Published online Jul 7, 2014. doi: 10.3748/wjg.v20.i25.8274
Revised: December 17, 2013
Accepted: March 19, 2014
Published online: July 7, 2014
AIM: To review the currently available literature comparing laparoscopic to open resection of hepatocellular carcinoma (HCC) in patients with known liver cirrhosis.
METHODS: A literature search of MEDLINE, EMBASE, and Cochrane databases was conducted. The search terms used included (laparoscopic OR laparoscopy) AND (hepatic or liver) AND (surgery or resection) AND “hepatocellular carcinoma” AND (cirrhosis or cirrhotic). Furthermore, to widen the search, we also used the “related articles” section. Studies reporting a comparison of outcomes and methods of open vs laparoscopic hepatic resection for HCC in patients with liver cirrhosis were included. Meta-analysis of results was performed using a random effects model to compute relative risk (RR) and for dichotomous variables and standard mean differences (SMD) for continuous variables.
RESULTS: A total of 420 patients from 4 cohort studies were included in final analysis. Patients undergoing laparoscopic procedures had statistically less blood loss compared to the open cohort, SMD of -1.01 (95%CI: -1.23-0.79), P < 0.001, with a reduced risk of transfusion, RR = 0.19 (95%CI: 0.09-0.38), P < 0.001. A wider clearance at tumour resection margins was achieved following a laparoscopic approach, SMD of 0.34 (95%CI: 0.08-0.60), P = 0.011. No significant difference was noted between laparoscopic and open resection operative times, SMD of -0.15 (95%CI: 0.35-0.05), P = 0.142. The overall RR of suffering from postoperative morbidity is 0.25 in favour of the open surgery cohort (95%CI: 0.17-0.37), P < 0.001. Patients under-going laparoscopic surgery had significantly shorter length of stays in hospital compared to the open cohort, SMD of -0.53 (95%CI: -0.73 to -0.32), P < 0.001.
CONCLUSION: This review suggests that laparoscopic resection of hepatocellular carcinoma in patients with cirrhosis is safe and may provide improved patient outcomes when compared to the open technique.
Core tip: Laparoscopic surgery is now considered the gold standard for the majority of surgical procedures. Minimally invasive surgery in oncological cases has been shown to provide enhanced recovery and overall better outcomes compared to an open approach. Although slower to be implemented, laparoscopic hepatic surgery is now considered safe and, in many situations, better than an open technique. Cirrhotic livers have always been considered technically difficult to approach surgically. This review suggests that not only is laparoscopic surgery for patients with hepatocellular carcinoma and known known cirrhosis safe, it may have improved outcomes compared to the open technique.
-
Citation: Twaij A, Pucher PH, Sodergren MH, Gall T, Darzi A, Jiao LR. Laparoscopic
vs open approach to resection of hepatocellular carcinoma in patients with known cirrhosis: Systematic review and meta-analysis. World J Gastroenterol 2014; 20(25): 8274-8281 - URL: https://www.wjgnet.com/1007-9327/full/v20/i25/8274.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i25.8274
The introduction and development of laparoscopic surgery has had an immense impact on outcomes in surgery since the first laparoscopic cholecystectomy performed in 1985[1]. Since its introduction, laparoscopy is now regularly used in the majority of elective and emergency surgical procedures. Laparoscopic techniques have gone through a slower rate of uptake for oncological procedures, but are now commonly used in gastrointestinal cancer surgery, particularly for bowel resections[2-5]. However, the use of laparoscopy in hepatic surgery is not yet widely established.
Hepatocellular carcinoma (HCC) is the most common primary cancer of the liver and the 5th and 8th most prevalent cancer worldwide in males and females respectively[6]. The incidence of HCC has been on the rise and is associated with an increase in hepatitis B or C-associated cirrhosis[7]. Approximately 80% of patients with HCC develop the tumour from such chronic liver diseases[8]. The incidence of HCC in cirrhotic patients varies from between 0.2%-8.0% per year depending on the cause of cirrhosis[9]. Mortality rate of HCC associated with liver cirrhosis is rising in developed countries with HCC now being a major cause of death in patients with compensated cirrhosis[10]. European cohort studies have suggested that HCC is responsible for 54%[11] to 70%[12] of deaths in patients who died of a liver related cause with compensated cirrhosis.
Patients suffering from liver cirrhosis complicated with HCC often have a narrow range of treatment options. Liver transplantation is potentially curative, yet, due to various limitations such as continued alcohol abuse associated with this patient cohort, as well donor availability and patient age, often limited in its application[13] . Liver resection is an alternative option and is now widely accepted as a potentially curative treatment for patients with HCC[14].
However, liver resection for cancer complicated by cirrhosis is not without risks[15,16]. Patients suffering from cirrhosis are at increased risk of developing significant postoperative complications including ascites, lung infection or pleural effusion, transient encephalopathy, kidney failure, portal vein thrombosis, hernias and upper gastrointestinal bleeding[17,18]. Risks can also be related to systemic changes related to poor hepatic function and cirrhosis, such as intraoperative haemorrhage due to primary haemostasis dysfunction[19] as well as the increased incidence of oesophageal varices[20], resulting in a potentially high risk of intraoperative blood loss. These factors have led to surgeons developing meticulous selection criteria for patients suitable for hepatic resection in the context of cirrhosis. For a number of years there has been a general consensus amongst physicians that patients with a Childs-Pugh classification of C should not have any elective surgical procedures performed due to the high mortality risk[21]. In a recent study by Neeff and colleagues it was noted that patients with cirrhosis had 10%, 17% and 63% mortality rates for Childs-Pugh classification A, B and C respectively[22]. As such, it remains at present unclear whether laparoscopic surgery is of benefit to patient outcomes following resection[23].
Laparoscopic surgery for hepatic procedures has been slow to develop. Initially introduced for staging procedures, it has now been implemented for uncomplicated liver resections in HCC. When compared to open procedures, studies have suggested that laparoscopic procedures result in reduced intraoperative blood loss[24] and thus reduced need for blood transfusions[25]. Moreover, reduced operative time[26] have been noted in laparoscopic cohorts with wider tumour resection margins[27] when compared to open resection. Postoperatively, lower morbidity rates also resulted in lower length of stays in hospitals in laparoscopic resection when compared to open procedures[25]. Differences in long-term outcomes have yet to be evaluated[28,29].
Whether this applies to the more complex group of patients suffering additionally from cirrhosis, however, is unclear. In the context of the known, and significant, additional risks of hepatic resection in cirrhosis, it would be inappropriate to assume such data could be extrapolated.
The aim of this study, therefore, was to review the currently available data comparing laparoscopic (LR) vs open liver resections (OR) for HCC in patients with known cirrhosis.
A systematic review was performed according to criteria were defined by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement[30].
A review of the literature was performed using MEDLINE and EMBASE databases as well as the Cochrane Library up to August 2013, without restriction on language or region. The search terms used included (laparoscopic OR laparoscopy) AND (hepatic or liver) AND (surgery or resection) AND “hepatocellular carcinoma” AND (cirrhosis or cirrhotic). Furthermore, to widen the search, we also used the “related articles” section.
Titles and abstracts were reviewed and candidate articles identified. These were then retrieved for full-text review and final inclusion of articles according to predefined criteria. The search was conducted by two independent researchers (AT and PP), any differences were resolved by consensus.
All published articles reporting a comparison of outcomes and methods of open vs laparoscopic hepatic resection for HCC in patients with known cirrhosis were included. Studies reporting outcomes in non-cirrhotic patients or patients with chronic liver disease and unproven cirrhosis were excluded. Furthermore, published abstracts were excluded from the review.
Outcome measures assessed included both perioperative and postoperative outcomes. Perioperative measures included operating time, blood loss volume, requirement of transfusions, and histological tumour margins. Postoperative measures included morbidity and mortality rates, length of stay in hospital, and long-term survival rates and disease free survival.
Meta-analysis of results was performed using a random effects model to compute relative risk (RR) and 95%CI for dichotomous variables and standard mean differences (SMD) for continuous variables, using Stata 12 (StataCorp, College Station, TX). I2 test was used to assess data heterogeneity; all cases a p value of less than 0.05 was deemed statistically significant.
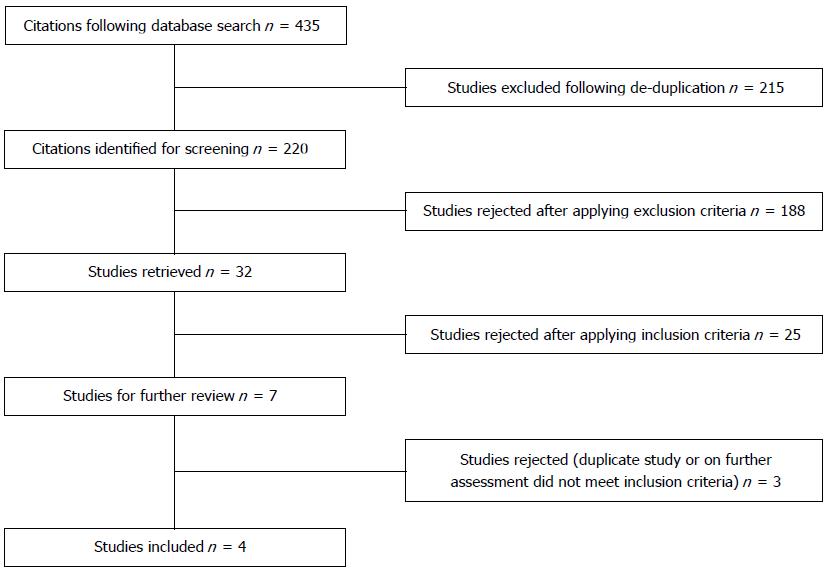
Following the database search 435 results were produced and following de-duplication, were narrowed down to 220 articles (Figure 1). Following article selection according to criteria as described, this resulted in final inclusion of 4 comparative cohort studies. These included a total of 420 patients (LR = 150, OR = 270). There were no significant differences in patient demographics between groups (Table 1). All studies were single centre retrospective cohort studies with 3 out of 4 being case matched studies. Cases were matched according to liver function tests, demographics, tumour and intraoperative technicalities. The majority of patients in both the LR and OR groups were classified as Child-Pugh class A, with the remainder being class B (88.7% and 90.7% of patients were Child-Pugh class A in the LR and OR cohorts respectively). Both cohorts, open vs laparoscopic, in all studies were performed by the same institution and same surgical teams.
| Study | Study type | Procedure | n (LR) | n (OR) | Age (lap) | Age (open) (yr) | M:F (lap) | M:F (open) | Conversion to open | CP A:B ratio (lap) | CP A:B ratio (open) |
| Kanazawa et al[18] | Cohort comparison | Single or multiple resection | 28 | 28 | 69 (40-85) | 68 (47-78) | 16:12 | 17:11 | 10.7% | 20:8 | 21:7 |
| Cheung et al[23] | Cohort with case-matched controls | Single resection | 32 | 64 | 59.5(39-79) | 61(29-82) | 22:10 | 50:14 | 18.8% | 32:0 | 62:4 |
| Truant et al[33] | Cohort with case-matched controls | Single or multiple resection | 36 | 53 | 60.6 +/- 10.2 | 63.3 +/- 7.6 | 31:5 | 47:6 | 19.4% | A only | A only |
| Belli et al[32] | Cohort comparison | Single or multiple resection | 54 | 125 | 63.3 +/- 6.1 | 61.5 +/- 7.8 | 31:23 | 78:47 | 7% | 49:5 | 117:8 |
The risk of bias was assessed using a modified Newcastle-Ottawa scale (NOS)[31] for assessing the quality of non-randomised studies and is demonstrated in Table 2. The overall quality of the studies included was of good quality, the NOS scores varied between 7 and 8 out of 9. An important factor to note is due to uncontrollable intraoperative complications, each study had a small portion of laparoscopic procedures converted to open, ranging from 7% (Belli[32]) to 19.4% (Truant[33]).
| Study | Selection | Comparability | Outcome | ||||||
| Representativeness of exposed cohort | Selection of non-exposed cohort | Exposure | Outcome of interest not present at start | Comparability of laparoscopic vs open | Assessment of outcome | Follow-up | Adequacy of follow-up/missing data | Score | |
| Kanazawa et al[18] | Truly representative | Same | Surgical records | Yes | No restrictions, not matched | Record linkage | 5 yr | Unclear | 7 |
| Cheung et al[23] | Truly representative | Same | Surgical records | Yes | No restrictions, matched | Record linkage | 5 yr | None | 8 |
| Truant et al[33] | Truly representative | Same | Surgical records | Yes | Restricted to subcapsular tumours located in the anterior or lateral segments II-VI, matched | Record linkage | 5 yr | Unclear | 7 |
| Belli et al[32] | Truly representative | Same | Surgical records | Yes | Restricted in exophytic or subcapsular tumours, no matching | Record linkage | 3 yr | Unclear | 7 |
The criteria for resectability included radiographic absence of extrahepatic involvement, anatomically suitable disease as well as lack of thrombus in the portal vein. The studies did not place a limit on tumour size, although initially Belli limited laparoscopic surgery to 5 cm lesions, which was extended to larger than 5 cm in the final year of their study[32]. All studies excluded patients with a Childs-Pugh score of C or greater, except Truant[33] who also excluded Childs-Pugh score of B (Table 1).
Belli limited the laparoscopic procedure to exophytic or subcapsular tumours localized to the left (Couinaud[34] segments II, III, IVb) or peripheral right (segments V, VI) segments[32]. Similarly, Truant limited the laparoscopic approach to subcapsular tumours located in the anterior or lateral segments II-VI[33]. Kanazawa[18] and Cheung[23] did not state any limitations to tumour location.
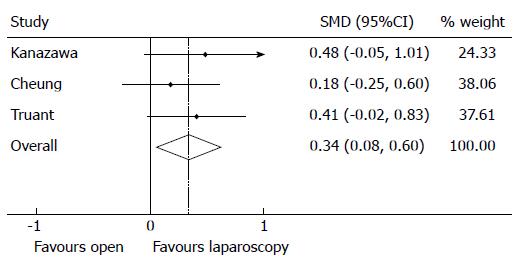
Intraoperative outcomes were subjected to meta-analysis, where appropriate. LR results in significantly larger tumour margins compared to OR (Figure 2), with an overall SMD of 0.34 (95%CI: 0.08-0.60), z-stat P = 0.011, in favour of LR with nil heterogeneity (I2 0.0% P = 0.631). Only one study (Belli[32]) reported rates of incomplete (R1) resection, reporting higher rates in the OR cohort compared to the LR cohort (8.4% vs 0% in the OR and LR cohorts respectively, P = 0.057).
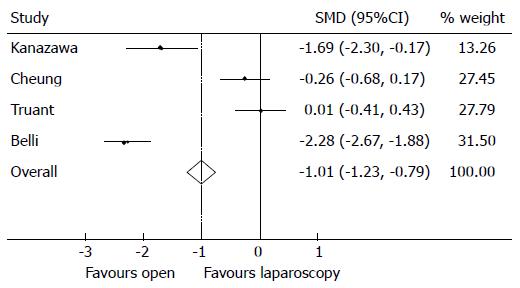
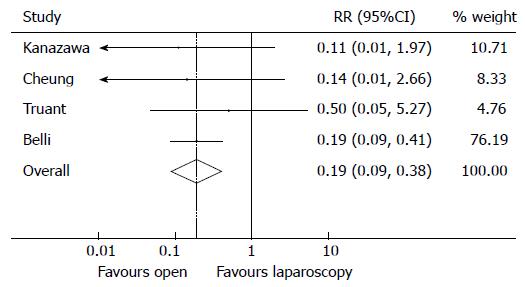
Intraoperative blood loss in the LR cohort was significantly less compared to patients undergoing OR (Figure 3) with an overall SMD of -1.01 (95%CI: -1.23-0.79), P < 0.001 in favour of open, though there was a significant degree of heterogeneity in the data reported by the included studies (I2 = 96.2%, P < 0.001). Inevitably, higher rates of blood loss resulted in patients undergoing OR requiring significantly greater rates of transfusion compared to LR (Figure 4), the overall RR was 0.19 (95%CI: 0.09-0.38), P < 0.001, I2 = 0.0% P = 0.845 in favour of open showing patients undergoing an OR are more likely to require a transfusion.
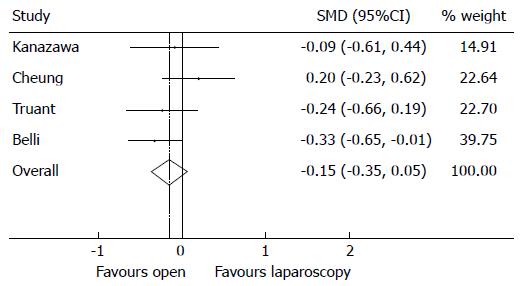
There was no statistically significant difference in operative time comparing the LR and OR techniques (Figure 5). Nonetheless, the results demonstrated a SMD of -0.15 in favour of open procedures requiring longer operative times (95%CI: 0.35-0.05), P = 0.142 with moderate heterogeneity (I2 = 24.2%, P = 0.266).
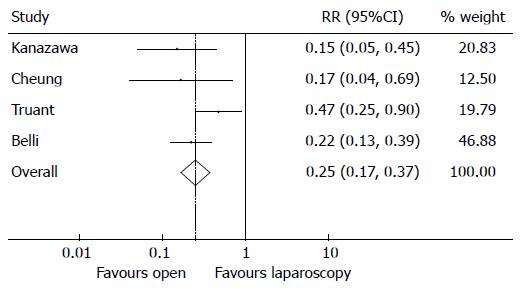
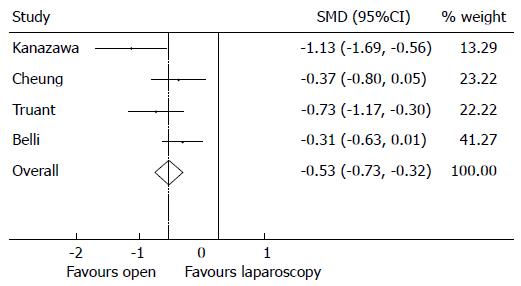
Postoperatively the OR cohort significantly suffered from higher morbidity rates compared to the LR cohort (Figure 6). The overall RR of suffering from postoperative morbidity is 0.25 in favour of the OR cohort (95%CI: 0.17-0.37), P < 0.001, with moderate heterogeneity (I2 = 41.1%, P = 0.165). The increased rates of morbidity and longer recovery times associated with OR resulted in the OR cohort having significantly longer in-hospital length of stays compared to the LR cohort (Figure 7). The SMD is -0.53 (95%CI -0.73,-0.32), P < 0.001, in favour of open with substantial heterogeneity (I2 = 59.8%, P = 0.058).
Though the heterogeneity of data reporting precluded meaningful meta-analysis, no statistically significant difference was reported across all studies with regards to both long-term survival and disease-free survival in the LR cohort compared to the OR cohort. Belli and colleagues reported 52% of the LR patients having a 3-year disease-free survival compared to 50% in the OR cohort which was not statistically significant[32]. Similarly, Truant and colleagues noted no statistically significant difference in 5-year disease-free survival between the two cohorts (35.5% vs 33.6% in the laparoscopic and open approaches respectively, P = 0.8)[33]. Comparable results were noted in overall survival rates, Cheung and colleagues noted 76.6% 5-year disease free in the LR cohort compared to 57.0% in the open cohort (P = 0.142)[23].
This review presents a summary and meta-analysis of intraoperative and postoperative outcomes of patients with known cirrhosis undergoing resection for HCC, comparing results for open and laparoscopic approaches. It suggests that a laparoscopic approach, compared to open surgery, may result in improved short-term outcomes in the form of wider resection margins, reduced intraoperative blood loss and need for transfusions, as well as reduced morbidity rates and shorter lengths of stay.
Laparoscopic techniques are known to provide reduced surgical trauma compared to open approaches and is associated with a reduction in postoperative pain, morbidity and in-hospital length of stay[35]. Laparoscopic surgery is now common practice for many oncological resections and has been shown to help with enhanced post-operative recovery, widened tumour margins and reduced intraoperative haemorrhage[36].
With the rapid development of surgical procedures and equipment for laparoscopic hepatic surgery[37], minimally invasive surgery is now common practice for liver surgery. This study suggests that the advantages of laparoscopy also apply in patients with known cirrhosis.
The progression of laparoscopic hepatic procedures, it has been suggested, has proceeded at a slower rate compared to other surgical procedures due to the perceived technical difficulties associated with maintaining haemostasis at the transection plane[38]. Though the main indication noted for conversion to open in the studies reviewed was due to uncontrollable haemorrhage, this review discounts the belief that a laparoscopic approach will lead to greater blood loss compared to the open technique. The meta-analysis presented here demonstrated significantly reduced volumes of blood loss in the LR cohort compared to open (SMD of -1.01, 95%CI: -1.23-0.79, P < 0.001). This could possible be related to the laparoscopic surgery allowing for smaller incisions to perform the operation, as well as the development of high-definition laparoscopic devices which allow magnification, enable the surgeons to obtain a decent view for performing haemostasis[23].
Additionally, due to the complex vasculature, clotting abnormalities and development of ascites, laparoscopic resection in cirrhotic livers has taken longer to receive endorsement by the wider surgical community. This review, however, suggests that laparoscopic surgery for HCC in cirrhotic livers is safe. Moreover, it suggests that laparoscopic procedures may, in fact, also provide oncological benefits compared to open approach. In a recent study by Shi and colleagues, it was shown that a resection margin of 2 cm provided better long-term outcomes for HCC compared to the traditional 1 cm[39]. The results of this meta-analysis have shown that surgeons performing laparoscopic procedures returned wider histological tumour margins following resection when compared to the open approach. Similarly to laparoscopic surgery resulting in reduced blood loss compared to the open approach, laparoscopic surgery, through high definition magnification, may provide easier assessment of affected tissue and aid the surgeon to resect a tumour-free wide margin. This can further be aided through the routine use of laparoscopic ultrasound during laparoscopic resection[40]. Moreover, reduced blood loss leads to a reduced need for transfusion in the laparoscopic approach when compared to the open technique. The post-operative recovery appears to be quicker in the LR cohort as indicated by reduced lengths of stay as well as reduced morbidity compared to open resection. Not only does this benefit patient outcomes and recovery, but also benefits healthcare systems economically by reducing the length of stay and cost of care.
There are limitations to this review which must be considered. To date, there have only been a small number of studies comparing laparoscopic hepatic resection for HCC specifically in patients with cirrhosis, with a lack of randomised trials. Though there was no reported significant difference in tumour size or patient demographics in the assessed studies, this cannot rule out the possibility of selection bias. Furthermore, the size of the cohort samples was relatively small, reducing the quality of conclusions reached. The quality of the studies included, assessed using the NOS was of moderate standard. As all studies were nonrandomised cohorts from single centres, an element of surgeon and selection bias is possible-affecting the potential generalisability of results. Further studies, with longer-term follow-up, are required to assess long-term outcomes and disease free survival for this patient cohort. Furthermore, the fact that all included studies were cohort studies, rather than randomised trials, incurs a risk of selection bias. It is possible that certain factors, such as a tumour’s anatomic location, may have influenced the choice of procedure. However, this was not commented upon by any of the included studies, which also controlled for other tumour-related factors such as size and staging to reduce bias risk.
Although higher quality data is desirable, the currently available data suggests that laparoscopic resection of HCC in cirrhotic patients is safe and potentially provides better outcomes for patients when compared to the open approach. In the modern surgical society, laparoscopic and minimally invasive surgery has become the gold standard for many surgical procedures. Similar to other areas of surgery, this review indicates that a laparoscopic approach to hepatic resection in cirrhotic patients should be considered as standard care.
Laparoscopic surgery for hepatic procedures has been slow to develop. Initially introduced for staging procedures, it has now been implemented for uncomplicated liver resections in hepatocellular carcinoma (HCC).
Laparoscopic surgery is now common practice for many oncological resections and has been shown to help with enhanced post-operative recovery, widened tumour margins and reduced intraoperative haemorrhage.
In the modern surgical society, laparoscopic and minimally invasive surgery has become the gold standard for many surgical procedures. Similar to other areas of surgery, this review indicates that a laparoscopic approach to hepatic resection in cirrhotic patients should be considered as standard care.
Authors reviewed the currently available literature comparing laparoscopic to open resection of HCC in patients with known liver cirrhosis. This review suggests that laparoscopic resection of hepatocellular carcinoma in patients with cirrhosis is safe and may provide improved patient outcomes when compared to the open technique. It has important guiding significance on the clinical treatment of HCC in patients with known liver cirrhosis.
P- Reviewers: Chu SH, Lunia MK, Shimizu Y, Wakim-Fleming J S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
| 1. | Reynolds W. The first laparoscopic cholecystectomy. JSLS. 2001;5:89-94. [PubMed] [Cited in This Article: ] |
| 2. | Veldkamp R, Kuhry E, Hop WC, Jeekel J, Kazemier G, Bonjer HJ, Haglind E, Påhlman L, Cuesta MA, Msika S. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005;6:477-484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1691] [Cited by in F6Publishing: 1611] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 3. | Berggren U, Gordh T, Grama D, Haglund U, Rastad J, Arvidsson D. Laparoscopic versus open cholecystectomy: hospitalization, sick leave, analgesia and trauma responses. Br J Surg. 1994;81:1362-1365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 202] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 4. | Golub R, Siddiqui F, Pohl D. Laparoscopic versus open appendectomy: a metaanalysis. J Am Coll Surg. 1998;186:545-553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 226] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 5. | Peters MJ, Mukhtar A, Yunus RM, Khan S, Pappalardo J, Memon B, Memon MA. Meta-analysis of randomized clinical trials comparing open and laparoscopic anti-reflux surgery. Am J Gastroenterol. 2009;104:1548-1561; quiz 1547, 1562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5-S16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1799] [Cited by in F6Publishing: 1760] [Article Influence: 88.0] [Reference Citation Analysis (0)] |
| 7. | Cherqui D, Laurent A, Tayar C, Chang S, Van Nhieu JT, Loriau J, Karoui M, Duvoux C, Dhumeaux D, Fagniez PL. Laparoscopic liver resection for peripheral hepatocellular carcinoma in patients with chronic liver disease: midterm results and perspectives. Ann Surg. 2006;243:499-506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 236] [Cited by in F6Publishing: 254] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 8. | Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907-1917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3241] [Cited by in F6Publishing: 3212] [Article Influence: 153.0] [Reference Citation Analysis (0)] |
| 9. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [PubMed] [Cited in This Article: ] |
| 10. | Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35-S50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1691] [Cited by in F6Publishing: 1670] [Article Influence: 83.5] [Reference Citation Analysis (2)] |
| 11. | Sangiovanni A, Del Ninno E, Fasani P, De Fazio C, Ronchi G, Romeo R, Morabito A, De Franchis R, Colombo M. Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance. Gastroenterology. 2004;126:1005-1014. [PubMed] [Cited in This Article: ] |
| 12. | Benvegnù L, Gios M, Boccato S, Alberti A. Natural history of compensated viral cirrhosis: a prospective study on the incidence and hierarchy of major complications. Gut. 2004;53:744-749. [PubMed] [Cited in This Article: ] |
| 13. | Yao FY, Bass NM, Nikolai B, Davern TJ, Kerlan R, Wu V, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: analysis of survival according to the intention-to-treat principle and dropout from the waiting list. Liver Transpl. 2002;8:873-883. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 305] [Cited by in F6Publishing: 326] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 14. | Mathurin P, Raynard B, Dharancy S, Kirzin S, Fallik D, Pruvot FR, Roumilhac D, Canva V, Paris JC, Chaput JC. Meta-analysis: evaluation of adjuvant therapy after curative liver resection for hepatocellular carcinoma. Aliment Pharmacol Ther. 2003;17:1247-1261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Thompson HH, Tompkins RK, Longmire WP. Major hepatic resection. A 25-year experience. Ann Surg. 1983;197:375-388. [PubMed] [Cited in This Article: ] |
| 16. | Tanabe G, Sakamoto M, Akazawa K, Kurita K, Hamanoue M, Ueno S, Kobayashi Y, Mitue S, Ogura Y, Yoshidome N. Intraoperative risk factors associated with hepatic resection. Br J Surg. 1995;82:1262-1265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Farges O, Malassagne B, Flejou JF, Balzan S, Sauvanet A, Belghiti J. Risk of major liver resection in patients with underlying chronic liver disease: a reappraisal. Ann Surg. 1999;229:210-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 231] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 18. | Kanazawa A, Tsukamoto T, Shimizu S, Kodai S, Yamazoe S, Yamamoto S, Kubo S. Impact of laparoscopic liver resection for hepatocellular carcinoma with F4-liver cirrhosis. Surg Endosc. 2013;27:2592-2597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 19. | Violi F, Leo R, Vezza E, Basili S, Cordova C, Balsano F. Bleeding time in patients with cirrhosis: relation with degree of liver failure and clotting abnormalities. C.A.L.C. Group. Coagulation Abnormalities in Cirrhosis Study Group. J Hepatol. 1994;20:531-536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922-938. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1229] [Cited by in F6Publishing: 1147] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 21. | Garrison RN, Cryer HM, Howard DA, Polk HC. Clarification of risk factors for abdominal operations in patients with hepatic cirrhosis. Ann Surg. 1984;199:648-655. [PubMed] [Cited in This Article: ] |
| 22. | Neeff H, Mariaskin D, Spangenberg HC, Hopt UT, Makowiec F. Perioperative mortality after non-hepatic general surgery in patients with liver cirrhosis: an analysis of 138 operations in the 2000s using Child and MELD scores. J Gastrointest Surg. 2011;15:1-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 23. | Cheung TT, Poon RT, Yuen WK, Chok KS, Jenkins CR, Chan SC, Fan ST, Lo CM. Long-term survival analysis of pure laparoscopic versus open hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a single-center experience. Ann Surg. 2013;257:506-511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 192] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 24. | Aldrighetti L, Guzzetti E, Pulitanò C, Cipriani F, Catena M, Paganelli M, Ferla G. Case-matched analysis of totally laparoscopic versus open liver resection for HCC: short and middle term results. J Surg Oncol. 2010;102:82-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 25. | Ker CG, Chen JS, Kuo KK, Chuang SC, Wang SJ, Chang WC, Lee KT, Chen HY, Juan CC. Liver Surgery for Hepatocellular Carcinoma: Laparoscopic versus Open Approach. Int J Hepatol. 2011;2011:596792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Shimada M, Hashizume M, Maehara S, Tsujita E, Rikimaru T, Yamashita Y, Tanaka S, Adachi E, Sugimachi K. Laparoscopic hepatectomy for hepatocellular carcinoma. Surg Endosc. 2001;15:541-544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 159] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 27. | Kim HH, Park EK, Seoung JS, Hur YH, Koh YS, Kim JC, Cho CK, Kim HJ. Liver resection for hepatocellular carcinoma: case-matched analysis of laparoscopic versus open resection. J Korean Surg Soc. 2011;80:412-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Mirnezami R, Mirnezami AH, Chandrakumaran K, Abu Hilal M, Pearce NW, Primrose JN, Sutcliffe RP. Short- and long-term outcomes after laparoscopic and open hepatic resection: systematic review and meta-analysis. HPB (Oxford). 2011;13:295-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 151] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 29. | Yin Z, Fan X, Ye H, Yin D, Wang J. Short- and long-term outcomes after laparoscopic and open hepatectomy for hepatocellular carcinoma: a global systematic review and meta-analysis. Ann Surg Oncol. 2013;20:1203-1215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 152] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 30. | Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264-269, W64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18463] [Cited by in F6Publishing: 16685] [Article Influence: 1112.3] [Reference Citation Analysis (0)] |
| 31. | Wells G, Shea B, O’connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses 2000. Accessed June 18. 2007; Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. [Cited in This Article: ] |
| 32. | Belli G, Limongelli P, Fantini C, D’Agostino A, Cioffi L, Belli A, Russo G. Laparoscopic and open treatment of hepatocellular carcinoma in patients with cirrhosis. Br J Surg. 2009;96:1041-1048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 166] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 33. | Truant S, Bouras AF, Hebbar M, Boleslawski E, Fromont G, Dharancy S, Leteurtre E, Zerbib P, Pruvot FR. Laparoscopic resection vs. open liver resection for peripheral hepatocellular carcinoma in patients with chronic liver disease: a case-matched study. Surg Endosc. 2011;25:3668-3677. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 34. | Couinaud C. Le foie: études anatomiques et chirurgicales. Paris: Masson Cie 1957; . [Cited in This Article: ] |
| 35. | McMahon AJ, Russell IT, Ramsay G, Sunderland G, Baxter JN, Anderson JR, Galloway D, O’Dwyer PJ. Laparoscopic and minilaparotomy cholecystectomy: a randomized trial comparing postoperative pain and pulmonary function. Surgery. 1994;115:533-539. [PubMed] [Cited in This Article: ] |
| 36. | Lacy AM, García-Valdecasas JC, Delgado S, Castells A, Taurá P, Piqué JM, Visa J. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet. 2002;359:2224-2229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1901] [Cited by in F6Publishing: 1768] [Article Influence: 80.4] [Reference Citation Analysis (0)] |
| 37. | Gan P. A novel liver retractor for reduced or single-port laparoscopic surgery. Surg Endosc. 2014;28:331-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Endo Y, Ohta M, Sasaki A, Kai S, Eguchi H, Iwaki K, Shibata K, Kitano S. A comparative study of the long-term outcomes after laparoscopy-assisted and open left lateral hepatectomy for hepatocellular carcinoma. Surg Laparosc Endosc Percutan Tech. 2009;19:e171-e174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 39. | Shi M, Guo RP, Lin XJ, Zhang YQ, Chen MS, Zhang CQ, Lau WY, Li JQ. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: a prospective randomized trial. Ann Surg. 2007;245:36-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 321] [Cited by in F6Publishing: 374] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 40. | Kleemann M, Hildebrand P, Birth M, Bruch HP. Laparoscopic ultrasound navigation in liver surgery: technical aspects and accuracy. Surg Endosc. 2006;20:726-729. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |