Published online Sep 7, 2014. doi: 10.3748/wjg.v20.i33.11815
Revised: February 23, 2014
Accepted: May 19, 2014
Published online: September 7, 2014
AIM: To assess whether non invasive blood flow measurement by arterial spin labeling in several brain regions detects minimal hepatic encephalopathy.
METHODS: Blood flow (BF) was analyzed by arterial spin labeling (ASL) in different brain areas of 14 controls, 24 cirrhotic patients without and 16 cirrhotic patients with minimal hepatic encephalopathy (MHE). Images were collected using a 3 Tesla MR scanner (Achieva 3T-TX, Philips, Netherlands). Pulsed ASL was performed. Patients showing MHE were detected using the battery Psychometric Hepatic Encephalopathy Score (PHES) consisting of five tests. Different cognitive and motor functions were also assessed: alterations in selective attention were evaluated using the Stroop test. Patients and controls also performed visuo-motor and bimanual coordination tests. Several biochemical parameters were measured: serum pro-inflammatory interleukins (IL-6 and IL-18), 3-nitrotyrosine, cGMP and nitrates+nitrites in plasma, and blood ammonia. Bivariate correlations were evaluated.
RESULTS: In patients with MHE, BF was increased in cerebellar hemisphere (P = 0.03) and vermis (P = 0.012) and reduced in occipital lobe (P = 0.017). BF in cerebellar hemisphere was also increased in patients without MHE (P = 0.02). Bimanual coordination was impaired in patients without MHE (P = 0.05) and much more in patients with MHE (P < 0.0001). Visuo-motor coordination was impaired only in patients with MHE (P < 0.0001). Attention was slightly affected in patients without MHE and more strongly in patients with MHE (P < 0.0001). BF in cerebellar hemisphere and vermis correlated with performance in most tests of PHES [(number connection tests A (NCT-A), B (NCT-B)and line tracing test] and in the congruent task of Stroop test. BF in frontal lobe correlated with NCT-A. Performance in bimanual and visuomotor coordination tests correlated only with BF in cerebellar hemisphere. BF in occipital lobe correlates with performance in the PHES battery and with CFF. BF in cerebellar hemisphere correlates with plasma cGMP and nitric oxide (NO) metabolites. BF in vermis cerebellar also correlates with NO metabolites and with 3-nitrotyrosine. IL-18 in plasma correlates with BF in thalamus and occipital lobe.
CONCLUSION: Non invasive BF determination in cerebellum using ASL may detect MHE earlier than the PHES. Altered NO-cGMP pathway seems to be associated to altered BF in cerebellum.
Core tip: Patients with minimal hepatic encephalopathy (MHE) show neurological impairment in specific tasks to which selective regional alterations in blood flow (BF) could contribute. Arterial spin labeling (ASL), a non-invasive magnetic resonance technique, quantitatively measures cerebral perfusion. We analyzed BF by ASL in different brain areas of controls and cirrhotic patients without and with MHE. We found that BF is more affected in cerebellum than in other areas of cirrhotic patients and that BF determination in cerebellum using ASL may detect MHE earlier than the Psychometric Hepatic Encephalopathy Score battery. Altered nitric oxide-cGMP pathway seems to be associated to altered BF in cerebellum.
- Citation: Felipo V, Urios A, Giménez-Garzó C, Cauli O, Andrés-Costa MJ, González O, Serra MA, Sánchez-González J, Aliaga R, Giner-Durán R, Belloch V, Montoliu C. Non invasive blood flow measurement in cerebellum detects minimal hepatic encephalopathy earlier than psychometric tests. World J Gastroenterol 2014; 20(33): 11815-11825
- URL: https://www.wjgnet.com/1007-9327/full/v20/i33/11815.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i33.11815
Patients with minimal hepatic encephalopathy (MHE) show selective alterations in specific functions and tasks such as visuo-spatial orientation and perception, complex tasks requiring attention and motor abilities[1-5]. This suggests that the brain areas modulating these tasks are more affected than others in patients with liver disease and MHE. The reasons for this “region selective” sensitivity may include selective alterations in blood flow (BF)[6].
Invasive positron emission tomography (PET) and single photon emission computed tomography (SPECT) techniques have shown altered cerebral blood flow (CBF) patterns in cirrhotic patients, but there is a large variability between the results published[6-9]. Some studies show redistribution of the BF from cortex to thalamus and cerebellum[6]. However, Iwasa et al[7,8] reported reduced BF both in cortex, cerebellum and cingulate gyrus of cirrhotic patients. These differences may reflect different grades in the progression of the disease.
The possible contribution of alterations in BF in specific brain areas to neurological alterations in patients with MHE remains unclear. Ahl et al[6] found that there was no association between changes in CBF and neuropsychiatric status. However, Catafau et al[9] reported a correlation between altered BF in basal ganglia and reduced performance in the “Luria Motor Alternances” and “Purdue Pegboard” tests. Iwasa et al[7,8] did not find correlations between reduced BF in frontal lobe and neuropsychological alterations, but later reported a correlation between reduced BF in cingulate gyrus and performance in psychometric tests. This suggests that BF alterations in some specific regions could be involved in the mechanisms leading to certain cognitive and motor alterations.
The above studies were performed using invasive techniques. It would be useful to have non-invasive procedures to analyze regional CBF and evaluate if BF in some region is useful to detect MHE and/or specific neurological alterations. This would allow early treatment and follow-up of the eficiency of therapeutic treatments.
Arterial spin labeling (ASL) is a completely non-invasive magnetic resonance technique that quantitatively measures cerebral perfusion by magnetically labeling protons in arterial blood water. The labeled protons travel through the vascular tree and exchange water with non labeled cerebral tissue. The perfusion cerebral image is obtained by substracting the images with the labeled and unlabeled protons[10].
An important modulator of CBF is the nitric oxide (NO)-cGMP system[11], which also modulates some forms of learning and memory. Altered function of the glutamate-NO-cGMP pathway in brain is responsible for some types of cognitive impairment in animal models of MHE. Normalizing this system by pharmacological treatments restores learning ability[12-14]. It is therefore likely that altered NO-cGMP system could contribute to alter BF in some brain areas which could contribute to cognitive impairment. Detection of altered CBF by magnetic resonance could serve as an indicator of mild cognitive impairment in MHE. Identifying some parameter in blood reflecting altered CBF in patients with liver disease would be also useful.
The aims of this work were to: (1) analyze by ASL the BF in brain areas of patients with liver cirrhosis; (2) assess whether patients with or without MHE show similar or different alterations; (3) assess if BF in some brain area may be a good indicator of MHE or of specific neurological alterations; and (4) assess whether some peripheral parameter correlates with BF. As possible peripheral indicators we determined parameters of the NO-cGMP system in blood. We also measured ammonia levels, parameters related with inflammation and 3-nitrotyrosine[15].
Forty patients with liver disease and 14 controls were enrolled after written informed consent. Inclusion criteria: patients were recruited from the outpatient clinics at Hospitals Clinico Universitario and Arnau de Vilanova, in Valencia and were included if they had clinical, biochemical and histological evidence of liver cirrhosis. For controls liver disease was discarded by clinical, analytical and serologic analysis. Patients were excluded if they had evidence of overt hepatic encephalopathy (HE) by the West Haven criteria, decompensate diabetes, renal dysfunction, hyponatremia, neurological disease, cardiovascular disease or antibiotic use. Patients had to be abstinent from alcohol for six months. Patients were not on any specific therapy for HE. After a standard history and physical examination, blood was drawn for biochemical measurements.
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Ethical Committee of the Hospital. After performing the psychometric tests, patients were classified as without or with MHE (see below). The study includes therefore three groups: (1) controls; (2) patients without MHE; and (3) patients with MHE. MRI examination was performed one week after the psychometric tests. The composition of groups, age, analytical data and aetiology of the disease are in Table 1.
| Range | Control | Patients without MHE | Patients with MHE | |
| Total individuals | 14 | 24 | 16 | |
| Age | 55 ± 9 | 58 ± 6 | 65 ± 9 | |
| Alcohol | 24 | 16 | ||
| Ascitis | 2 | 5 | ||
| Child Pugh A/B/C | 21/3/0 | 11/3/2 | ||
| MELD | 8.9 ± 3.5 | 9.5 ± 3.4 | ||
| AST (mU/mL) | 1-37 | 20 ± 4.0 | 73 ± 56b | 82.5 ± 58b |
| ALT (mU/mL) | 1-41 | 18 ± 6.0 | 77 ± 24b | 90.1 ± 24b |
| GGT (mU/mL) | 10-49 | 26.7 ± 5.0 | 86.4 ± 60b | 106 ± 64b |
| Uric acid (mg/dL) | 2.5-7 | 4.0 ± 1.0 | 6.2 ± 2.0 | 5.73 ± 2.3 |
| Creatinine (mg/dL) | 0.5-1.3 | 0.92 ± 0.1 | 1.1 ± 0.2 | 1.2 ± 0.2 |
| Cholesterol (mg/dL) | 140-200 | 172 ± 22 | 175 ± 44 | 167 ± 55 |
| Triglycerides (mg/dL) | 40-160 | 95 ± 32 | 111 ± 64 | 119 ± 64 |
| Bilirubin (mg/dL) | 0.1-1 | 0.6 ± 0.2 | 1.7 ± 0.7b | 2.3 ± 0.6b |
| Albumin (g/dL) | 3.5-5 | 4.4 ± 0.2 | 3.7 ± 0.6b | 2.9 ± 0.6bc |
| Prothrombin time (s) | 13 ± 1.3 | 24 ± 4b | 30 ± 4b | |
| Fibrinogen (g/L) | 2-4 | 3.1 ± 1.0 | 3.3 ± 1.3 | 3.6 ± 1.2 |
| Alkaline phosphatase (mU/mL) | 50-250 | 147 ± 53 | 216 ± 77b | 314 ± 96bc |
| Erythrocytes | 4.2-6.1 | 4.6 ± 0.4 | 4.3 ± 0.7 | 3.4 ± 0.6 |
| Leucocytes | 4.8-10.8 | 6.5 ± 1.3 | 6 ± 2.6 | 5.5 ± 2.0 |
| Neutrophils (%) | 55-75 | 55 ± 7.4 | 54 ± 6.2 | 59 ± 9.3 |
| Lymphocytes (%) | 17-45 | 35 ± 6.0 | 29 ± 10 | 27 ± 9.4 |
| Monocytes (%) | 2-8 | 6.0 ± 1.3 | 8.4 ± 3.0b | 10 ± 2.6b |
| Eosinophils (%) | 1-4 | 3.3 ± 2.0 | 2.4 ± 1.2 | 1.7 ± 1.0 |
| Basophils (%) | 0.05-0.5 | 0.5 ± 0.2 | 0.6 ± 0.3 | 0.6 ± 0.1 |
Diagnosis of MHE: Patients showing MHE were detected using the battery Psychometric Hepatic Encephalopathy Score (PHES). Classification of patients as “with” or “without” MHE was based only on PHES performance, considered as the “gold standard”[16]. PHES comprises 5 psychometric tests: digit symbol test (DST) evaluates processing speed and working memory, number connection tests A (NCT-A) and B (NCT-B) mental processing speed and attention; serial dotting test (SD) and line tracing test (LTT) visuo-spatial coordination. The scores were adjusted for age and education level using Spanish normality tables (http://www.redeh.org). Patients were classified as having MHE when the score was less than −4 points[17].
Evaluation of attention with the Stroop test: The Stroop task evaluates selective attention. We used a colour-word version of the Stroop task[18]. Each individual performed sequentially the congruent, neutral and incongruent tasks, 45 s per task. The number of items correctly named was quantified and adjusted by age[19].
Visuo-motor coordination: This task was performed as described in reference[20].
Bimanual coordination: This task was performed as described in reference[21].
Critical flicker frequency: The CFF was measured as in reference references[22,23].
Venous blood (5 mL) was taken in BD Vacutainer tubes with or without EDTA (plasma and serum, respectively) and centrifuged at 500 g for 10 min. The supernatant was collected, and stored frozen at -80 °C.
Nitrates + nitrites in plasma were measured as previously[24]. Serum IL-6 and IL-18 were determined using ELISA kits (Pierce Biotechnology, United States) and Bender MedSystems GmbH (Vienna, Austria), respectively. Plasma cGMP was determined using the BIOTRAK™ cGMP enzyme immunoassay kit (GE Healthcare, United Kingdom). Ammonia was determined with the Ammonia Test Kit II (Arkay, Inc., Kyoto, Japan). 3-nitrotyrosine in serum was determined by HPLC as in reference[15].
Images were collected using a 3 Tesla MR scanner (Achieva 3T-TX, Philips, the Netherlands). Pulsed ASL was performed using EPISTAR strategy for tagging and control images with 150 mm thick slab of tagging pulse 30 mm far from the imaging region. The plane resolution was 3.5 mm × 3.5 mm. Multi-Slice (10 slices) multiphase (11 phases) acquisition was performed with a phase interval of 350 ms. No rest slabs or fat suppression pulses were applied during acquisition. Thirty repetitions were acquired in order to increase the signal to noise ratio. ASL data were processed using homemade analysis software written in Interactive Data Language 6.3 (Research System, Inc.). This analysis program fit the multi-phase data of every pixel to the Günther model[25] for a lock-looker ASL acquisition, and estimation of blood flow was performed with equation [A2] from reference[25]. The analysis program calculated automatically the M0 image from ASL data. After image analysis CBF maps are generated.
Values are given as mean ± SEM. Results were analysed by one-way ANOVA followed by post-hoc Bonferroni’s multiple comparison test. Differences between groups were analyzed by Games-Howell test for multiple comparisons in the parameters with non-homogeneous variances. Adjusted P values are shown. Variables that were not previously age-adjusted (bimanual coordination and visuo-motor coordination) were analysed using univariate analysis of covariance (ANCOVA) with age included as covariate, followed by post-hoc Bonferroni. Bivariate correlations among variables were evaluated using the Pearson correlation test. The diagnostic performance for MHE was assessed using a logistic regression analysis, followed by receiver operating characteristic (ROC) curve to determine sensitivity and specificity and to identify the optimal threshold value. Analyses were performed using SPSS Version 19.0 (SPSS Inc, Chicago, United states) and two-sided P values < 0.05 were considered significant.
Sixteen (40%) of the 40 patients showed MHE according to PHES performance. The composition of groups, age, analytical data and aetiology of the disease are in Table 1.
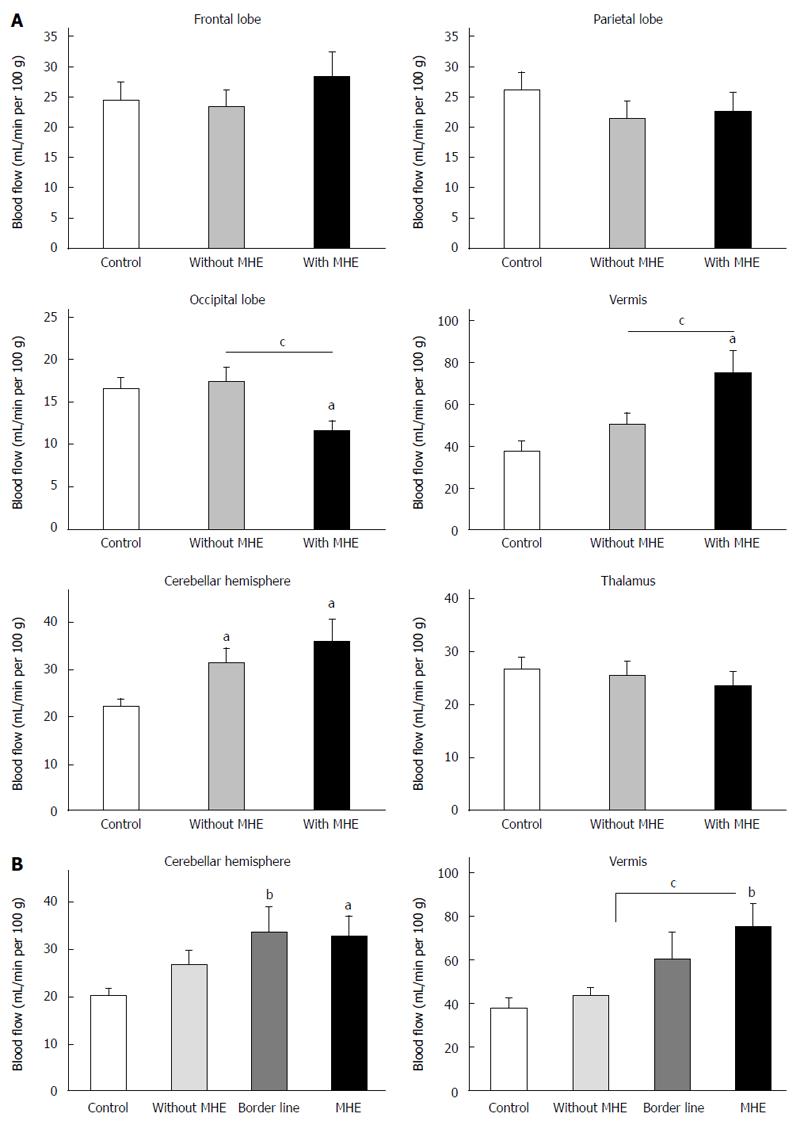
BF was increased in cerebellar hemisphere and vermis in patients with MHE and reduced in occipital lobe (Figure 1). BF in cerebellar hemisphere was 22 ± 2 mL/min per 100 g in controls, 32 ± 3 mL/min per 100 g in patients without MHE (P = 0.02) and increased further in patients with MHE (36 ± 5 mL/min per 100 g) compared to control group (P = 0.03). BF in vermis were 38 ± 5 and 51 ± 5 mL/min per 100 g in controls and cirrhotics without MHE respectively, and increased in patients with MHE (75 ± 11 mL/min per 100 g) respect to control group (P = 0.012) and to patients without MHE (P = 0.03). In occipital lobe, BF was 16.6 ± 1.2 mL/min per 100 g in controls and 17.4 ± 1.7 mL/min per 100 g in cirrhotics without MHE, and decreased in patients with MHE (11.7 ± 1.1 mL/min per 100 g) compared to controls (P = 0.017) and to patients without MHE (P = 0.02).
Cirrhotic patients with or without MHE show increased blood levels of cGMP, NO metabolites, 3-nitrotyrosine, IL-6, IL-18 and ammonia (Table 2). The increase in cGMP (P < 0.05), 3-nitrotyrosine (P < 0.0001), IL-6 (P = 0.001) and IL-18 (P < 0.05) was higher in patients with than without MHE (Table 2).
| Parameter | Controls(n = 14) | Patients without MHE(n = 24) P vs control | Patients with mHE(n = 16) P vs control | Patients with MHEPvs without MHE | P values |
| cGMP in plasma (pmol/mL) | 0.64 ± 0.1 | 6.1 ± 0.6 | 8.7 ± 1.1 | ||
| P < 0.0001 | P < 0.0001 | < 0.05 | < 0.0001 | ||
| Nitrates + Nitrites (μmol/L) | 18 ± 1 | 38 ± 4 | 44 ± 9 | ||
| P = 0.004 | P = 0.004 | NS | 0.001 | ||
| 3-Nitro-tyrosine (nmol/L) | 3.5 ± 1.2 | 10 ± 1 | 43 ± 6 | < 0.0001 | < 0.0001 |
| P < 0.0001 | |||||
| IL-6 (pg/mL) | 0.7 ± 0.2 | 2.3 ± 0.3 | 4.4 ± 0.5 | 0.001 | < 0.0001 |
| P < 0.05 | P < 0.0001 | ||||
| IL-18 (pg/mL) | 103 ± 13 | < 0.05 | < 0.0001 | ||
| 268 ± 39 | 404 ± 44 | ||||
| P = 0.02 | P < 0.0001 | ||||
| Ammonia (μmol/L) | 39 ± 4 | 84 ± 14 | 103 ± 33 | NS | 0.005 |
| P = 0.04 | P = 0.006 |
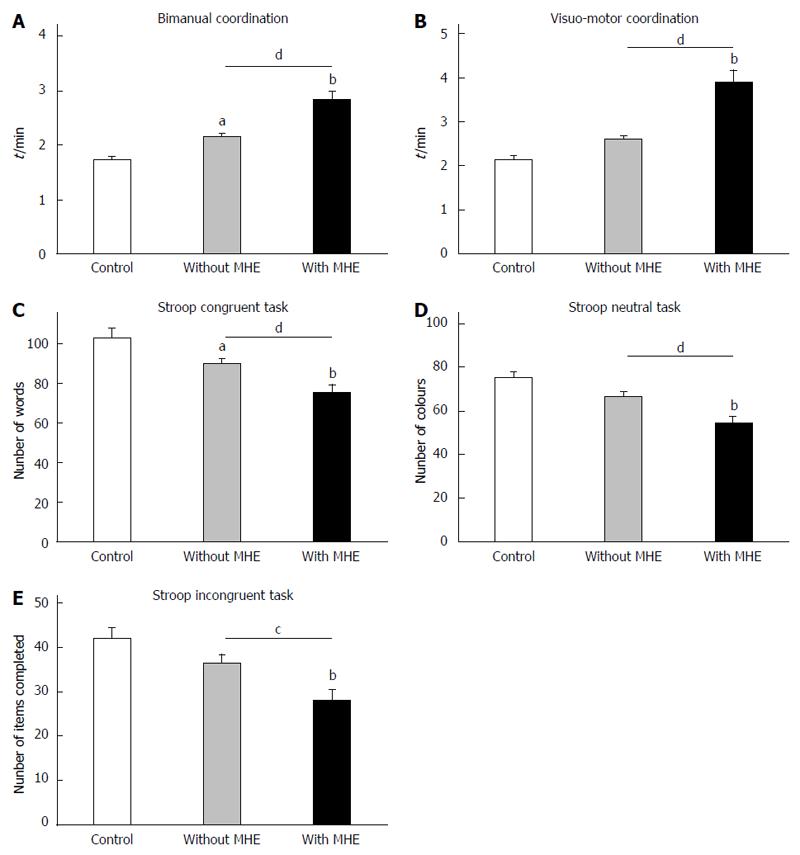
Controls completed the bimanual coordination test in 1.73 ± 0.05 min. Patients without MHE needed 2.16 ± 0.05 min (P = 0.05) and patients with MHE 2.8 ± 0.1 min, more than controls (P < 0.0001) or patients without MHE (P < 0.001) (Figure 2A, Table 3). In the visuo-motor coordination test, controls completed the task in 2.15 ± 0.08 min. The score was not affected in patients without MHE (2.61 ± 0.08 min). Patients with MHE needed more time (3.9 ± 0.3 min, P < 0.0001) (Figure 2B, Table 3). In the congruent task of the Stroop test of selective attention, controls read 102 ± 5 words. Patients without MHE read 90 ± 2 words (P = 0.03) and patients with MHE even less words (76 ± 4) which was lower than for controls (P < 0.0001) and patients without MHE (P = 0.009) (Figure 2C, Table 3). In the neutral task controls named 75 ± 3 colours. Patients without MHE named 67 ± 2 colours (P = 0.07) and patients with MHE 54 ± 3 colours, which was lower than for controls (P < 0.0001) and patients without MHE (P = 0.003) (Figure 2D, Table 3). In the incongruent task, controls named 42 ± 2 colours. Patients without MHE 36 ± 2 colours and patients with MHE 28 ± 2 colours, which was lower than for controls (P = 0.001) and patients without MHE (P = 0.03) (Figure 2E, Table 3). Performance in the 5 individual tests of the PHES battery is shown in Table 3.
| Test | Sub-test | Controls(n = 14) | Patients without MHE(n = 24)P vs Control | Patients with MHE(n = 16)P vs control | Patients with MHEP vs without MHE | P values |
| CFF | 43.2 ± 0.7 | 40.5 ± 0.7 | 36 ± 1 | P = 0.001 | < 0.001 | |
| P < 0.001 | ||||||
| PHES Global score | -0.13 ± 0.25 | -1.23 ± 0.30 | -6.31 ± 0.72 | |||
| P < 0.001 | P < 0.001 | < 0.001 | ||||
| Symbol digit test | -0.07 ± 0.12 | -0.11 ± 0.08 | -0.87 ± 0.12 | |||
| P < 0.001 | P < 0.001 | < 0.001 | ||||
| NCT-A | -0.07 ± 0.15 | -0.04 ± 0.07 | -1.19 ± 0.28 | |||
| P < 0.001 | P < 0.001 | < 0.001 | ||||
| NCT-B | -0.07 ± 0.18 | -0.27 ± 0.14 | -2.07 ± 0.30 | |||
| P < 0.001 | P < 0.001 | < 0.001 | ||||
| Serial dotting test | 0.00 ± 0.00 | -0.57 ± 0.17 | -1.19 ± 0.29 | NS | ||
| P = 0.001 | 0.002 | |||||
| Line tracing test | -0.07 ± 0.07 | -0.15 ± 0.07 | -1.25 ± 0.33 | P < 0.001 | ||
| P < 0.001 | < 0.001 | |||||
| Stroop | Congruent task | 102 ± 5 | 90 ± 2 | 76 ± 4 | ||
| P = 0.03 | P < 0.0001 | P = 0.009 | < 0.0001 | |||
| Neutral Task | 75 ± 3 | 67 ± 2 | 54 ± 3 | |||
| P < 0.0001 | P = 0.003 | < 0.0001 | ||||
| Incongruent Task | 42 ± 2 | 36 ± 2 | 28 ± 2 | P = 0.030 | 0.001 | |
| P = 0.001 | ||||||
| Bimanual coordination | 1.73 ± 0.05 | 2.16 ± 0.05 | 2.83 ± 0.15 | |||
| P = 0.05 | P < 0.0001 | P < 0.0001 | < 0.0001 | |||
| Visuo-motor coordination | 2.15 ± 0.08 | 2.61 ± 0.08 | 3.89 ± 0.28 | < 0.0001 | ||
| P < 0.0001 | P < 0.0001 |
BF in cerebellar hemisphere and vermis correlated with performance in most tests of PHES (NCT-A, NCT-B and line tracing test) and in the congruent task of Stroop test. BF in frontal lobe correlated with NCT-A (Table 4). Performance in bimanual and visuomotor coordination tests correlated only with BF in cerebellar hemisphere. BF in occipital lobe correlates with global performance in the PHES battery and with CFF but not with any individual psychometric tests (Table 4). Global performance in the PHES correlated with BF in frontal lobe (P = 0.007, r = -0.36), occipital lobe (P = 0.031, r = 0.309), cerebellar hemisphere (P = 0.01, r = -0.348), and especially in vermis (P < 0.001, r = -0.504).
| Studied parameter/test | Sub-test | Frontal lobe | Parietal lobe | Occipital | Vermis | Cerebellar hemisphere | Thalamus |
| CFF | NS | NS | r = 0.353 | r = -0.326 | NS | NS | |
| P = 0.017 | P = 0.025 | ||||||
| PHES Global score | r = -0.36 | NS | r = 0.309 | r = -0.504 | r = -0.348 | NS | |
| P = 0.007 | P = 0.031 | P < 0.001 | P = 0.01 | ||||
| Symbol digit test | NS | NS | NS | NS | NS | NS | |
| NCT-A | r = -0.469 | NS | NS | r = -0.530 | r = -0.320 | NS | |
| P < 0.001 | P < 0.001 | P = 0.018 | |||||
| NCT-B | NS | NS | NS | r = -0.531 | r = -0.307 | NS | |
| P < 0.001 | P = 0.027 | ||||||
| Serial dotting test | NS | NS | NS | NS | NS | NS | |
| Line tracing test | NS | NS | NS | r = -0.330 | r = -0.364 | NS | |
| P = 0.018 | P = 0.007 | ||||||
| STROOP | Congruent task | NS | NS | NS | r = -0.320 | r = -0.354 | NS |
| P = 0.027 | P = 0.012 | ||||||
| Neutral task | NS | NS | NS | NS | NS | NS | |
| Incongruent task | NS | NS | NS | NS | NS | ||
| NS | |||||||
| Bimanual coordination | NS | NS | NS | NS | r = 0.362 | NS | |
| P = 0.011 | |||||||
| Visuo-motor coordination | NS | NS | NS | NS | r = 0.335 | NS | |
| P = 0.017 | |||||||
| cGMP in plasma (pmoles/mL) | NS | NS | NS | NS | r = 0.301 | NS | |
| P = 0.034 | |||||||
| Nitrates + Nitrites (mmol/L) | NS | NS | NS | r = 0.339 | r = 0.362 | NS | |
| P = 0.043 | P = 0.023 | ||||||
| 3-Nitro-tyrosine (nmol/L) | NS | NS | NS | r = 0.358 | NS | NS | |
| P = 0.011 | |||||||
| IL-6 (pg/mL) | NS | NS | NS | NS | NS | NS | |
| IL-18 (pg/mL) | NS | NS | r = -0.353 | NS | NS | r = -0.405 | |
| P = 0.041 | P = 0.032 | ||||||
| Ammonia (mmol/L) | NS | NS | NS | NS | NS | NS |
BF in cerebellar hemisphere correlates with plasma cGMP and NO metabolites (Table 4). BF in vermis cerebellar also correlates with NO metabolites and with 3-nitrotyrosine. IL-18 in plasma correlates with BF in thalamus and occipital lobe (Table 4).
To assess whether altered BF may be useful to predict MHE we performed logistic regression analyses. Univariate logistic regression using the presence of MHE as the dependent variable and blood flow in vermis as independent variable shows that BF significantly predicts MHE, with an OR of 1.042 (95%CI: 1.006-1.078, P = 0.021).
ROC curve analysis were performed to determine the cut-off, the area under the curve (AUC), and specificity and sensitivity for MHE detection. The ROC curve has an AUC of 0.714 (95%CI: 0.54-0.88, P = 0.027) for BF in vermis. At the cut-off of 46 mL/min per 100 g, the specificity was 57% and the sensitivity was 69%. Although these values are not excellent for diagnosis, the data show that BF in cerebellum is altered in patients with MHE and also in some patients without MHE who also show impaired bimanual coordination. This supports that altered BF in cerebellum detects some motor deficits earlier that the PHES battery. To get further insight on this matter, we sub-classified the patients showing a PHES of -3 (who are usually classified as without MHE) as “borderline” patients. There were 7 patients with PHES-3. BF in vermis was 60 ± 12 mL/min per 100 g, which was nearly significantly different (P = 0.055) from controls (Figure 1B). BF in cerebellar hemisphere of these “borderline patients” was 37 ± 6 mL/min per 100 g which was significantly different (P < 0.01) from controls and not different from patients with MHE (Figure 1B). This supports that altered BF in cerebellum detects some motor deficits earlier than the PHES battery in its present form.
The results reported show that CBF is increased selectively in the cerebellar vermis and reduced in the occipital lobe in patients with MHE compared with patients without MHE. CBF was increased in cerebellar hemispheres in cirrhotic patients irrespective of the presence of MHE as defined according to PHES. This suggests that (1) cerebellar hemisphere is the most sensitive region in cirrhotic patients; and (2) non invasive determination of BF in cerebellum using ASL would be useful for early detection of MHE even before it is detectable with PHES.
Treatment of HE is associated with lower hospitalization frequency and duration, better clinical status, and fewer adverse events[26]. We show that non invasive BF determination in cerebellum by ASL would allow early detection and treatment of MHE. This would reduce societal costs by reducing the number of motor vehicle accidents[27], improve quality of life and prevent progression to overt HE.
The data reported show that some motor alterations may appear before MHE is detectable using the PHES battery. This agrees with previous reports suggesting that impairment of some motor coordination functions are early markers for cerebral dysfunction in some patients with MHE even prior to neuropsychometric alterations becoming detectable[4]. These motor functions are mainly modulated in cerebellum, supporting that cerebellar alterations would contribute to these early alterations.
In addition to increased BF in cerebellar hemisphere, patients with MHE also show increased BF in cerebellar vermis and reduced BF in occipital lobe. Changes in BF in specific cortical areas are sensitive early markers of progression of mild cognitive impairment in Alzheimer’s disease[28]. Changes in regional BF could also serve as markers of the presence and progression of MHE. Altered BF may contribute to neurological impairment and to impair performance in the PHES and in bimanual and visuo-motor coordination and/or in attention tests. BF in cerebellar hemisphere correlates with performance in most tests, suggesting that increased BF in cerebellar hemisphere may be an early relevant contributor to neurological impairment in MHE.
BF in cerebellar hemisphere is also increased in cirrhotic patients without MHE, who also show impaired bimanual coordination and performance in the congruent task in the Stroop test. This suggests that increased BF in cerebellar hemisphere is an early event which may contribute to neurological deterioration and reduced performance in bimanual coordination and the congruent Stroop task and predisposing to impair visuomotor coordination, executive functioning (NCT-B) and cognitive processing speed (NCT-A).
BF in cerebellar hemisphere and vermis correlate with performance in NCT-A and NCT-B (also known as trail making test). NCT-B examines executive functioning and NCT-A cognitive processing speed[29]. Cerebellar alterations result in impaired executive function and performance in NCT test in schizophrenia[30,31], Parkinsonism[32] or Cerebellar Cognitive Affective Syndrome[33]. Altered BF in cerebellum may contribute to impair executive functioning and performance in NCT tests in MHE.
Bimanual coordination correlates with BF in cerebellar hemisphere but not in other areas. This agrees with functional magnetic resonance imaging studies showing that cerebellum is a critical site for the control of bimanual coordination[34] and that intercerebellar coupling is key for execution of simultaneous bimanual movements[35]. Moreover, both bimanual coordination and BF in cerebellar hemisphere are impaired in patients without MHE according to PHES, supporting the idea that altered BF in cerebellar hemisphere would contribute to impair bimanual coordination.
The data reported show that patients which are classified as “without MHE” according to the PHES battery have significantly reduced bimanual coordination. We have previously shown that patients who do not show MHE as detected using the PHES already have some psychomotor slowing and impaired bimanual coordination, indicating that some mild neurological alterations are not detected with the PHES but can be detected by more sensitive procedures[5]. Butz et al[4] also showed that ataxia, tremor, and slowing of finger movements are early markers for cerebral dysfunction in at least a subgroup of cirrhotic patients even prior to alterations in performance in the PHES become detectable. These data suggest that, although the PHES battery is currently the gold standard for detection of the presence of MHE, and it has been very useful to unify the assessment of neurological alterations in cirrhotic patients, is not sensitive enough to detect some mild alterations in motor coordination and mental processing speed.
Alterations in both bimanual coordination and BF in cerebellum are early events, occurring before altered performance in the PHES, this suggests that bimanual coordination tests should be also performed for early MHE detection and that non invasive determination of BF in cerebellum using ASL would detect MHE earlier than the currently used PHES battery.
Visuo-motor coordination also correlates with BF in cerebellar hemisphere but not in other areas. This could be expected, as cerebellum is crucial in visuomotor coordination[36]. This further supports that many functions modulated by cerebellum are affected in patients with MHE and that cerebellum is more susceptible than other areas in cirrhotic patients.
Magnetic resonance studies show that cerebellum is involved in the Stroop task[37]. Nabeyama et al[37] showed that in patients with obsessive-compulsive disorder reduced performance in the Stroop test is associated with reduced cerebellum activation. Moreover, patients with Cerebellar Cognitive Affective Syndrome show reduced performance in the Stroop test[33]. This suggests that altered BF in cerebellum could also contribute to impair performance in the Stroop test in patients with MHE.
To shed some initial insight on possible mechanisms involved in altered CBF in each area in MHE we also assessed the correlations between CBF and some biochemical parameters in blood. Ammonia or IL-6 levels do not correlate with CBF in any area, suggesting that hyperammonemia and IL-6 related inflammation are not main direct contributors to alterations in CBF. Serum IL-18 correlates with CBF in occipital lobe and thalamus, but not in cerebellum. This suggests that some inflammatory factors would contribute to alter CBF in these areas but not in cerebellum.
CBF in cerebellar hemisphere correlates with NO metabolites and cGMP, suggesting an association between alterations in the NO-cGMP system and in BF in cerebellum but not in thalamus or cortex. This agrees with the role of NO in CBF modulation. Neuronal NO plays an essential role in coupling neuronal activity with CBF in cerebellum but not in cortex[38,39]. NO modulates BF in cerebellum via stimulation of soluble guanylyl cyclase and cGMP formation[38-40]. This suggests that altered NO-cGMP pathway could contribute to increase BF in cerebellum in patients with MHE and even in cirrhotic patients without MHE.
The NO-cGMP pathway is strongly altered in blood[25,41-43] and cerebellum[44] of cirrhotic patients with MHE or died in HE. Moreover, altered activation of guanylate cyclase by NO in lymphocytes correlates with the MHE grade in cirrhotic patients. It has been suggested that altered NO-cGMP pathway contributes to cognitive impairment in MHE[42]. The contribution of altered NO-cGMP pathway in cerebellum to cognitive impairment has been clearly established in animal models of MHE. Rats with MHE have reduced ability to learn a Y maze task, which is a consequence of impaired function of the glutamate-NO-cGMP pathway in cerebellum. Learning ability is restored by treatments that restore the pathway and cGMP levels[12-14,45].
These data suggest the idea that, in patients with MHE, altered NO-cGMP pathway is associated with altered CBF in cerebellum which, in turn, may contribute to induce MHE, impairing bimanual and visuo-motor coordination, executive functioning, cognitive processing speed and performance in the PHES. If this were the case, treatments normalizing the NO-cGMP pathway could improve CBF in cerebellum and cognitive function in cirrhotic patients with MHE.
In summary, the data support that: (1) CBF is more sensitive in cerebellum than in other areas of cirrhotic patients; (2) altered CBF in cerebellum is an early event contributing to initiate neurological deterioration, impairing bimanual coordination and the congruent Stroop task and predisposing to impair visuomotor coordination, executive functioning and cognitive processing speed; (3) altered NO-cGMP pathway would contribute to alter CBF in cerebellum; and (4) non invasive determination of CBF in cerebellum using ASL may detect MHE earlier than the currently used PHES battery.
Patients with minimal hepatic encephalopathy (MHE) show neurological impairment in specific tasks to which selective regional alterations in blood flow (BF) could contribute. Invasive positron emission tomography and single photon emission computed tomography techniques have shown altered cerebral blood flow patterns in cirrhotic patients, but there is a large variability between the results published. The possible contribution of alterations in BF in specific brain areas to neurological alterations in patients with MHE remains unclear. Previous reports suggest that impairment of some motor coordination functions are early markers for cerebral dysfunction in some patients with MHE even prior to neuropsychometric alterations becoming detectable. These motor functions are mainly modulated in cerebellum, supporting that cerebellar alterations would contribute to these early alterations.
Arterial spin labeling (ASL) is a non-invasive magnetic resonance technique that measures quantitatively cerebral perfusion by magnetically labeling protons in arterial blood water. This study assess whether non invasive BF measurement by ASL in several brain regions detects MHE and/or specific neurological alterations.
The authors analyzed BF by ASL in different brain areas of controls and cirrhotic patients without and with MHE. The authors found that BF is more affected in cerebellum than in other areas of cirrhotic patients and that BF determination in cerebellum using ASL may detect MHE earlier than the Psychometric Hepatic Encephalopathy Score (PHES) battery. Altered NO-cGMP pathway seems to be associated to altered BF in cerebellum.
ASL technique would be useful to detect MHE and/or specific neurological alterations. This would allow early treatment and follow-up of the eficiency of therapeutic treatments.
ASL is a non-invasive magnetic resonance technique that measures quantitatively cerebral perfusion by magnetically labeling protons in arterial blood water. PHES is a battery of five psychometric tests that has been recommended as the “gold standard” in the diagnosis of MHE. NO-cGMP pathway is an intracellular signaling pathway involved in important brain functions.
In this cross-sectional study, the authors report a moderate correlation between non-invasive cerebral blood flow, in the cerebellum and minimal hepatic encephalopathy as well as other psychometric tests in patients with cirrhosis. Overall, this is a rigorous and well-conducted study.
P- Reviewer: Singh S S- Editor: Qi Y L- Editor: A E- Editor: Ma S
| 1. | Amodio P, Montagnese S, Gatta A, Morgan MY. Characteristics of minimal hepatic encephalopathy. Metab Brain Dis. 2004;19:253-267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 196] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 2. | Weissenborn K, Giewekemeyer K, Heidenreich S, Bokemeyer M, Berding G, Ahl B. Attention, memory, and cognitive function in hepatic encephalopathy. Metab Brain Dis. 2005;20:359-367. [PubMed] [Cited in This Article: ] |
| 3. | Bajaj JS. Minimal hepatic encephalopathy matters in daily life. World J Gastroenterol. 2008;14:3609-3615. [PubMed] [Cited in This Article: ] |
| 4. | Butz M, Timmermann L, Braun M, Groiss SJ, Wojtecki L, Ostrowski S, Krause H, Pollok B, Gross J, Südmeyer M. Motor impairment in liver cirrhosis without and with minimal hepatic encephalopathy. Acta Neurol Scand. 2010;122:27-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Felipo V, Ordoño JF, Urios A, El Mlili N, Giménez-Garzó C, Aguado C, González-Lopez O, Giner-Duran R, Serra MA, Wassel A. Patients with minimal hepatic encephalopathy show impaired mismatch negativity correlating with reduced performance in attention tests. Hepatology. 2012;55:530-539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Ahl B, Weissenborn K, van den Hoff J, Fischer-Wasels D, Köstler H, Hecker H, Burchert W. Regional differences in cerebral blood flow and cerebral ammonia metabolism in patients with cirrhosis. Hepatology. 2004;40:73-79. [PubMed] [Cited in This Article: ] |
| 7. | Iwasa M, Matsumura K, Kaito M, Ikoma J, Kobayashi Y, Nakagawa N, Watanabe S, Takeda K, Adachi Y. Decrease of regional cerebral blood flow in liver cirrhosis. Eur J Gastroenterol Hepatol. 2000;12:1001-1006. [PubMed] [Cited in This Article: ] |
| 8. | Iwasa M, Matsumura K, Nakagawa Y, Yamamoto M, Tanaka H, Horiike S, Ikoma J, Kaito M, Takeda K, Adachi Y. Evaluation of cingulate gyrus blood flow in patients with liver cirrhosis. Metab Brain Dis. 2005;20:7-17. [PubMed] [Cited in This Article: ] |
| 9. | Catafau AM, Kulisevsky J, Bernà L, Pujol J, Martin JC, Otermin P, Balanzó J, Carrió I. Relationship between cerebral perfusion in frontal-limbic-basal ganglia circuits and neuropsychologic impairment in patients with subclinical hepatic encephalopathy. J Nucl Med. 2000;41:405-410. [PubMed] [Cited in This Article: ] |
| 10. | Golay X, Hendrikse J, Lim TC. Perfusion imaging using arterial spin labeling. Top Magn Reson Imaging. 2004;15:10-27. [PubMed] [Cited in This Article: ] |
| 11. | Keynes RG, Garthwaite J. Nitric oxide and its role in ischaemic brain injury. Curr Mol Med. 2004;4:179-191. [PubMed] [Cited in This Article: ] |
| 12. | Erceg S, Monfort P, Hernández-Viadel M, Rodrigo R, Montoliu C, Felipo V. Oral administration of sildenafil restores learning ability in rats with hyperammonemia and with portacaval shunts. Hepatology. 2005;41:299-306. [PubMed] [Cited in This Article: ] |
| 13. | Erceg S, Monfort P, Hernandez-Viadel M, Llansola M, Montoliu C, Felipo V. Restoration of learning ability in hyperammonemic rats by increasing extracellular cGMP in brain. Brain Res. 2005;1036:115-121. [PubMed] [Cited in This Article: ] |
| 14. | Cauli O, Rodrigo R, Piedrafita B, Boix J, Felipo V. Inflammation and hepatic encephalopathy: ibuprofen restores learning ability in rats with portacaval shunts. Hepatology. 2007;46:514-519. [PubMed] [Cited in This Article: ] |
| 15. | Montoliu C, Cauli O, Urios A, ElMlili N, Serra MA, Giner-Duran R, González-Lopez O, Del Olmo JA, Wassel A, Rodrigo JM. 3-nitro-tyrosine as a peripheral biomarker of minimal hepatic encephalopathy in patients with liver cirrhosis. Am J Gastroenterol. 2011;106:1629-1637. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1468] [Cited by in F6Publishing: 1364] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 17. | Weissenborn K, Ennen JC, Schomerus H, Rückert N, Hecker H. Neuropsychological characterization of hepatic encephalopathy. J Hepatol. 2001;34:768-773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 540] [Cited by in F6Publishing: 528] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 18. | Glaser MO, Glaser WR. Time course analysis of the Stroop phenomenon. J Exp Psychol Hum Percept Perform. 1982;8:875-894. [PubMed] [Cited in This Article: ] |
| 19. | Golden CJ. Stroop Test de Colores y Palabras. Aplicada. Madrid: TEA Ediciones 2001; . [Cited in This Article: ] |
| 20. | Yela M. López Ladrón L. Un test de coordinación visomotora. Rev Psic Gral y Apl. 1955;34:409-421. [Cited in This Article: ] |
| 21. | Yela M. Un test de rapidez motora. Rev Psic Gral y Apl. 1955;33:137-148. [Cited in This Article: ] |
| 22. | Kircheis G, Wettstein M, Timmermann L, Schnitzler A, Häussinger D. Critical flicker frequency for quantification of low-grade hepatic encephalopathy. Hepatology. 2002;35:357-366. [PubMed] [Cited in This Article: ] |
| 23. | Romero-Gómez M, Córdoba J, Jover R, del Olmo JA, Ramírez M, Rey R, de Madaria E, Montoliu C, Nuñez D, Flavia M. Value of the critical flicker frequency in patients with minimal hepatic encephalopathy. Hepatology. 2007;45:879-885. [PubMed] [Cited in This Article: ] |
| 24. | Montoliu C, Kosenko E, Del Olmo JA, Serra MA, Rodrigo JM, Felipo V. Correlation of nitric oxide and atrial natriuretic peptide changes with altered cGMP homeostasis in liver cirrhosis. Liver Int. 2005;25:787-795. [PubMed] [Cited in This Article: ] |
| 25. | Günther M, Bock M, Schad LR. Arterial spin labeling in combination with a look-locker sampling strategy: inflow turbo-sampling EPI-FAIR (ITS-FAIR). Magn Reson Med. 2001;46:974-984. [PubMed] [Cited in This Article: ] |
| 26. | Leevy CB, Phillips JA. Hospitalizations during the use of rifaximin versus lactulose for the treatment of hepatic encephalopathy. Dig Dis Sci. 2007;52:737-741. [PubMed] [Cited in This Article: ] |
| 27. | Bajaj JS, Pinkerton SD, Sanyal AJ, Heuman DM. Diagnosis and treatment of minimal hepatic encephalopathy to prevent motor vehicle accidents: a cost-effectiveness analysis. Hepatology. 2012;55:1164-1171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 28. | Encinas M, De Juan R, Marcos A, Gil P, Barabash A, Fernández C, De Ugarte C, Cabranes JA. Regional cerebral blood flow assessed with 99mTc-ECD SPET as a marker of progression of mild cognitive impairment to Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2003;30:1473-1480. [PubMed] [Cited in This Article: ] |
| 29. | Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19:203-214. [PubMed] [Cited in This Article: ] |
| 30. | Segarra N, Bernardo M, Valdes M, Caldu X, Falcón C, Rami L, Bargallo N, Parramon G, Junque C. Cerebellar deficits in schizophrenia are associated with executive dysfunction. Neuroreport. 2008;19:1513-1517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Kühn S, Romanowski A, Schubert F, Gallinat J. Reduction of cerebellar grey matter in Crus I and II in schizophrenia. Brain Struct Funct. 2012;217:523-529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 32. | Camicioli R, Gee M, Bouchard TP, Fisher NJ, Hanstock CC, Emery DJ, Martin WR. Voxel-based morphometry reveals extra-nigral atrophy patterns associated with dopamine refractory cognitive and motor impairment in parkinsonism. Parkinsonism Relat Disord. 2009;15:187-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Braga-Neto P, Pedroso JL, Alessi H, Dutra LA, Felício AC, Minett T, Weisman P, Santos-Galduroz RF, Bertolucci PH, Gabbai AA. Cerebellar cognitive affective syndrome in Machado Joseph disease: core clinical features. Cerebellum. 2012;11:549-556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 34. | Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP. Cerebellar and premotor function in bimanual coordination: parametric neural responses to spatiotemporal complexity and cycling frequency. Neuroimage. 2004;21:1416-1427. [PubMed] [Cited in This Article: ] |
| 35. | Pollok B, Butz M, Gross J, Schnitzler A. Intercerebellar coupling contributes to bimanual coordination. J Cogn Neurosci. 2007;19:704-719. [PubMed] [Cited in This Article: ] |
| 36. | Miall RC, Reckess GZ, Imamizu H. The cerebellum coordinates eye and hand tracking movements. Nat Neurosci. 2001;4:638-644. [PubMed] [Cited in This Article: ] |
| 37. | Nabeyama M, Nakagawa A, Yoshiura T, Nakao T, Nakatani E, Togao O, Yoshizato C, Yoshioka K, Tomita M, Kanba S. Functional MRI study of brain activation alterations in patients with obsessive-compulsive disorder after symptom improvement. Psychiatry Res. 2008;163:236-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 38. | Yang G, Chen G, Ebner TJ, Iadecola C. Nitric oxide is the predominant mediator of cerebellar hyperemia during somatosensory activation in rats. Am J Physiol. 1999;277:R1760-R1770. [PubMed] [Cited in This Article: ] |
| 39. | Hayashi T, Katsumi Y, Mukai T, Inoue M, Nagahama Y, Oyanagi C, Yamauchi H, Shibasaki H, Fukuyama H. Neuronal nitric oxide has a role as a perfusion regulator and a synaptic modulator in cerebellum but not in neocortex during somatosensory stimulation--an animal PET study. Neurosci Res. 2002;44:155-165. [PubMed] [Cited in This Article: ] |
| 40. | Yang G, Iadecola C. Activation of cerebellar climbing fibers increases cerebellar blood flow: role of glutamate receptors, nitric oxide, and cGMP. Stroke. 1998;29:499-507; discussion 507-508. [PubMed] [Cited in This Article: ] |
| 41. | Corbalán R, Miñana MD, Del Olmo JA, Serra MA, Rodrigo JM, Felipo V. Altered modulation of soluble guanylate cyclase in lymphocytes from patients with liver disease. J Mol Med (Berl). 2002;80:117-123. [PubMed] [Cited in This Article: ] |
| 42. | Montoliu C, Piedrafita B, Serra MA, del Olmo JA, Ferrandez A, Rodrigo JM, Felipo V. Activation of soluble guanylate cyclase by nitric oxide in lymphocytes correlates with minimal hepatic encephalopathy in cirrhotic patients. J Mol Med (Berl). 2007;85:237-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 43. | Montoliu C, Rodrigo R, Monfort P, Llansola M, Cauli O, Boix J, Elmlili N, Agusti A, Felipo V. Cyclic GMP pathways in hepatic encephalopathy. Neurological and therapeutic implications. Metab Brain Dis. 2010;25:39-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 44. | Corbalán R, Chatauret N, Behrends S, Butterworth RF, Felipo V. Region selective alterations of soluble guanylate cyclase content and modulation in brain of cirrhotic patients. Hepatology. 2002;36:1155-1162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 45. | Cauli O, Mansouri MT, Agusti A, Felipo V. Hyperammonemia increases GABAergic tone in the cerebellum but decreases it in the rat cortex. Gastroenterology. 2009;136:1359-1367, e1-2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |