antibiotic prophylaxis should be considered in these patients[95]. However, in the light of increasing antimicrobial resistance in cirrhotic patients and of decreased efficacy of antibiotic prophylaxis over time adherence to these guidelines is poor among practitioners resulting in cases of SBP that could have been prevented[107,108]. Genetic association studies may help identifying patients at the highest need for antibiotic prophylaxis. Frequent polymorphisms in genes involved in the innate antimicrobial defense, such as NOD2, TLR2 and MCP-1, have been reported to confer a three- to four-fold increased life-time risk to develop SBP[109-113]. NOD2 gene variants are of particular interest because they regulate intestinal immunity via expression of antimicrobial peptides and intracellular bacterial killing[114,115] thereby linking Paneth cell defense with pathological BT in cirrhosis[13,116]. In a recent study including four patients with NOD2 variants, five patients with TLR2 variants and 29 wild type controls, the presence of any of these variants was associated with markers of impaired intestinal permeability and higher systemic inflammation[26]. However, subsequent studies including a higher number of patients are required to answer the question on the influence of these gene variants on intestinal integrity and pathological BT as a driver of SBP per se. Whether genotype-based risk-stratification for antibiotic prophylaxis is a feasible approach to reduce infectious complications, at least in populations of European descent where these polymorphisms are frequently found, currently remains an open question but will hopefully be answered soon.
Whereas host factors contributing to SBP, such as genetic background or severity of liver disease[117], cannot be easily modified, environmental factors such as alcohol use in less advanced cirrhosis[118] or prescribed concurrent medication are more susceptible to therapeutic intervention. In contrast to the protective effects of NSBB that have already been discussed above[24], the use of proton pump inhibitors (PPI) is associated with a three-fold increased risk for developing SBP in hospitalized patients according to a recent meta-analysis of observational studies[119] suggesting that the indication for PPI treatment in liver cirrhosis has to be very carefully weighed against the potential risks[120].
Studies on specific risk factors for extraperitoneal bacterial infections are scarce. Urinary tract infections (UTI) are very common and represent 20% up to 40% of bacterial infections in prospective studies of hospitalized patients with cirrhosis[2,121]. The majority of identified uropathogens are Gram-negative bacteria with E. coli as the most commonly isolated microorganism still, but multiresistant bacteria are increasingly observed, especially in nosocomial infection in southern Europe[4,84]. In a large retrospective cohort of almost 400 cirrhotic patients we could recently show that predominantly women develop UTI and that the risk of infection increases stronger with age than with the severity of underlying liver disease in contrast to other bacterial infections in cirrhosis[84]. Notably, in this and other series UTI was frequently accompanied by SIRS (42%-65% of cases)[83], and up to one third of patients with UTI presents with or develops concomitant bacterial infections[84,122] making UTI a bacterial infection that should not be underestimated in the cirrhotic patient. Although strategies for UTI prevention have not been investigated in the cirrhotic population specifically, evaluation and reduction of unnecessary urinary catheter use applies as in patients without liver disease.
Among the extraperitoneal manifestation of bacterial infections, lower respiratory tract infections and pneumonia are associated with the highest risk of mortality in cirrhosis[6,123]. Animal models suggest that low serum complement levels in rats with decompensated cirrhosis increase susceptibility to pulmonary infection with Streptococcus pneumoniae (S. pneumoniae)[124]. In agreement with these findings, patients with cirrhosis are more susceptible to pneumonia, present with a more complicated course of disease and develop more often bacteremia than non-cirrhotic patients[125,126]. Since S. pneumoniae represents the most common etiologic agent isolated in community-acquired pneumonia, pneumococcal vaccination with the 23-valent vaccine is recommended as a preventive measure despite reduced immunological responses and accelerated antibody decline in patients with cirrhosis[127,128].
Skin and soft tissue infections in cirrhosis can be caused by Gram-positive bacteria entering the edematous skin as well as by translocated Gram-negative bacteria. They are often recurrent, complicated by renal failure and lead to a mortality of approximately 20%[122,129,130]. Walking barefoot has been identified as a risk factor for cellulitis in patients with decompensated cirrhosis and edema by an Indian study and should be avoided[130].
Patients with cirrhosis have an increased risk of infectious endocarditis compared to the general population[131]. Infectious endocarditis in cirrhosis is predominantly hospital-acquired with Staphylococcus aureus (S. aureus), β-hemolytic streptococci and Enterococcus spp. as most frequent infectious agents, predominantly involves the aortic valve, and is associated with 60% renal failure and 50% mortality[132].
Bacterial Meningitis is a rare complication in cirrhosis (< 1%) often with atypical presentation and Gram-negative pathogen spectrum associated with bacteremia in the majority of cases and a mortality exceeding 40%[133,134].
The meta-analysis by Arvaniti et al[6] comprising data from almost 12000 patients concluded a four-fold increased mortality owing to bacterial infections in cirrhotic patients - with respiratory tract infections, SBP and bacteremia as major contributors. We and others[84,123] could recently demonstrate that urinary tract infections in decompensated cirrhosis are less detrimental as these three major infections, but are still associated with a significantly increased risk of mortality, especially in the presence of concomitant SIRS. Fueled by excessive inflammation in cirrhosis, organ failure frequently occurs in the absence of septic shock, making bacterial infections a distinct stage in the natural history of cirrhosis progression[6] and the major precipitating event for the development of decompensation and ACLF[5]. There are three major factors that determine mortality of bacterial infection in cirrhosis irrespective of etiology: severity of underlying liver disease, concomitant renal failure and non-resolution of infection due to antimicrobial resistance.
Among the 18 variables that were significantly associated with death after bacterial infections in three or more studies evaluated by Arvaniti et al[6] five were related to liver function or severity of cirrhosis (Child-Pugh score, prothrombin time/INR, bilirubin, albumin, and MELD score) and three related to cirrhosis-associated complications (hepatic encephalopathy, gastrointestinal bleeding, and hepatocellular carcinoma). Therefore, using composite scores like Child-Pugh or MELD score to estimate prognosis is tempting, since they reflect severity of underlying disease as well as infection-triggered organ failure and are well-established scores to predict short-term and long-term mortality under a variety of conditions in cirrhosis. However, the independent contribution of systemic inflammation and bacterial infection to the deterioration of liver function needs to be considered: In the absence of SIRS a MELD score of > 18 is associated with 12% in-hospital mortality - in the presence of SIRS the mortality increases to 43%[83]; in the absence of leukocytosis a MELD score of ≥ 22 is associated with 30% one-month mortality after SBP - in the presence of leukocytosis the mortality increases to 52%[135].
Clinically significant portal hypertension and pathological BT in cirrhosis contribute to mesenteric vasodilation and splanchnic pooling, which results in reduced central blood volume with compensatory but insufficient hyperdynamic circulation, activation of neurohumoral vasoconstrictor systems, and sodium retention in the kidneys[136,137]. The markedly reduced renal blood flow in decompensated cirrhosis[138] renders the kidney susceptible to infection-triggered renal failure and hepatorenal syndrome (HRS)[139]. Renal failure occurs in approximately one third of patients with cirrhosis and bacterial infections, especially after UTI, SBP, and skin infections, and is non-reversible or progressive in 25% to 33% of cases[121,140-142]. Compared to parenchymal nephropathy in cirrhosis, hypovolemia-related renal failure (OR = 2.32) and infection-related renal failure (OR = 2.61) are both associated with a two-fold increased risk of mortality within 90 d[143]. Given the large impact of progressive renal failure on mortality in patients with cirrhosis, current strategies implement the criteria of the Acute Kidney Injury Network (AKIN) [creatinine increase ≥ 0.3 mg/dL (27 μmol/L) within 48 h or 50% creatinine increase from a stable baseline] for all acute deteriorations of renal function in cirrhosis under the term “hepatorenal dysfunction”[144]. Patients with cirrhosis and infections that develop renal failure according to the AKIN criteria have lower mean arterial pressure, higher bilirubin, lower albumin, higher WBC count, higher platelet count, and lower serum sodium in univariate analyses[142]. Mortality increases with occurrence of AKI (34% vs 7% 90-d mortality), with severity of AKI (2%, 7%, and 21% in-hospital mortality in stages 1, 2, and 3, respectively) and with progression of renal failure (15% 90-d mortality after complete recovery, 40% after partial renal recovery, and 80% in patients without renal recovery or progression)[142,144].
Notably, one third of patients with AKI due to bacterial infections develop a second infection, which aggravates renal failure and reduces survival presumably by superimposing inflammation and exhaustion of innate defense mechanisms[65,122,142]. Therefore, prevention of secondary infection in cirrhotic patients with infection-induced renal failure seems a legitimate approach to reduce mortality due to infections. In addition, intravenous albumin substitution has proven effective to reduce renal failure, in-hospital mortality, and 90-d mortality in patients with SBP[145] and improves serum creatinine but not survival in cirrhotic patients with infections other than SBP[146]. Additional approaches using anti-inflammatory strategies with pentoxifylline have been proven effective in improving renal function during alcoholic hepatitis[147] and might have prophylactic potential for preventing HRS in decompensated cirrhosis in selected patients[148,149].
Failure of first-line empiric antibiotic therapy for bacterial infections in cirrhosis is associated with increased mortality, as it has been shown for SBP[43,150-152]. Since escalation of therapy after unsuccessful empiric therapy still carries an increased risk of mortality[151], knowing the patient’s previous contact to the health-care system, used antibiotics, co-morbidities, and the local antibiotic susceptibility spectrum[153] is crucial for successful implementation of effective antimicrobial strategies. Whereas the frequency of multiresistant bacteria isolated from culture-positive bacterial infections in cirrhosis remained small for community-acquired infections, it has reached 40% in several tertiary hospitals around the globe resulting in a less than 50% resolution rate for nosocomial infections in cirrhosis[4,150,154]. Both, extended spectrum beta-lactamases (ESBL)-producing Enterobacteriaceae (predominantly in Southern Europe) and Enterococcus spp. (predominantly in Central Europe) are of increasing relevance in nosocomial SBP in Europe because both are associated with resistance to third-generation cephalosporins (TGC) which are currently used as the first line empiric therapy for community-acquired and healthcare-acquired SBP[43,150,151,154]. As a consequence, nosocomial acquisition has emerged as an important determinant for not surviving SBP, doubling the risk of mortality within 30 d[150].
Since colonization with ESBL-producing Enterobacteriaceae seems not to correlate with the development of TGC-resistant infections in cirrhosis[155], stool screening has currently no role for identifying hospitalized patients at risk. Therefore, clinicians are advised to carefully check the following risk factors for TGC-resistant and multiresistant bacteria in infected cirrhotic patients before initiating empiric antibiotic therapy: nosocomial acquisition, previous antibiotic treatment (norfloxacin prophylaxis, β-lactam use within the last three months), previous infection by multiresistant bacteria, diabetes mellitus, and upper GI bleeding[4,43,154]. Until now, no randomized controlled trials have evaluated the efficacy of empiric therapy with carbapenems, tigecycline, or addition of anti-enterococcal antibiotics (ampicillin, vancomycin) in the setting of hospital-acquired infections in cirrhosis. Therefore, clinicians are advised to implement the five main aspects summarized as the Tarragona strategy[153]: (1) Recognize individual risk factors; (2) Know local epidemiology; (3) Treat immediately and broad enough; (4) Select the ideal antibiotic for the site of infection; and (5) Re-evaluate your therapy after three days.
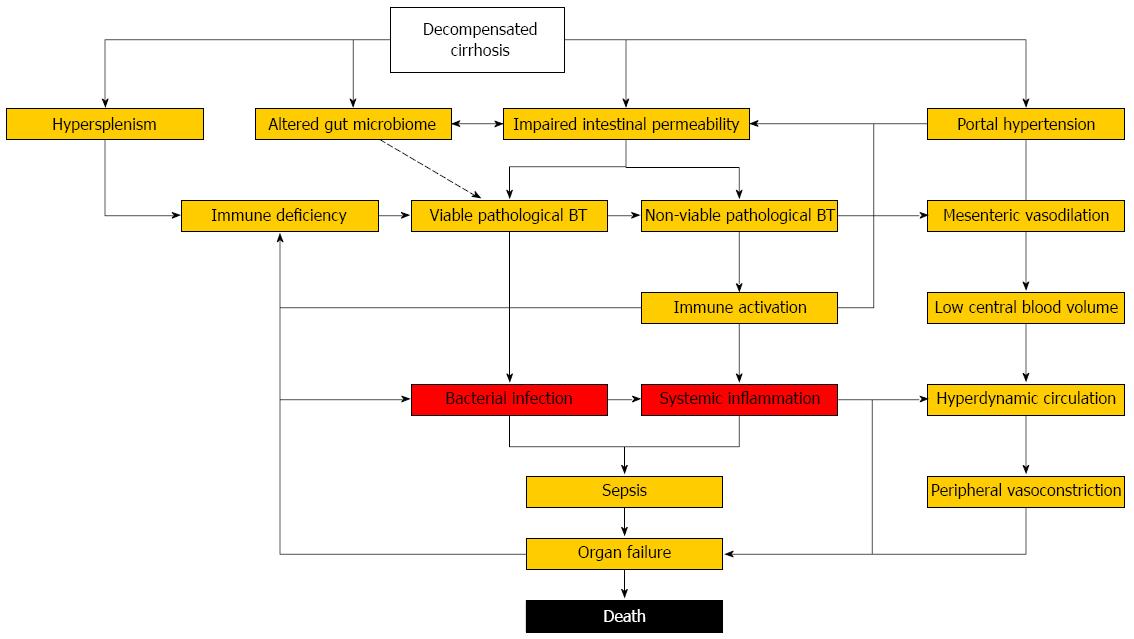
Patients with decompensated cirrhosis represent a highly vulnerable population with structural, immunological and hemodynamic abnormalities, which render them susceptible to bacterial infections, pronounced systemic inflammation, organ failure, and death (Figure 1). Despite increasing understanding of underlying pathophysiology and mechanisms of organ failure, the outcome of bacterial infections in cirrhosis has remained poor over last decades. Antibacterial prophylaxis is effective in preventing infectious complications but increasing antimicrobial resistance demands its restriction to patients at the highest risk. Non-antibiotic approaches to decrease the risk of bacterial infections by reducing pathological BT (probiotics, prokinetics, bile acids) or restoring dysfunctional immune responses (anti-inflammatory strategies, immune therapy) are desperately needed as we enter the post-antibiotic era. Until then, basic measures of infection prevention (vaccinations, hygiene, nutrition), antimicrobial de-escalation strategies, and surveillance for early signs of organ dysfunction are required to reduce the burden associated with bacterial infections in cirrhosis.
P- Reviewers: Garcia-Fernandez MI, Guo XZ, Ruiz-Gaspa S, Zhang SJ S- Editor: Wen LL L- Editor: A E- Editor: Zhang DN
| 1. | Caly WR, Strauss E. A prospective study of bacterial infections in patients with cirrhosis. J Hepatol. 1993;18:353-358. [PubMed] [Cited in This Article: ] |
| 2. | Borzio M, Salerno F, Piantoni L, Cazzaniga M, Angeli P, Bissoli F, Boccia S, Colloredo-Mels G, Corigliano P, Fornaciari G. Bacterial infection in patients with advanced cirrhosis: a multicentre prospective study. Dig Liver Dis. 2001;33:41-48. [PubMed] [Cited in This Article: ] |
| 3. | Fernández J, Navasa M, Gómez J, Colmenero J, Vila J, Arroyo V, Rodés J. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002;35:140-148. [PubMed] [Cited in This Article: ] |
| 4. | Fernández J, Acevedo J, Castro M, Garcia O, de Lope CR, Roca D, Pavesi M, Sola E, Moreira L, Silva A. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology. 2012;55:1551-1561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 403] [Cited by in F6Publishing: 392] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 5. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-137, 1426-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1720] [Cited by in F6Publishing: 1866] [Article Influence: 169.6] [Reference Citation Analysis (3)] |
| 6. | Arvaniti V, D’Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, Burroughs AK. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246-156, 1246-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 720] [Cited by in F6Publishing: 749] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 7. | Wiest R, Krag A, Gerbes A. Spontaneous bacterial peritonitis: recent guidelines and beyond. Gut. 2012;61:297-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 255] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 8. | Bonnel AR, Bunchorntavakul C, Reddy KR. Immune dysfunction and infections in patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9:727-738. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 243] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 9. | Bruns T, Stallmach A. [Spontaneous and Secondary Bacterial Peritonitis in Cirrhotic Patients with Ascites.]. Zentralbl Chir. 2011;Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 10. | Arabi YM, Dara SI, Memish Z, Al Abdulkareem A, Tamim HM, Al-Shirawi N, Parrillo JE, Dodek P, Lapinsky S, Feinstein D. Antimicrobial therapeutic determinants of outcomes from septic shock among patients with cirrhosis. Hepatology. 2012;56:2305-2315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 11. | Runyon BA, Squier S, Borzio M. Translocation of gut bacteria in rats with cirrhosis to mesenteric lymph nodes partially explains the pathogenesis of spontaneous bacterial peritonitis. J Hepatol. 1994;21:792-796. [PubMed] [Cited in This Article: ] |
| 12. | Scarpellini E, Valenza V, Gabrielli M, Lauritano EC, Perotti G, Merra G, Dal Lago A, Ojetti V, Ainora ME, Santoro M. Intestinal permeability in cirrhotic patients with and without spontaneous bacterial peritonitis: is the ring closed? Am J Gastroenterol. 2010;105:323-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Teltschik Z, Wiest R, Beisner J, Nuding S, Hofmann C, Schoelmerich J, Bevins CL, Stange EF, Wehkamp J. Intestinal bacterial translocation in rats with cirrhosis is related to compromised Paneth cell antimicrobial host defense. Hepatology. 2012;55:1154-1163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 139] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 14. | Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41:422-433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 492] [Cited by in F6Publishing: 484] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 15. | Steffen EK, Berg RD, Deitch EA. Comparison of translocation rates of various indigenous bacteria from the gastrointestinal tract to the mesenteric lymph node. J Infect Dis. 1988;157:1032-1038. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 151] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Bert F, Johnson JR, Ouattara B, Leflon-Guibout V, Johnston B, Marcon E, Valla D, Moreau R, Nicolas-Chanoine MH. Genetic diversity and virulence profiles of Escherichia coli isolates causing spontaneous bacterial peritonitis and bacteremia in patients with cirrhosis. J Clin Microbiol. 2010;48:2709-2714. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Katouli M, Nettebladt CG, Muratov V, Ljungqvist O, Bark T, Svenberg T, Möllby R. Selective translocation of coliform bacteria adhering to caecal epithelium of rats during catabolic stress. J Med Microbiol. 1997;46:571-578. [PubMed] [Cited in This Article: ] |
| 18. | Macutkiewicz C, Carlson G, Clark E, Dobrindt U, Roberts I, Warhurst G. Characterisation of Escherichia coli strains involved in transcytosis across gut epithelial cells exposed to metabolic and inflammatory stress. Microbes Infect. 2008;10:424-431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Clark E, Hoare C, Tanianis-Hughes J, Carlson GL, Warhurst G. Interferon gamma induces translocation of commensal Escherichia coli across gut epithelial cells via a lipid raft-mediated process. Gastroenterology. 2005;128:1258-1267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 133] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Cirera I, Bauer TM, Navasa M, Vila J, Grande L, Taurá P, Fuster J, García-Valdecasas JC, Lacy A, Suárez MJ. Bacterial translocation of enteric organisms in patients with cirrhosis. J Hepatol. 2001;34:32-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 302] [Cited by in F6Publishing: 294] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 21. | O’Boyle CJ, MacFie J, Mitchell CJ, Johnstone D, Sagar PM, Sedman PC. Microbiology of bacterial translocation in humans. Gut. 1998;42:29-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 265] [Cited by in F6Publishing: 285] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 22. | Garcia-Tsao G, Lee FY, Barden GE, Cartun R, West AB. Bacterial translocation to mesenteric lymph nodes is increased in cirrhotic rats with ascites. Gastroenterology. 1995;108:1835-1841. [PubMed] [Cited in This Article: ] |
| 23. | Pérez-Paramo M, Muñoz J, Albillos A, Freile I, Portero F, Santos M, Ortiz-Berrocal J. Effect of propranolol on the factors promoting bacterial translocation in cirrhotic rats with ascites. Hepatology. 2000;31:43-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 185] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 24. | Senzolo M, Cholongitas E, Burra P, Leandro G, Thalheimer U, Patch D, Burroughs AK. beta-Blockers protect against spontaneous bacterial peritonitis in cirrhotic patients: a meta-analysis. Liver Int. 2009;29:1189-1193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 25. | Úbeda M, Muñoz L, Borrero MJ, Díaz D, Francés R, Monserrat J, Lario M, Lledó L, Such J, Álvarez-Mon M. Critical role of the liver in the induction of systemic inflammation in rats with preascitic cirrhosis. Hepatology. 2010;52:2086-2095. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Reiberger T, Ferlitsch A, Payer BA, Mandorfer M, Heinisch BB, Hayden H, Lammert F, Trauner M, Peck-Radosavljevic M, Vogelsang H. Non-selective betablocker therapy decreases intestinal permeability and serum levels of LBP and IL-6 in patients with cirrhosis. J Hepatol. 2013;58:911-921. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 222] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 27. | Kasravi FB, Wang L, Wang XD, Molin G, Bengmark S, Jeppsson B. Bacterial translocation in acute liver injury induced by D-galactosamine. Hepatology. 1996;23:97-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Garcia-Tsao G, Albillos A, Barden GE, West AB. Bacterial translocation in acute and chronic portal hypertension. Hepatology. 1993;17:1081-1085. [PubMed] [Cited in This Article: ] |
| 29. | Bauer TM, Schwacha H, Steinbrückner B, Brinkmann FE, Ditzen AK, Aponte JJ, Pelz K, Berger D, Kist M, Blum HE. Small intestinal bacterial overgrowth in human cirrhosis is associated with systemic endotoxemia. Am J Gastroenterol. 2002;97:2364-2370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 144] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 30. | Jun DW, Kim KT, Lee OY, Chae JD, Son BK, Kim SH, Jo YJ, Park YS. Association between small intestinal bacterial overgrowth and peripheral bacterial DNA in cirrhotic patients. Dig Dis Sci. 2010;55:1465-1471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 31. | Chang CS, Chen GH, Lien HC, Yeh HZ. Small intestine dysmotility and bacterial overgrowth in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 1998;28:1187-1190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 191] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 32. | Lorenzo-Zúñiga V, Bartolí R, Planas R, Hofmann AF, Viñado B, Hagey LR, Hernández JM, Mañé J, Alvarez MA, Ausina V. Oral bile acids reduce bacterial overgrowth, bacterial translocation, and endotoxemia in cirrhotic rats. Hepatology. 2003;37:551-557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 230] [Cited by in F6Publishing: 228] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 33. | Van Bossuyt H, Desmaretz C, Gaeta GB, Wisse E. The role of bile acids in the development of endotoxemia during obstructive jaundice in the rat. J Hepatol. 1990;10:274-279. [PubMed] [Cited in This Article: ] |
| 34. | Suzuki T, Yoshinaga N, Tanabe S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J Biol Chem. 2011;286:31263-31271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 336] [Cited by in F6Publishing: 377] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 35. | Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171:6164-6172. [PubMed] [Cited in This Article: ] |
| 36. | Campillo B, Pernet P, Bories PN, Richardet JP, Devanlay M, Aussel C. Intestinal permeability in liver cirrhosis: relationship with severe septic complications. Eur J Gastroenterol Hepatol. 1999;11:755-759. [PubMed] [Cited in This Article: ] |
| 37. | Campillo B, Richardet JP, Kheo T, Dupeyron C. Nosocomial spontaneous bacterial peritonitis and bacteremia in cirrhotic patients: impact of isolate type on prognosis and characteristics of infection. Clin Infect Dis. 2002;35:1-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 134] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 38. | Benjamin J, Singla V, Arora I, Sood S, Joshi YK. Intestinal permeability and complications in liver cirrhosis: A prospective cohort study. Hepatol Res. 2013;43:200-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 39. | Chen Y, Yang F, Lu H, Wang B, Chen Y, Lei D, Wang Y, Zhu B, Li L. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54:562-572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 674] [Cited by in F6Publishing: 701] [Article Influence: 53.9] [Reference Citation Analysis (1)] |
| 40. | Lu H, Wu Z, Xu W, Yang J, Chen Y, Li L. Intestinal microbiota was assessed in cirrhotic patients with hepatitis B virus infection. Intestinal microbiota of HBV cirrhotic patients. Microb Ecol. 2011;61:693-703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 145] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 41. | Liu Q, Duan ZP, Ha DK, Bengmark S, Kurtovic J, Riordan SM. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology. 2004;39:1441-1449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 444] [Cited by in F6Publishing: 426] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 42. | Kuo CH, Changchien CS, Yang CY, Sheen IS, Liaw YF. Bacteremia in patients with cirrhosis of the liver. Liver. 1991;11:334-339. [PubMed] [Cited in This Article: ] |
| 43. | Reuken PA, Pletz MW, Baier M, Pfister W, Stallmach A, Bruns T. Emergence of spontaneous bacterial peritonitis due to enterococci - risk factors and outcome in a 12-year retrospective study. Aliment Pharmacol Ther. 2012;35:1199-1208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 44. | Bajaj JS, Hylemon PB, Ridlon JM, Heuman DM, Daita K, White MB, Monteith P, Noble NA, Sikaroodi M, Gillevet PM. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am J Physiol Gastrointest Liver Physiol. 2012;303:G675-G685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 354] [Cited by in F6Publishing: 375] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 45. | Bajaj JS, Ridlon JM, Hylemon PB, Thacker LR, Heuman DM, Smith S, Sikaroodi M, Gillevet PM. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol. 2012;302:G168-G175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 347] [Cited by in F6Publishing: 373] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 46. | Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon JI. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145:745-757. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1392] [Cited by in F6Publishing: 1476] [Article Influence: 113.5] [Reference Citation Analysis (0)] |
| 47. | Sun Y, Zhang M, Chen CC, Gillilland M, Sun X, El-Zaatari M, Huffnagle GB, Young VB, Zhang J, Hong SC. Stress-induced corticotropin-releasing hormone-mediated NLRP6 inflammasome inhibition and transmissible enteritis in mice. Gastroenterology. 2013;144:1478-187, 1478-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 48. | Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1620] [Cited by in F6Publishing: 1727] [Article Influence: 143.9] [Reference Citation Analysis (0)] |
| 49. | Wiest R, Lawson M, Geuking M. Pathological bacterial translocation in liver cirrhosis. J Hepatol. 2014;60:197-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 494] [Cited by in F6Publishing: 506] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 50. | Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723-737. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3097] [Cited by in F6Publishing: 3475] [Article Influence: 267.3] [Reference Citation Analysis (0)] |
| 51. | Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451-483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1913] [Cited by in F6Publishing: 1981] [Article Influence: 132.1] [Reference Citation Analysis (0)] |
| 52. | Zimmermann HW, Reuken PA, Koch A, Bartneck M, Adams DH, Trautwein C, Stallmach A, Tacke F, Bruns T. Soluble urokinase plasminogen activator receptor is compartmentally regulated in decompensated cirrhosis and indicates immune activation and short-term mortality. J Intern Med. 2013;274:86-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 53. | Saba TM. Physiology and physiopathology of the reticuloendothelial system. Arch Intern Med. 1970;126:1031-1052. [PubMed] [Cited in This Article: ] |
| 54. | Zimmermann HW, Trautwein C, Tacke F. Functional role of monocytes and macrophages for the inflammatory response in acute liver injury. Front Physiol. 2012;3:56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 181] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 55. | Rimola A, Soto R, Bory F, Arroyo V, Piera C, Rodes J. Reticuloendothelial system phagocytic activity in cirrhosis and its relation to bacterial infections and prognosis. Hepatology. 1984;4:53-58. [PubMed] [Cited in This Article: ] |
| 56. | Bolognesi M, Merkel C, Bianco S, Angeli P, Sacerdoti D, Amodio P, Gatta A. Clinical significance of the evaluation of hepatic reticuloendothelial removal capacity in patients with cirrhosis. Hepatology. 1994;19:628-634. [PubMed] [Cited in This Article: ] |
| 57. | Petermann H, Heymann S, Vogl S, Dargel R. Phagocytic function and metabolite production in thioacetamide-induced liver cirrhosis: a comparative study in perfused livers and cultured Kupffer cells. J Hepatol. 1996;24:468-477. [PubMed] [Cited in This Article: ] |
| 58. | Homann C, Garred P, Hasselqvist P, Graudal N, Thiel S, Thomsen AC. Mannan-binding protein and complement dependent opsonization in alcoholic cirrhosis. Liver. 1995;15:39-44. [PubMed] [Cited in This Article: ] |
| 59. | Albillos A, Hera Ad Ade L, Reyes E, Monserrat J, Muñoz L, Nieto M, Prieto A, Sanz E, Alvarez-Mon M. Tumour necrosis factor-alpha expression by activated monocytes and altered T-cell homeostasis in ascitic alcoholic cirrhosis: amelioration with norfloxacin. J Hepatol. 2004;40:624-631. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 108] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 60. | Zimmermann HW, Seidler S, Nattermann J, Gassler N, Hellerbrand C, Zernecke A, Tischendorf JJ, Luedde T, Weiskirchen R, Trautwein C. Functional contribution of elevated circulating and hepatic non-classical CD14CD16 monocytes to inflammation and human liver fibrosis. PLoS One. 2010;5:e11049. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 237] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 61. | Holland-Fischer P, Grønbæk H, Sandahl TD, Moestrup SK, Riggio O, Ridola L, Aagaard NK, Møller HJ, Vilstrup H. Kupffer cells are activated in cirrhotic portal hypertension and not normalised by TIPS. Gut. 2011;60:1389-1393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 62. | Fiuza C, Salcedo M, Clemente G, Tellado JM. In vivo neutrophil dysfunction in cirrhotic patients with advanced liver disease. J Infect Dis. 2000;182:526-533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 147] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 63. | Ono Y, Watanabe T, Matsumoto K, Ito T, Kunii O, Goldstein E. Opsonophagocytic dysfunction in patients with liver cirrhosis and low responses to tumor necrosis factor-alpha and lipopolysaccharide in patients’ blood. J Infect Chemother. 2004;10:200-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 64. | Shawcross DL, Wright GA, Stadlbauer V, Hodges SJ, Davies NA, Wheeler-Jones C, Pitsillides AA, Jalan R. Ammonia impairs neutrophil phagocytic function in liver disease. Hepatology. 2008;48:1202-1212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 123] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 65. | Bruns T, Peter J, Hagel S, Herrmann A, Stallmach A. The augmented neutrophil respiratory burst in response to Escherichia coli is reduced in liver cirrhosis during infection. Clin Exp Immunol. 2011;164:346-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 66. | Tritto G, Bechlis Z, Stadlbauer V, Davies N, Francés R, Shah N, Mookerjee RP, Such J, Jalan R. Evidence of neutrophil functional defect despite inflammation in stable cirrhosis. J Hepatol. 2011;55:574-581. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 67. | Mookerjee RP, Stadlbauer V, Lidder S, Wright GA, Hodges SJ, Davies NA, Jalan R. Neutrophil dysfunction in alcoholic hepatitis superimposed on cirrhosis is reversible and predicts the outcome. Hepatology. 2007;46:831-840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 238] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 68. | Rajkovic IA, Williams R. Abnormalities of neutrophil phagocytosis, intracellular killing and metabolic activity in alcoholic cirrhosis and hepatitis. Hepatology. 1986;6:252-262. [PubMed] [Cited in This Article: ] |
| 69. | Márquez M, Fernández-Gutiérrez C, Montes-de-Oca M, Blanco MJ, Brun F, Rodríguez-Ramos C, Girón-González JA. Chronic antigenic stimuli as a possible explanation for the immunodepression caused by liver cirrhosis. Clin Exp Immunol. 2009;158:219-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 70. | Lario M, Muñoz L, Ubeda M, Borrero MJ, Martínez J, Monserrat J, Díaz D, Alvarez-Mon M, Albillos A. Defective thymopoiesis and poor peripheral homeostatic replenishment of T-helper cells cause T-cell lymphopenia in cirrhosis. J Hepatol. 2013;59:723-730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 71. | Keeffe EB, Iwarson S, McMahon BJ, Lindsay KL, Koff RS, Manns M, Baumgarten R, Wiese M, Fourneau M, Safary A. Safety and immunogenicity of hepatitis A vaccine in patients with chronic liver disease. Hepatology. 1998;27:881-886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 187] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 72. | Villeneuve E, Vincelette J, Villeneuve JP. Ineffectiveness of hepatitis B vaccination in cirrhotic patients waiting for liver transplantation. Can J Gastroenterol. 2000;14 Suppl B:59B-62B. [PubMed] [Cited in This Article: ] |
| 73. | Cheong HJ, Song JY, Park JW, Yeon JE, Byun KS, Lee CH, Cho HI, Kim TG, Kim WJ. Humoral and cellular immune responses to influenza vaccine in patients with advanced cirrhosis. Vaccine. 2006;24:2417-2422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 74. | Schirren CA, Jung MC, Zachoval R, Diepolder H, Hoffmann R, Riethmüller G, Pape GR. Analysis of T cell activation pathways in patients with liver cirrhosis, impaired delayed hypersensitivity and other T cell-dependent functions. Clin Exp Immunol. 1997;108:144-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 75. | Peter J, Frey O, Stallmach A, Bruns T. Attenuated antigen-specific T cell responses in cirrhosis are accompanied by elevated serum interleukin-10 levels and down-regulation of HLA-DR on monocytes. BMC Gastroenterol. 2013;13:37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 76. | Hsu CC, Leevy CM. Inhibition of PHA-stimulated lymphocyte transformation by plasma from patients with advanced alcoholic cirrhosis. Clin Exp Immunol. 1971;8:749-760. [PubMed] [Cited in This Article: ] |
| 77. | Taga K, Mostowski H, Tosato G. Human interleukin-10 can directly inhibit T-cell growth. Blood. 1993;81:2964-2971. [PubMed] [Cited in This Article: ] |
| 78. | Chuang WL, Liu HW, Chang WY, Chen SC, Hsieh MY, Wang LY. Natural killer cell activity in patients with liver cirrhosis relative to severity of liver damage. Dig Dis Sci. 1991;36:299-302. [PubMed] [Cited in This Article: ] |
| 79. | Zimmermann HW, Mueller JR, Seidler S, Luedde T, Trautwein C, Tacke F. Frequency and phenotype of human circulating and intrahepatic natural killer cell subsets is differentially regulated according to stage of chronic liver disease. Digestion. 2013;88:1-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 80. | Doi H, Iyer TK, Carpenter E, Li H, Chang KM, Vonderheide RH, Kaplan DE. Dysfunctional B-cell activation in cirrhosis resulting from hepatitis C infection associated with disappearance of CD27-positive B-cell population. Hepatology. 2012;55:709-719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 81. | Byl B, Roucloux I, Crusiaux A, Dupont E, Devière J. Tumor necrosis factor alpha and interleukin 6 plasma levels in infected cirrhotic patients. Gastroenterology. 1993;104:1492-1497. [PubMed] [Cited in This Article: ] |
| 82. | Abdel-Khalek EE, El-Fakhry A, Helaly M, Hamed M, Elbaz O. Systemic inflammatory response syndrome in patients with liver cirrhosis. Arab J Gastroenterol. 2011;12:173-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 83. | Cazzaniga M, Dionigi E, Gobbo G, Fioretti A, Monti V, Salerno F. The systemic inflammatory response syndrome in cirrhotic patients: relationship with their in-hospital outcome. J Hepatol. 2009;51:475-482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 167] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 84. | Reuken PA, Stallmach A, Bruns T. Mortality after urinary tract infections in patients with advanced cirrhosis - Relevance of acute kidney injury and comorbidities. Liver Int. 2013;33:220-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 85. | Thabut D, Massard J, Gangloff A, Carbonell N, Francoz C, Nguyen-Khac E, Duhamel C, Lebrec D, Poynard T, Moreau R. Model for end-stage liver disease score and systemic inflammatory response are major prognostic factors in patients with cirrhosis and acute functional renal failure. Hepatology. 2007;46:1872-1882. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 182] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 86. | Rosenbloom AJ, Pinsky MR, Bryant JL, Shin A, Tran T, Whiteside T. Leukocyte activation in the peripheral blood of patients with cirrhosis of the liver and SIRS. Correlation with serum interleukin-6 levels and organ dysfunction. JAMA. 1995;274:58-65. [PubMed] [Cited in This Article: ] |
| 87. | Lee FY, Lu RH, Tsai YT, Lin HC, Hou MC, Li CP, Liao TM, Lin LF, Wang SS, Lee SD. Plasma interleukin-6 levels in patients with cirrhosis. Relationship to endotoxemia, tumor necrosis factor-alpha, and hyperdynamic circulation. Scand J Gastroenterol. 1996;31:500-505. [PubMed] [Cited in This Article: ] |
| 88. | Cervoni JP, Thévenot T, Weil D, Muel E, Barbot O, Sheppard F, Monnet E, Di Martino V. C-reactive protein predicts short-term mortality in patients with cirrhosis. J Hepatol. 2012;56:1299-1304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 128] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 89. | Tsiakalos A, Karatzaferis A, Ziakas P, Hatzis G. Acute-phase proteins as indicators of bacterial infection in patients with cirrhosis. Liver Int. 2009;29:1538-1542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 90. | Reuken PA, Kiehntopf M, Stallmach A, Bruns T. Mid-regional pro-adrenomedullin (MR-proADM): an even better prognostic biomarker than C-reactive protein to predict short-term survival in patients with decompensated cirrhosis at risk of infection? J Hepatol. 2012;57:1156-118; author reply 1156-118;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 91. | Lazzarotto C, Ronsoni MF, Fayad L, Nogueira CL, Bazzo ML, Narciso-Schiavon JL, de Lucca Schiavon L, Dantas-Corrêa EB. Acute phase proteins for the diagnosis of bacterial infection and prediction of mortality in acute complications of cirrhosis. Ann Hepatol. 2013;12:599-607. [PubMed] [Cited in This Article: ] |
| 92. | Bruns T, Sachse S, Straube E, Assefa S, Herrmann A, Hagel S, Lehmann M, Stallmach A. Identification of bacterial DNA in neutrocytic and non-neutrocytic cirrhotic ascites by means of a multiplex polymerase chain reaction. Liver Int. 2009;29:1206-1214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 93. | Guggenbichler JP, Allerberger FJ, Dierich M. Influence of cephalosporines III generation with varying biliary excretion on fecal flora and emergence of resistant bacteria during and after cessation of therapy. Padiatr Padol. 1986;21:335-342. [PubMed] [Cited in This Article: ] |
| 94. | Gerbes AL, Gülberg V, Sauerbruch T, Wiest R, Appenrodt B, Bahr MJ, Dollinger MM, Rössle M, Schepke M. [German S 3-guideline “ascites, spontaneous bacterial peritonitis, hepatorenal syndrome”]. Z Gastroenterol. 2011;49:749-779. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 95. | European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1125] [Cited by in F6Publishing: 1078] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 96. | Chavez-Tapia NC, Barrientos-Gutierrez T, Tellez-Avila FI, Soares-Weiser K, Uribe M. Antibiotic prophylaxis for cirrhotic patients with upper gastrointestinal bleeding. Cochrane Database Syst Rev. 2010;CD002907. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 63] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 97. | Chavez-Tapia NC, Barrientos-Gutierrez T, Tellez-Avila F, Soares-Weiser K, Mendez-Sanchez N, Gluud C, Uribe M. Meta-analysis: antibiotic prophylaxis for cirrhotic patients with upper gastrointestinal bleeding - an updated Cochrane review. Aliment Pharmacol Ther. 2011;34:509-518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 153] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 98. | Banerjee S, Shen B, Baron TH, Nelson DB, Anderson MA, Cash BD, Dominitz JA, Gan SI, Harrison ME, Ikenberry SO. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2008;67:791-798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 277] [Cited by in F6Publishing: 291] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 99. | Llach J, Elizalde JI, Bordas JM, Gines A, Almela M, Sans M, Mondelo F, Pique JM. Prospective assessment of the risk of bacteremia in cirrhotic patients undergoing lower intestinal endoscopy. Gastrointest Endosc. 1999;49:214-217. [PubMed] [Cited in This Article: ] |
| 100. | Hirota WK, Petersen K, Baron TH, Goldstein JL, Jacobson BC, Leighton JA, Mallery JS, Waring JP, Fanelli RD, Wheeler-Harbough J. Guidelines for antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2003;58:475-482. [PubMed] [Cited in This Article: ] |
| 101. | Baron TH. Antibiotic prophylaxis for colonoscopy in cirrhotics. Liver Transpl. 2006;12:493-493. [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 102. | Guarner C, Solà R, Soriano G, Andreu M, Novella MT, Vila MC, Sàbat M, Coll S, Ortiz J, Gómez C. Risk of a first community-acquired spontaneous bacterial peritonitis in cirrhotics with low ascitic fluid protein levels. Gastroenterology. 1999;117:414-419. [PubMed] [Cited in This Article: ] |
| 103. | Titó L, Rimola A, Ginès P, Llach J, Arroyo V, Rodés J. Recurrence of spontaneous bacterial peritonitis in cirrhosis: frequency and predictive factors. Hepatology. 1988;8:27-31. [PubMed] [Cited in This Article: ] |
| 104. | Runyon BA. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49:2087-2107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 628] [Cited by in F6Publishing: 588] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 105. | Fernández J, Navasa M, Planas R, Montoliu S, Monfort D, Soriano G, Vila C, Pardo A, Quintero E, Vargas V. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology. 2007;133:818-824. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 508] [Cited by in F6Publishing: 428] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 106. | Saab S, Hernandez JC, Chi AC, Tong MJ. Oral antibiotic prophylaxis reduces spontaneous bacterial peritonitis occurrence and improves short-term survival in cirrhosis: a meta-analysis. Am J Gastroenterol. 2009;104:993-1001; quiz 1002. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 107. | Thevenot T, Degand T, Grelat N, Elkrief L, Christol C, Moreau R, Henrion J, Cadranel JF, Sheppard F, Bureau C, di Martino V, Pauwels A. A French national survey on the use of antibiotic prophylaxis in cirrhotic patients. Liver Int. 2013;33:389-397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 108. | Ngamruengphong S, Nugent K, Rakvit A, Parupudi S. Potential preventability of spontaneous bacterial peritonitis. Dig Dis Sci. 2011;56:2728-2734. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 109. | Gäbele E, Mühlbauer M, Paulo H, Johann M, Meltzer C, Leidl F, Wodarz N, Wiest R, Schölmerich J, Hellerbrand C. Analysis of monocyte chemotactic protein-1 gene polymorphism in patients with spontaneous bacterial peritonitis. World J Gastroenterol. 2009;15:5558-5562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 110. | Appenrodt B, Grünhage F, Gentemann MG, Thyssen L, Sauerbruch T, Lammert F. Nucleotide-binding oligomerization domain containing 2 (NOD2) variants are genetic risk factors for death and spontaneous bacterial peritonitis in liver cirrhosis. Hepatology. 2010;51:1327-1333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 111. | Bruns T, Peter J, Reuken PA, Grabe DH, Schuldes SR, Brenmoehl J, Schölmerich J, Wiest R, Stallmach A. NOD2 gene variants are a risk factor for culture-positive spontaneous bacterial peritonitis and monomicrobial bacterascites in cirrhosis. Liver Int. 2012;32:223-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 112. | Nischalke HD, Berger C, Aldenhoff K, Thyssen L, Gentemann M, Grünhage F, Lammert F, Nattermann J, Sauerbruch T, Spengler U. Toll-like receptor (TLR) 2 promoter and intron 2 polymorphisms are associated with increased risk for spontaneous bacterial peritonitis in liver cirrhosis. J Hepatol. 2011;55:1010-1016. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 113. | Bruns T, Reuken PA, Fischer J, Berg T, Stallmach A. Further evidence for the relevance of TLR2 gene variants in spontaneous bacterial peritonitis. J Hepatol. 2012;56:1207-128; author reply 1207-128;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 114. | Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nuñez G, Flavell RA. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731-734. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1334] [Cited by in F6Publishing: 1302] [Article Influence: 68.5] [Reference Citation Analysis (2)] |
| 115. | Travassos LH, Carneiro LA, Ramjeet M, Hussey S, Kim YG, Magalhães JG, Yuan L, Soares F, Chea E, Le Bourhis L. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat Immunol. 2010;11:55-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 916] [Cited by in F6Publishing: 972] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 116. | Lala S, Ogura Y, Osborne C, Hor SY, Bromfield A, Davies S, Ogunbiyi O, Nuñez G, Keshav S. Crohn’s disease and the NOD2 gene: a role for paneth cells. Gastroenterology. 2003;125:47-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 388] [Cited by in F6Publishing: 356] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 117. | Obstein KL, Campbell MS, Reddy KR, Yang YX. Association between model for end-stage liver disease and spontaneous bacterial peritonitis. Am J Gastroenterol. 2007;102:2732-2736. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 118. | Rosa H, Silvério AO, Perini RF, Arruda CB. Bacterial infection in cirrhotic patients and its relationship with alcohol. Am J Gastroenterol. 2000;95:1290-1293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 119. | Deshpande A, Pasupuleti V, Thota P, Pant C, Mapara S, Hassan S, Rolston DD, Sferra TJ, Hernandez AV. Acid-suppressive therapy is associated with spontaneous bacterial peritonitis in cirrhotic patients: a meta-analysis. J Gastroenterol Hepatol. 2013;28:235-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 120. | de Vos M, De Vroey B, Garcia BG, Roy C, Kidd F, Henrion J, Deltenre P. Role of proton pump inhibitors in the occurrence and the prognosis of spontaneous bacterial peritonitis in cirrhotic patients with ascites. Liver Int. 2013;33:1316-1323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 121. | Fasolato S, Angeli P, Dallagnese L, Maresio G, Zola E, Mazza E, Salinas F, Donà S, Fagiuoli S, Sticca A. Renal failure and bacterial infections in patients with cirrhosis: epidemiology and clinical features. Hepatology. 2007;45:223-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 189] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 122. | Bajaj JS, O’Leary JG, Reddy KR, Wong F, Olson JC, Subramanian RM, Brown G, Noble NA, Thacker LR, Kamath PS. Second infections independently increase mortality in hospitalized patients with cirrhosis: the North American consortium for the study of end-stage liver disease (NACSELD) experience. Hepatology. 2012;56:2328-2335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 284] [Cited by in F6Publishing: 296] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 123. | Hung TH, Tseng CW, Hsieh YH, Tseng KC, Tsai CC, Tsai CC. High mortality of pneumonia in cirrhotic patients with ascites. BMC Gastroenterol. 2013;13:25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 124. | Mellencamp MA, Preheim LC. Pneumococcal pneumonia in a rat model of cirrhosis: effects of cirrhosis on pulmonary defense mechanisms against Streptococcus pneumoniae. J Infect Dis. 1991;163:102-108. [PubMed] [Cited in This Article: ] |
| 125. | Falguera M, Trujillano J, Caro S, Menéndez R, Carratalà J, Ruiz-González A, Vilà M, García M, Porcel JM, Torres A. A prediction rule for estimating the risk of bacteremia in patients with community-acquired pneumonia. Clin Infect Dis. 2009;49:409-416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 126. | Di Pasquale M, Esperatti M, Crisafulli E, Ferrer M, Bassi GL, Rinaudo M, Escorsell A, Fernandez J, Mas A, Blasi F. Impact of chronic liver disease in intensive care unit acquired pneumonia: a prospective study. Intensive Care Med. 2013;39:1776-1784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 127. | Leise MD, Talwalkar JA. Immunizations in chronic liver disease: what should be done and what is the evidence. Curr Gastroenterol Rep. 2013;15:300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 128. | McCashland TM, Preheim LC, Gentry MJ. Pneumococcal vaccine response in cirrhosis and liver transplantation. J Infect Dis. 2000;181:757-760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 113] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 129. | Pereira G, Guevara M, Fagundes C, Solá E, Rodríguez E, Fernández J, Pavesi M, Arroyo V, Ginès P. Renal failure and hyponatremia in patients with cirrhosis and skin and soft tissue infection. A retrospective study. J Hepatol. 2012;56:1040-1046. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 130. | Mohan P, Ramu B, Bhaskar E, Venkataraman J. Prevalence and risk factors for bacterial skin infection and mortality in cirrhosis. Ann Hepatol. 2011;10:15-20. [PubMed] [Cited in This Article: ] |
| 131. | Hung TH, Hsieh YH, Tseng KC, Tsai CC, Tsai CC. The risk for bacterial endocarditis in cirrhotic patients: a population-based 3-year follow-up study. Int J Infect Dis. 2013;17:e391-e393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 132. | Fernández Guerrero ML, González López J, Górgolas M. Infectious endocarditis in patients with cirrhosis of the liver: a model of infection in the frail patient. Eur J Clin Microbiol Infect Dis. 2010;29:1271-1275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 133. | Barahona-Garrido J, Hernández-Calleros J, Téllez-Avila FI, Chávez-Tapia NC, Remes-Troche JM, Torre A. Bacterial meningitis in cirrhotic patients: case series and description of the prognostic role of acute renal failure. J Clin Gastroenterol. 2010;44:e218-e223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 134. | Pauwels A, Pinès E, Abboura M, Chiche I, Lévy VG. Bacterial meningitis in cirrhosis: review of 16 cases. J Hepatol. 1997;27:830-834. [PubMed] [Cited in This Article: ] |
| 135. | Tandon P, Kumar D, Seo YS, Chang HJ, Chaulk J, Carbonneau M, Qamar H, Keough A, Mansoor N, Ma M. The 22/11 risk prediction model: a validated model for predicting 30-day mortality in patients with cirrhosis and spontaneous bacterial peritonitis. Am J Gastroenterol. 2013;108:1473-1479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 136. | Ginès P, Schrier RW. Renal failure in cirrhosis. N Engl J Med. 2009;361:1279-1290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 577] [Cited by in F6Publishing: 508] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 137. | Newby DE, Hayes PC. Hyperdynamic circulation in liver cirrhosis: not peripheral vasodilatation but ‘splanchnic steal’. QJM. 2002;95:827-830. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 138. | Tristani FE, Cohn JN. Systemic and renal hemodynamics in oliguric hepatic failure: effect of volume expansion. J Clin Invest. 1967;46:1894-1906. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 123] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 139. | Ginès P, Guevara M, Arroyo V, Rodés J. Hepatorenal syndrome. Lancet. 2003;362:1819-1827. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 443] [Cited by in F6Publishing: 366] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 140. | Terra C, Guevara M, Torre A, Gilabert R, Fernández J, Martín-Llahí M, Baccaro ME, Navasa M, Bru C, Arroyo V. Renal failure in patients with cirrhosis and sepsis unrelated to spontaneous bacterial peritonitis: value of MELD score. Gastroenterology. 2005;129:1944-1953. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 164] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 141. | Barreto R, Fagundes C, Guevara M, Solà E, Pereira G, Rodríguez E, Graupera I, Martín-Llahí M, Ariza X, Cárdenas A. Type-1 hepatorenal syndrome associated with infections in cirrhosis. Natural history, outcome of kidney function and survival. Hepatology. 2013;Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 142. | Wong F, O’Leary JG, Reddy KR, Patton H, Kamath PS, Fallon MB, Garcia-Tsao G, Subramanian RM, Malik R, Maliakkal B. New consensus definition of acute kidney injury accurately predicts 30-day mortality in patients with cirrhosis and infection. Gastroenterology. 2013;145:1280-8.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 182] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 143. | Martín-Llahí M, Guevara M, Torre A, Fagundes C, Restuccia T, Gilabert R, Solá E, Pereira G, Marinelli M, Pavesi M. Prognostic importance of the cause of renal failure in patients with cirrhosis. Gastroenterology. 2011;140:488-496.e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 227] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 144. | Belcher JM, Garcia-Tsao G, Sanyal AJ, Bhogal H, Lim JK, Ansari N, Coca SG, Parikh CR. Association of AKI with mortality and complications in hospitalized patients with cirrhosis. Hepatology. 2013;57:753-762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 249] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 145. | Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-del-Arbol L, Castells L, Vargas V, Soriano G, Guevara M. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403-409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1132] [Cited by in F6Publishing: 948] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 146. | Guevara M, Terra C, Nazar A, Solà E, Fernández J, Pavesi M, Arroyo V, Ginès P. Albumin for bacterial infections other than spontaneous bacterial peritonitis in cirrhosis. A randomized, controlled study. J Hepatol. 2012;57:759-765. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 147. | Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:1637-1648. [PubMed] [Cited in This Article: ] |
| 148. | Lebrec D, Thabut D, Oberti F, Perarnau JM, Condat B, Barraud H, Saliba F, Carbonell N, Renard P, Ramond MJ. Pentoxifylline does not decrease short-term mortality but does reduce complications in patients with advanced cirrhosis. Gastroenterology. 2010;138:1755-1762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 149. | Tyagi P, Sharma P, Sharma BC, Puri AS, Kumar A, Sarin SK. Prevention of hepatorenal syndrome in patients with cirrhosis and ascites: a pilot randomized control trial between pentoxifylline and placebo. Eur J Gastroenterol Hepatol. 2011;23:210-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 150. | Cheong HS, Kang CI, Lee JA, Moon SY, Joung MK, Chung DR, Koh KC, Lee NY, Song JH, Peck KR. Clinical significance and outcome of nosocomial acquisition of spontaneous bacterial peritonitis in patients with liver cirrhosis. Clin Infect Dis. 2009;48:1230-1236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 151. | Umgelter A, Reindl W, Miedaner M, Schmid RM, Huber W. Failure of current antibiotic first-line regimens and mortality in hospitalized patients with spontaneous bacterial peritonitis. Infection. 2009;37:2-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 152. | Song KH, Jeon JH, Park WB, Park SW, Kim HB, Oh MD, Lee HS, Kim NJ, Choe KW. Clinical outcomes of spontaneous bacterial peritonitis due to extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella species: a retrospective matched case-control study. BMC Infect Dis. 2009;9:41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 153. | Sandiumenge A, Diaz E, Bodí M, Rello J. Therapy of ventilator-associated pneumonia. A patient-based approach based on the ten rules of “The Tarragona Strategy”. Intensive Care Med. 2003;29:876-883. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 127] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 154. | Ariza X, Castellote J, Lora-Tamayo J, Girbau A, Salord S, Rota R, Ariza J, Xiol X. Risk factors for resistance to ceftriaxone and its impact on mortality in community, healthcare and nosocomial spontaneous bacterial peritonitis. J Hepatol. 2012;56:825-832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 155. | Campillo B, Dupeyron C, Richardet JP. Epidemiology of hospital-acquired infections in cirrhotic patients: effect of carriage of methicillin-resistant Staphylococcus aureus and influence of previous antibiotic therapy and norfloxacin prophylaxis. Epidemiol Infect. 2001;127:443-450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |